Abstract
The NF-κB family of transcription factors is an important component of stress-activated cytoprotective signal transduction pathways. Previous studies demonstrated that some activation mechanisms require phosphorylation, ubiquitination and degradation of the inhibitor protein, IκBα. Herein, it is demonstrated that ionizing radiation in the therapeutic dose range stimulates NF-κB activity by a mechanism in which IκBα tyrosine-181 is nitrated as a consequence of constitutive NO• synthase activation, leading to dissociation of intact IκBα from NF-κB. This mechanism does not appear to require IκBα kinase-dependent phosphorylation or proteolytic degradation of IκBα. Tyrosine-181 is involved in several noncovalent interactions with the p50 subunit of NF-κB stabilizing the IκBα/NF-κB complex. Evaluation of hydropathic interactions of IκBα/p50 based on the crystal structure of the complex is consistent with nitration disrupting these interactions, and dissociating the IκBα/NF-κB complex. Tyrosine nitration is not commonly studied in the context of signal transduction. However the present results indicate that tyrosine nitration is an important post-translational regulatory modification for NF-κB activation and possibly for other signaling molecules modulated by mild and transient oxidative and nitrosative stresses.
The Rel/NF-κB family of transcription factors mediate cellular responses to oxidative and other stresses (1). NF-κB transcription factors are formed by the homo-or heterodimerization of proteins of the Rel family including p50, p52, p65 (RelA), c-Rel and RelB. The most abundant and best-understood dimer is p65/p50. This dimer exists in the cytoplasm complexed with an inhibitor protein, IκBα, that masks the NF-κB nuclear localization sequence (2).Stimulation of cells with cytokines such as tumor necrosis factor (TNFα) results in the phosphorylation, ubiquitination and degradation of IκBα, permitting the unmasked p65/p50 heterodimer to translocate into the nucleus (2).
Ionizing radiation (IR) also activates NF-κB by mechanisms not fully understood. At high IR doses (>10Gy) activation appears similar to that observed for TNFα. DNA damage-inducible kinases, ATM and DNA-PK, activate IκBα kinases (e.g., IKKβ) stimulating the phosphorylation, ubiquitination and proteasome degradation of IκBα (1, 3). IKKβ activity and IκBα degradation, however, do not appear necessary for NF-κB activation at lower, therapeutic doses of IR, e.g. (4, 5). Indeed, IR inhibits proteasomal activities at doses as low as 0.2 Gy with maximal inhibition at 2 Gy (4, 6). IKKβ has other functions including phosphorylation of S536 in the transactivation domain of the p65 subunit (7–10). Serine-536 phosphorylation is independent of IκBα phosphorylation and important for enhancing p65 transactivation potential and modulating gene target selection.
Recent studies demonstrate that NO• and a metabolic derivative, peroxynitrite (ONOO−) modulate NF-κB activity (11–15). For example, addition of a ONOO− donor to cultured cells stimulates NF-κB reporter activity without IκBα degradation (11). These findings and the evidence that a Ca2+ dependent constitutive NO• synthase activity in epithelial cells is transiently stimulated by low IR doses (16–19) prompted the following investigation of NO• signaling in IR-induced activation of NF-κB.
Materials and Methods
Cell Culture, Irradiation, and Transfection
CHO-K1 and MCF-7 cells were cultured and irradiated at a dose rate of 2 Gy/min with a 60Co source as previously described (18). Cells were transfected with the LipofectAMINE PLUS™ kit (Invitrogen).
Reagents
Primary antibodies used: actin, nuclear lamin A/C, IκBα, NOS1, IKKβ and p65 (Santa Cruz Biotechnology); nitro-tyrosine (Upstate Biotechnology); p50, phospho-S32/36-IκBα, and 9E10 epitope of c-Myc (Cell Signaling).
Wild type pCMV-IκBα from Clontech has two ATG start sites with the second having an optimal Kozak sequence. For this reason an IκBα doublet was detected after SDS polyacrylamide gel electrophoresis in early experimentation. Both proteins were, however, equally nitrated (e.g. Fig. 2A,E; 4A). Amino acid substitution mutants were constructed from pairs of point mutation primers by PCR technology with wild type pCMV-IκBα as the initial template. Mutations were verified by full-length sequencing. Another set of mutants was prepared with an N-terminal c-Myc epitope tag to facilitate analysis.
Figure 2.
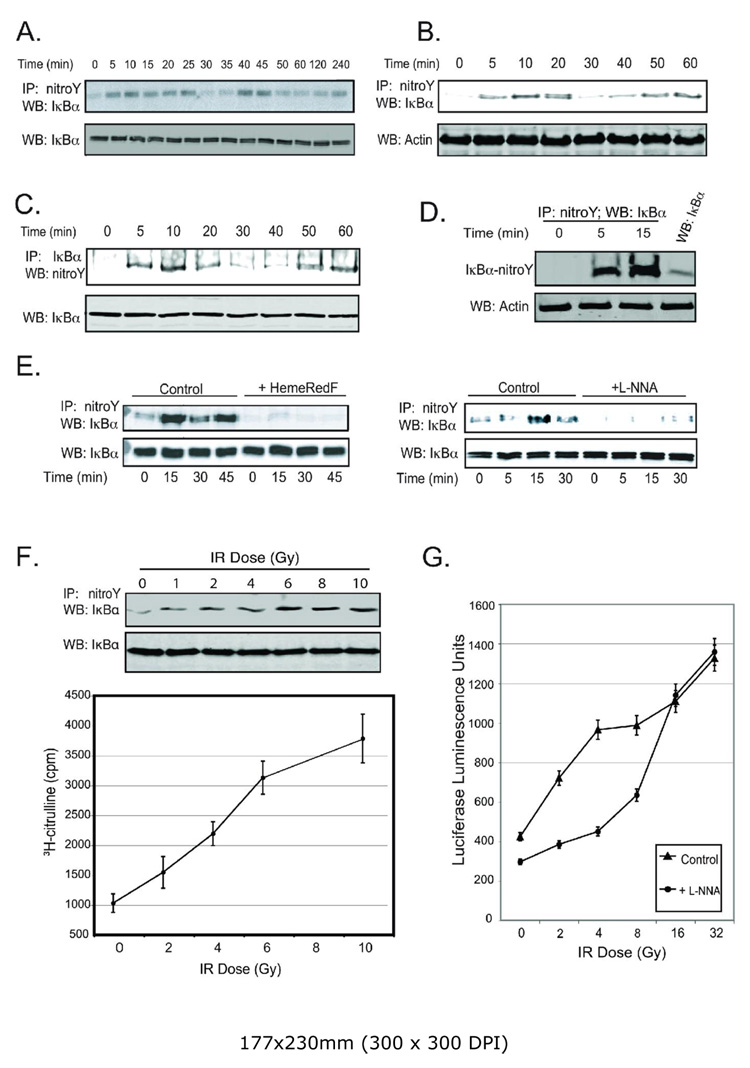
IR dose dependence of NOS-1 activity, NF-κB induction and IκBα tyrosine-nitration. A. Tyrosine nitration of IκBα after IR (5 Gy) was monitored in CHO-cells transfected with pCMV-IκBα-wild type and irradiated 48 h after transfection. Anti-nitro-tyrosine immunoprecipitates analyzed for IκBα by Western blotting. Equal loading was verified by immunoblotting cell lysates with anti-IκBα antibody (bottom panel). B. Radiation-stimulated tyrosine nitration of endogenous IκBα in MCF-7 cells. MCF-7 cells were radiated (5 Gy) and cell lysate prepared at the indicated time points. Anti-nitro-tyrosine immunoprecipitates were analyzed with anti-IκBα antibody. For loading controls, equal amounts of each cell lysate were fractionated by electrophoresis and immunoblotted with anti-actin antibody. C. MCF-7 cells were radiated as in Fig. 2B. Anti-IκBα immunoprecipitates were probed with anti-nitro-tyrosine antibody. For loading controls, equal amounts of each immunoprecipitate were fractionated by electrophoresis and blotted with anti-IκBα. D. Whole-cell extracts from non-transfected cells were prepared at 0 (no IR), and 5 and 15 min post-IR (5 Gy). Anti-nitro-tyrosine immunoprecipitates from total cell extracts were analyzed by immunoblotting with anti-IκBα and compared with 2% of total cellular IκBα (lane 4). Cell lysates were probed with anti-actin antibody as loading control (bottom panel). E. NOS activity modulates IκBα tyrosine-nitration after IR (5 Gy). CHO cells were co-transfected with pIκBα and pHemeRedF or an empty vector. Alternatively, cells transfected with pIκBα were treated with 100nM L-NNA 4 h prior to irradiation. The experimental protocol in Fig. 2C was used to determine the effects of inhibiting NOS activity by HemeRedF expression or incubating cells with L-NNA (upper panel). Bottom panels are loading controls of cell lysates probed with anti-IκBα antibody. F. MCF-7 cells were lysed 15 minutes after exposure to different IR doses. Anti-nitro-tyrosine immunoprecipitates were analyzed by immunoblotting with anti-IκBα. Cell lysates were probed with anti-IκBα to verify equal loading (lower panel); Graph: Arginine-citrulline conversion assay with different doses of IR was performed as previously described (17,20). Results are presented as the average of triplicate samples ±SD; G. L-NNA inhibition of NF-κB activity after different doses of IR. MCF-7 cells were transfected with pNF-κB-luc and irradiated 48 h after transfection. Luciferase activity was measured in cell lysates 24 h later. Cells were incubated with 100 nM L-NNA for 4 h prior to a IR exposure.
Figure 4.
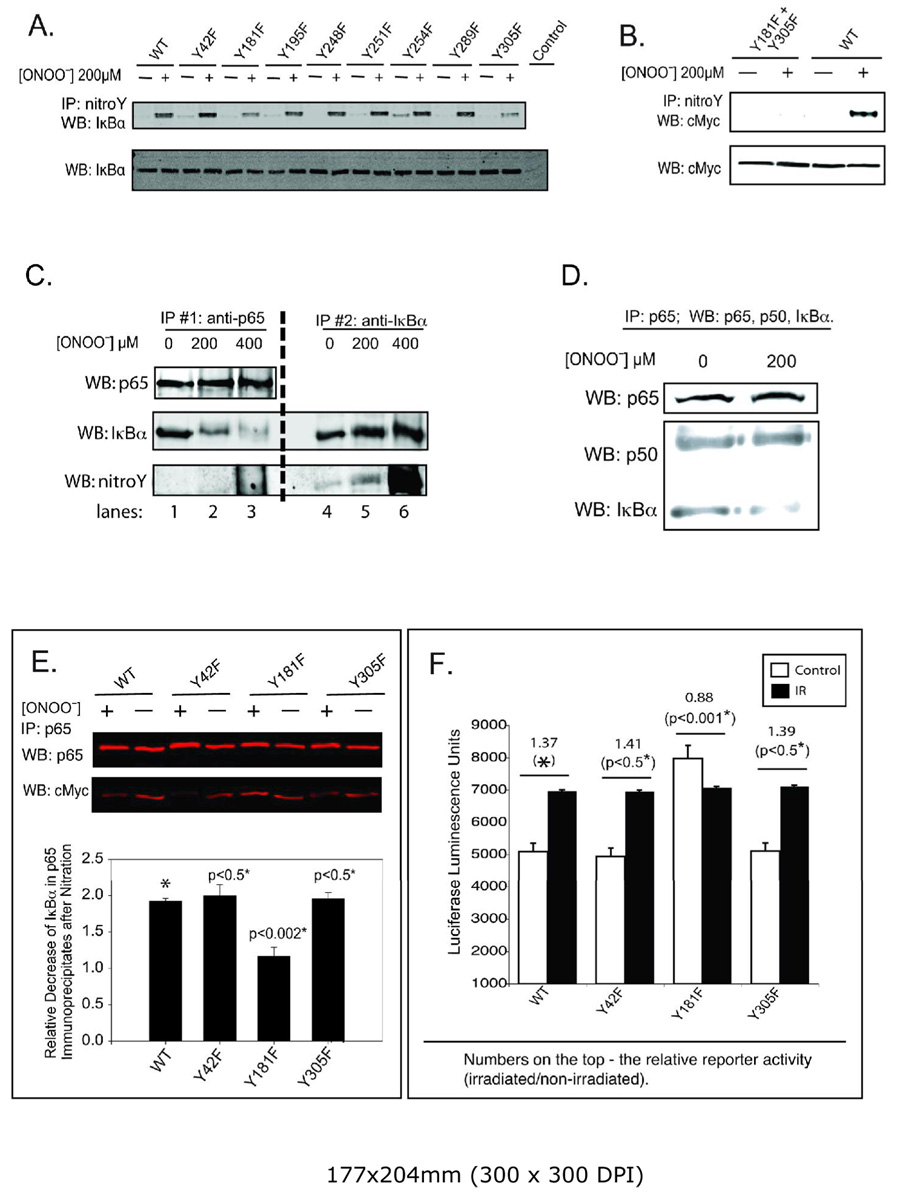
Tyrosine-nitration of IκBα dissociates the IκBα/NF-κB complex. A. Cell lysates from CHO cells expressing IκBα wild type and different Y→F mutants were treated with ONOO−. Anti-nitro-tyrosine immunoprecipitates were analyzed by immunoblotting with anti-IκBα. Cell lysates were probed with anti-IκBα to verify equal loading (lower panel). Control –untransfected sample. B. Cell lysates from CHO cells expressing cMyc-tagged IκBα wild type and double mutant 181/305YY→FF were treated with ONOO−. Anti-nitro-tyrosine immunoprecipitates were analyzed by immunoblotting with anti-cMyc. Cell lysates were probed with anti-cMyc to verify equal loading of cMyc-tagged IκBα (lower panel). C. p65 and IκBα immunoprecipitates were obtained from ONOO− treated cell lysates as described in the text. Preliminary control experiments demonstrated that the amount of antibody used was sufficient to fully immunoprecipitate the target antigen. Samples from first (lanes 1, 2, 3) and second (lanes 4, 5, 6) immunoprecipitations were analyzed for p65 (upper panel) and IκBα (middle panel) by immunoblotting. The blots were probed simultaneously with anti-nitro-tyrosine antibody (bottom panel). D. After ONOO− treatment, anti-p65 immunoprecipitates were analyzed for p50 and IκBα. The loading control was p65 (upper panel). E. CHO-cells were transfected with plasmids encoding c-myc-tagged wild type and the Y→F mutants of IκBα. Cell lysates prepared 24 hours after transfection were treated with 200 µM ONOO−. Anti-p65 immunoprecipitates were analyzed by Western blot for p65 and c-myc. The fluorogram from one of two experiments is shown. The fluorescence intensity readings from two different experiments were used to calculate the relative nitration-induced decrease of IκBα associated with p65 calculated as average intensity of IκBα normalized to the loading control (p65 intensity) of +/−standard deviation. * – The p values were determined by Student’s t-test relative to wild type transfected cells. F. DNA-binding activity of NF-κB was estimated as in Fig. 1 after co-transfection of pNF-κB-luc with IκBα wild type and the indicated Y→F mutants. The increase in activity after IR was normalized relative to cells co-transfected with pNF-κB-luc and empty vector. Data are means ±SD for quadruplicate samples and representative for experiments performed in triplicate. * – The p values were determined by Student’s t-test relative to the wild type transfected cells.
The luciferase-based reporter construct of NF-κB (pNF-κB-luc) was also purchased from Clontech. Luciferase activity was measured in cell lysates 24 hr after IR exposure with a Luciferase Reporter Gene Assay Kit (Packard Bioscience) according to the manufacturer’s directions.
The human shRNA IKKβ plasmid was provided by Upstate Biotechnology. The dominant negative NOS-1 mutant (HemeRedF) and its effects on expression and activity of NOS-1 in CHO and other cells have been described (17, 20, 21). Mouse NOS-1 siRNAs and All Stars Negative Control siRNA were purchased from QIAGEN. HiPerFect Transfection Reagent (QIAGEN) was used for transfection of CHO-cells with siRNAs. Cells were seeded and transfected on the same day according to the manufacturer’s reverse transfection protocol.
Measurement of Nuclear NF-κB DNA Binding Activity
CHO-cells were seeded 48 h before radiation in 100-mm dishes and transfected the same day with the NOS1 siRNAs or 24 h later with a plasmid expressing the HemeRedF NOS-1 mutant. Incubation with 100nM L-NNA was performed 4 h before radiation. NF-κB DNA binding activity in nuclear extracts prepared as in (22) was measured with a NF-κB p65 ELISA Kit (Stressgen Bioreagents) according to manufacturer’s recommendations.
Biochemical Analyses
Immunoprecipitation and Western blotting methods have been described (17) (20). Protein detection was by chemiluminescence with alkaline phosphatase-conjugated secondary antibodies or with secondary antibodies conjugated with infrared fluorescent dyes and imaging with the Odyssey® Infrared Imaging System (Li-Cor® Biosciences). Cellular NOS activity was measured with an arginine to citrulline assay as previously described (17, 20).
Mass Spectrometry
All proteins were resolved by one-dimensional SDS-PAGE and silver stained. After destaining, proteins were in-gel digested with modified porcine trypsin (0.6 µg, Promega) alone or with S. aureus V8 protease (0.6 µg, Sigma Chemical Co.) for 12 hrs according to the method of Shevchenko (23). The resultant peptides were purified with Poros 20 R2 reverse phase packing (Applied Biosystems) and subjected to direct infusion nanospray using NanoES spray capillaries (PROXEON, Odense, Denmark) on an Applied Biosystems QSTAR® pulsar XL mass spectrometer. MS spectra were collected in positive mode with an ion spray voltage of 800 volts. Subsequent MS/MS spectra were collected and amino acid sequences obtained using BioAnalyst software.
Structural Analysis
To determine the optimal geometry of nitro-tyrosine, we performed quantum mechanical calculations using density functional theory with 6-311+G(d,p) basis set, B3PW91 hybrid functionals, default spin configuration, and net molecular charge of zero (24). Self-consistent field was calculated directly with convergence limit of 2 × 10−5. These parameters ensured that optimized structure of nitro-tyrosine was available for calculation of its various structural and chemical properties. The force constants for bonds, bond angles, and dihedrals were calculated using the Hessian matrix. Subsequently, charge distribution and electronic polarizability of nitro-tyrosine were calculated in water solution to fully characterize the properties of this modified amino acid. These properties were properly entered in the CHARMM22 (25) parameter and topology files for use in NAMD2, a molecular dynamics algorithm (26, 27). The validity of quantum mechanical calculations was established by 13CNMR spectroscopy using commercially available 3-nitro-tyrosine ethyl ester in D2O (see supplemental data).
Two X-ray structures of IκBα/NF-κB complex are found in the Protein Data Bank – 1NFI (2.7Å resolution) (28) and 1IKN (2.3 Å resolution) (29). The R-values for both structures were 0.223. The sequence enumerations are identical for IκBα and p65 but are shifted by 3 residues for the p50 subunit. The 1IKN structure was used for our analysis. Energy minimization was performed for 2500 steps enabling energetic relaxation of the system.
For the HINT calculations, the PDB coordinates of control and nitrated IκBα/NF-κB complexes at the end of minimization were used. We defined Y181 or nitro-Y181 as structure “A” and the remainder of the IκBα/NF-κB complex as structure “B”. The HINT program was then used to evaluate a comprehensive set of non-bonded interactions between structures “A” and “B” (hydrogen bonding, acid-base, hydrophobic-hydrophobic, acid-acid, base-base, and hydrophobic-polar) (30–35).
Results
Inhibiting NOS-1 blocks IR-stimulated NF-κB activity
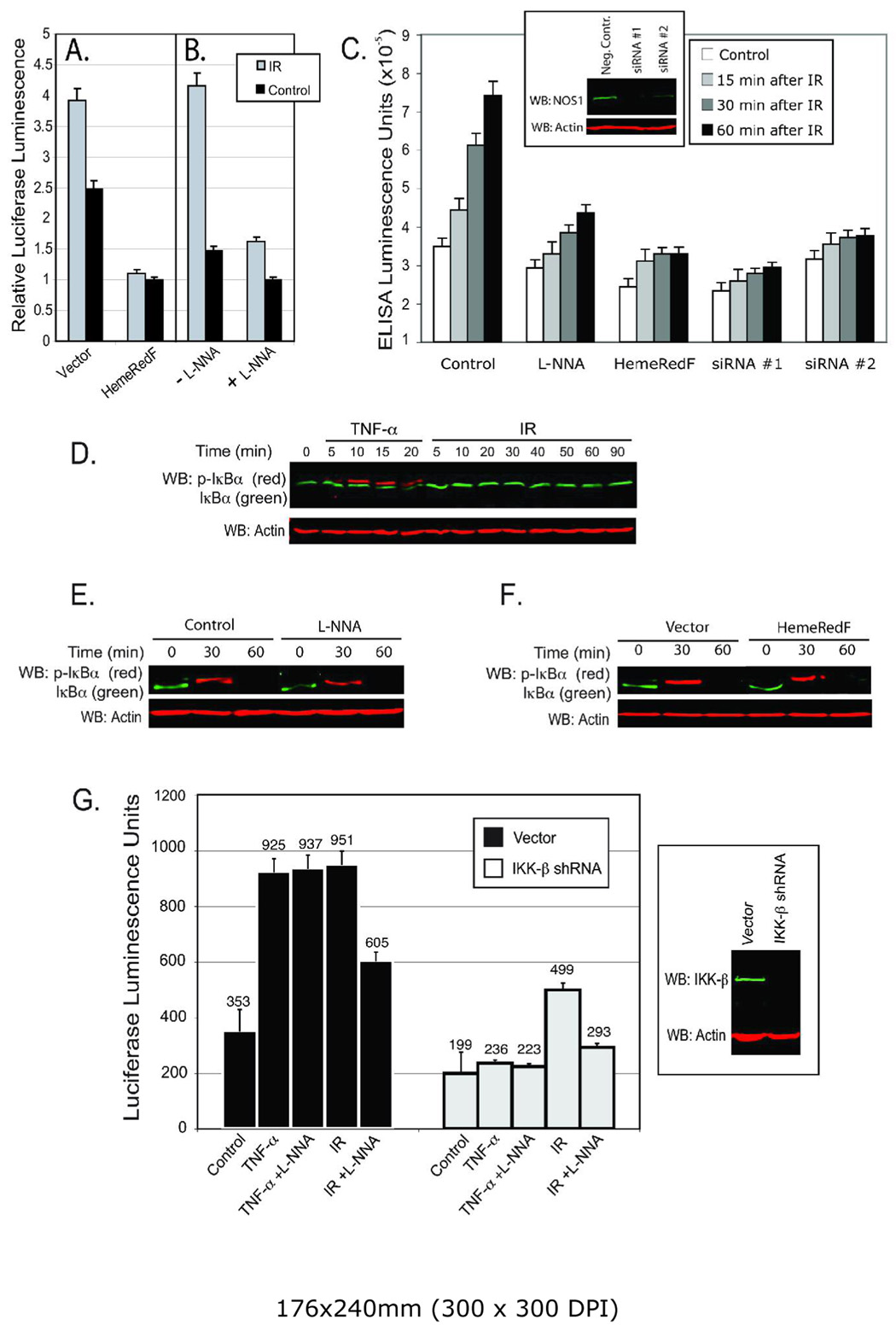
Initial experiments tested whether radiation induced NOS-1 activity contributed to an increase of NF-κB promoter activity measured with a luciferase based reporter assay. CHO cells were co-transfected with the reporter plasmid and either an empty vector or the dominant negative mutant of NOS-1 (HemeRedF) whose expression was previously shown to inhibit IR-activated NOS activity in CHO cells (17, 20). A single IR exposure of 5 Gy stimulated a 1.5–3 fold increase in NF-κB dependent luciferase activity measured at 24 h post-IR (e.g. Fig. 1A,B,G; 2G). This increase in activity was similar to what others have observed with diverse cell types after an exposure to IR (36–38). Expression of HemeRedF completely inhibited IR-stimulated reporter gene expression and reduced basal reporter activity by 40 to 50% (Fig. 1A). The effect of genetically inhibiting NOS-1 activity on NF-κB promoter activity was confirmed pharmacologically with the NOS inhibitor, NG-Nitro-L-arginine (L-NNA). Incubating cells with L-NNA 4 hr prior to radiation significantly reduced both basal and IR-induced NF-κB promoter activity (Fig. 1B,G; 2G).
Figure 1.
IR and TNFα activation of NF-κB. A. CHO-cells were co-transfected with pNF-κB-luc and either pHemeRedF (dominant negative mutant of NOS-1) or an empty vector as a control. Cells were radiated (5 Gy) 48 h after transfection and luciferase activity was measured in cell lysates 24 h later. B. 44 hrs after transfection with pNF-κB-luc, cells were treated with 100 nM L-NNA for 4 h prior to a 5 Gy IR exposure. Experimental data (for A and B) are presented as means ±SD for quadruplicate samples and are representative of experiments performed in triplicate. C. CHO-cells were seeded and transfected with NOS1 siRNAs on the same day or with HemeRedF plasmid 24 hours later. Incubation with 100nM L-NNA was performed 4 h before radiation. Cells were radiated (5 Gy) 48 h after seeding and harvested at the given time-points after IR. Nuclear extracts of the cells were prepared and normalized relative to the total protein concentration. The ELISA assay was performed to measure specific DNA binding activity of NF-κB in the nuclear extracts. Experimental data are presented as means ±SD for triplicate samples. Embedded panel shows level of NOS1 48-h after isRNAs transfection. D. MCF-7 cells were irradiated at 5 Gy and cell lysates analyzed by immunoblotting for phospho-S32/36-IκBα (red) and IκBα (green). As a positive control cells were treated with 10 nM TNFα. Equal loading was verified by blotting with anti-actin (bottom panel). E. Cells were pretreated with 100 nM L-NNA for 4 h prior to adding 10nM of TNFα. Equal loading was verified by western blotting of cell lysates with anti-actin (bottom panel). F. MCF-7 cells were transfected with pHemeRedF or empty vector as a control. Cells were treated with TNFα (10nM) 48 h after transfection. Equal loading was verified by immunoblotting cell lysates with anti-actin (bottom panel). G. MCF-7 cells were co-transfected with pNF-κB-luc and either pIKK-β shRNA or the empty vector as a control. Cells were radiated (5 Gy) or treated with TNFα 48 h after transfection and luciferase activity was measured in cell lysates 24 h later. L-NNA (100nM) was added to the cell cultures 4 hrs prior to IR exposure or TNFα treatment. Experimental data are presented as means ±SD for triplicate samples. Right panel: Western blots of cell lysates validating the effectiveness of IKKβ shRNA treatment – 48 h after transfection. Cell lysates were probed with anti-IKKβ (green) and anti-actin antibody (red) as a loading control.
To further validate the role for NOS-1 in NF-κB activation by IR, cells were transfected with control and siRNA directed against NOS-1. The inset of Figure 1C demonstrates that both siRNAs tested were efficient in knocking down expression of NOS-1 in CHO cells. We compared the relative effects of siRNA transfection, expression of the NOS-1 mutant, HemeRedF, and the chemical inhibitor, L-NNA, with respect to their relative abilities to inhibit basal and IR-induced NF-κB. NF-κB activity for these analyses was measured using nuclear extracts in an ELISA assay for p65 binding to a NF-κB specific oligonucleotide consensus sequence. Results in Figure 1C show that all three methods of inhibiting NOS-1 activity were effective at blocking IR-stimulated NF-κB activity although the molecular approaches appeared more effective. Further validation was obtained by following nuclear translocation of p65 subsequent to radiation. As shown in Fig. S3 of Supplemental Data, nuclear isolates were probed for p65 by Western blot analysis normalized with respect to nuclear lamin levels. Nuclear accumulation of p65 increased within 10 min of irradiation by a mechanism inhibited by L-NNA.
Activation of NF-κB by a low IR dose does not stimulate IκBα phosphorylation
We tested whether low IR doses activated IKK measured as IκBα S32/36 phosphorylation and proteolysis. By this assay, IKK activity was not stimulated at the IR doses used (≤5 Gy) (Fig. 1D). Control experiments with TNFα stimulation showed enhanced, but transient IκBα Ser32/36 phosphorylation and progressive decrease in IκBα protein levels indicating that this IKK-dependent activation mechanism was intact. Incubation with L-NNA or expression of HemeRedF did not inhibit IκBα S32/36 phosphorylation stimulated by TNFα (Fig. 1E,F).
NF-κB activation by TNFκ or IR at 5 Gy were also compared in cells transfected with shRNA specific to IKKβ to abrogate this activation pathway for NF-κB (Fig. 1G). A co-transfected luciferase reporter construct was used to assess cellular NF-κB activity. Cells expressing shRNA showed IKKβ protein levels less then 10% of control cells, transfected with empty vector. This inhibition of IKKβ expression with shRNA reduced NF-κB basal activity by 50% and completely blocked stimulation by TNFα. In contrast to these observations with TNFα, IR-stimulated NF-κB activity was only reduced by approximately 50% with IKKβ shRNA expression. The remaining activity was inhibited by treatment with L-NNA (Fig. 1G). Since both basal and IR-stimulated NF-κB activities were reduced by IKKβ shRNA expression, the fold-activation with IR achieved in these cells was not significantly different from that of control, IKKβ-expressing cells.
Radiation stimulates the tyrosine nitration of IκBα
IR-activation of NOS stimulates ONOO− generation detected as tyrosine nitration of a number of proteins (17). We tested whether IR at 5 Gy stimulated the nitration of IκBα using CHO cells transfected with human wild type IκBα. Anti-nitro-tyrosine immunoprecipitates from lysates of control and irradiated cells were analyzed by Western blot for IκBα (Fig. 2A). IR stimulated oscillating changes in IκBα tyrosine nitration with an initial maximum at 10–20 min post-IR and a second maximum at ≈40 min. Similar results were obtained with endogenous IκBα in MCF-7 breast carcinoma cells (Fig. 2B). The reciprocal experiment with immunoprecipitation of IκBα followed by blotting with anti-nitro-tyrosine IgG showed the same oscillations in tyrosine nitration of IκBα without changes in IκBα protein expression levels (Fig. 2C). Comparing amounts of endogenous tyrosine-nitrated IκBα with total IκBα suggests that up to 25% is transiently nitrated 15 min post-IR (Fig. 2D). Basal and IR-induced tyrosine nitration of IκBα are both significantly inhibited by expression of HemeRedF or by incubating cells with L-NNA (Fig. 2E).
Previous studies demonstrated oscillations of NF-κB DNA binding that correlated with oscillations in total IκBα protein levels following TNFα treatment of cells (39, 40). The present work with low doses of IR, in contrast, showed no measurable change in total cellular IκBα levels after IR at 5 Gy (Fig. 2A,C,E). Thus, it is unlikely that selective proteolysis of nitrated IκBα accounts for the observed oscillations in IκBα nitration following an IR exposure. Proteolytic degradation of IκBα is not a requirement for NF-κB activation, e.g. (11, 41). Furthermore, IR inhibits proteasome activities (4, 6).
Radiation dose response analyses comparing cellular NOS activity, IκBα tyrosine nitration and NF-κB transcription reporter activity
Our previous studies (17, 18) using a fluorescent dye to measure reactive oxygen/nitrogen species demonstrated a dose response which saturated at doses >6 Gy. These findings were confirmed by measuring as a function of IR dose cellular NOS activity directly with an arginine-citrulline conversion assay or indirectly by measuring tyrosine nitration of IκBα (Fig. 2F). Both measures of NO• activity progressively increased with IR dose and reached relative plateaus at doses greater than 6 Gy. A similar dose response curve was observed for NF-κB reporter activity (Fig. 2G). At IR doses above 8 Gy, L-NNA was a less effective inhibitor of NF-κB activation. At 16 Gy or higher, inhibition of NOS-1 activity with LNNA had no effect on IR-induced NF-κB activity.
Tyrosines 181 and 305 of IκBα are nitrated after irradiating intact cells
A genetic approach was also used to determine sites of nitration. Each tyrosine of IκBα was individually mutated to phenylalanine. CHO cells were transfected with plasmids expressing all 8 myc-tagged Y-F mutants and the myc tagged wild type IκBα. Tyrosine nitration of the mutants and wild type were compared as a function of time following an IR exposure of 5 Gy by immunoaffinity purification of the nitrated proteins followed by Western blot detection with anti-myc (Fig. 3). To facilitate comparisons between blots, each blot included one lane of wild type of myc-tagged IκBα obtained from cell lysates nitrated with 50 µM ONOO− and subsequently immunopurified with anti-nitro-tyrosine conjugated agarose beads. All single mutants demonstrated with approximately identical frequencies oscillating levels of nitrated IκBα following radiation. However, for the Y181F and Y305F single mutants, the amplitude in IR-induced nitration was significantly less than that observed for wild type and the other tyrosine mutants. A double mutant (Y181F+Y305F) was constructed to test whether these two tyrosines were exclusively nitrated following radiation. As shown in the bottom panels of Fig. 3, the double mutant was not nitrated after a 5 Gy radiation exposure.
Figure 3.
Site-directed mutagenesis and identification of IκBα tyrosines nitrated after radiation. CHO cells were transfected with cMyc-tagged IκBα wild type, different Y→F mutants, and double mutant 181/305YY→FF and irradiated with 5 Gy 48 h after transfection. Cells were lysed at the certain time-points after IR. Anti-nitro-tyrosine immunoprecipitates were analyzed by immunoblotting with anti-cMyc (left panels). Cell lysates were probed with anti-cMyc to verify equal loading of cMyc-tagged IκBα (right panels). As a positive control and to facilitate comparisons, cMyc-tagged IκBα in cell lysates of over-expressing cells was nitrated with 50µM ONOO−. The nitrated IκBα as immunopurified as described and run on each blot (the last line of the each panel).
Peroxynitrite treatment of the NF-κB/IκBα nitrates tyrosines 181 and 305 of IκBα and dissociates the IκBα/NF-κB complex
Initial experiments using the different IκBα mutants and treatment of cell lysates with ONOO− also demonstrated a high degree of specificity in the ONOO− induced nitration of IκBα. As observed with radiation only mutant proteins for tyrosines 181 and 305 showed significantly reduced nitration following a single bolus addition of ONOO−. The double mutant for these two tyrosines was not nitrated at all (Fig. 4A,B).
We attempted to validate the genetic evidence for ONOO− induced nitration of these two tyrosines by mass spectrometry. The cell lysate with overexpressed IκBα was nitrated with ONOO− and nitrated IκBα isolated by precipitation with anti-nitro-tyrosine IgG, resolved by gel electrophoresis and processed for mass spectroscopy. After proteolysis, five IκBα peptides were identified for coverage of 21% and this included 4 of the 8 tyrosines of IκBα (Table S1 of supplemental data). The tryptic peptide containing tyrosine-181 is over 40 amino acids long and was not detected in the mass spectra. Attempts with different peptide cutting agents to obtain an identifiable peptide containing tyrosine-181 proved unsuccessful. However, a 20 amino acid peptide of IκBα (aa295–314) was sequenced and identified in the un-nitrated form and also as a peptide with a mass consistent with tyrosine nitration (+45Da, supplemental data, Fig. S1, S2). Our initial attempts to sequence the only tyrosine in this peptide, Y305, to confirm its nitration, have not been successful. Additional mass spectroscopic analysis of the IκBα 295–314 peptide indicated that C308 was modified by propionamide (an acrylamide adduct) indicating that C308 was not oxidized by the ONOO− treatment. Methionine-91 in the amino acid 88–95 peptide was also not oxidized. Both findings support the conclusion that a bolus ONOO− treatment is relatively specific in its effects on amino acid modification of IκBα (42, 43).
We tested whether in vitro nitration with exogenous ONOO− dissociated the IκBα/NF-κB complex. A single bolus addition of ONOO− was used since the short half life of ONOO− (<1 sec) enhances specificity in its reactions, e.g. (42, 43). Cell lysates prepared under mild non-denaturing conditions were treated with 200 or 400 µM ONOO−. Two consecutive immunoprecipitations were performed. Agarose-conjugated anti-p65 IgG was used to pull down the NF-κB/IκBα and free NF-κB complexes. After centrifugation to remove these complexes, the resulting supernatants were incubated with agarose-conjugated anti-IκBα IgG to pull down free IκBα. Western blots of the immunoprecipitates were probed with antibodies against p65, IκBα and nitro-tyrosine. With increasing concentrations of ONOO−, a decreasing amount of IκBα associated with p65 with a corresponding increase in free tyrosine-nitrated IκBα (Fig. 4C). Tyrosine-nitrated IκBα did not co-immunoprecipitate with NF-κB (lanes 5, 6). For long exposure times and at high [ONOO−] a broad smear of nitro-tyrosine staining was observed in p65 immunoprecipitates but with no distinct band for IκBα (lane 3). Neither p50 nor p65 were nitrated under these conditions (data is not shown) and the p50/p65 dimer remained intact as shown by co-immunoprecipitation (Fig. 4D). These results suggest that tyrosine-nitration of IκBα dissociates the NF-κB/IκBα complex releasing the p50/p65 dimer.
Cell lysates were prepared from cells transfected with myc-tagged wild type IκBα and mutants (Y42F, Y181F and Y305F) and treated with 200 µM ONOO−. NF-κB immunoprecipitates obtained with anti-p65 were probed for p65 and myc-tagged IκBα. ONOO− treatment decreases the amount of IκBα associated with p65 in wild type and all mutants tested except for the Y181F mutant (Fig. 4E). These results suggest that Y181 is critical to the stability of the NF-κB/IκBα complex following ONOO− treatment.
CHO cells were co-transfected with the NF-κB reporter gene and either myc-tagged wild type or the myc-tagged IκBα Y→F mutants. As expected, over-expression of wild type or mutants inhibited basal NF-κB reporter activity (≈90%). However, a significant IR-induced activation (~1.4) was still observed in cells expressing wild type or the Y42F and Y305F mutants (Fig. 4F). This is observed if promoter activity is expressed in terms of absolute values or as ratios of reporter activities of irradiated to control cells. In contrast, IR-stimulated NF-κB reporter activity was completely blocked in cells expressing the Y181F mutant, demonstrating an important role for Y181 in the mechanism for IR-induced activation of NF-κB. Basal NF-κB activity of Y181F IκBα-mutant expressing cells was moderately higher relative to the basal NF-κB activity of cells overexpressing wild type or the other IκBα mutants (Fig. 4F). This is consistent with a previous analysis of different IκBα mutants showing that a Y181A mutant had a slightly lower affinity for p50/p65 (44). The variability in basal activity (e.g. Fig. 5A) probably reflects difference in expression levels.
Figure 5.
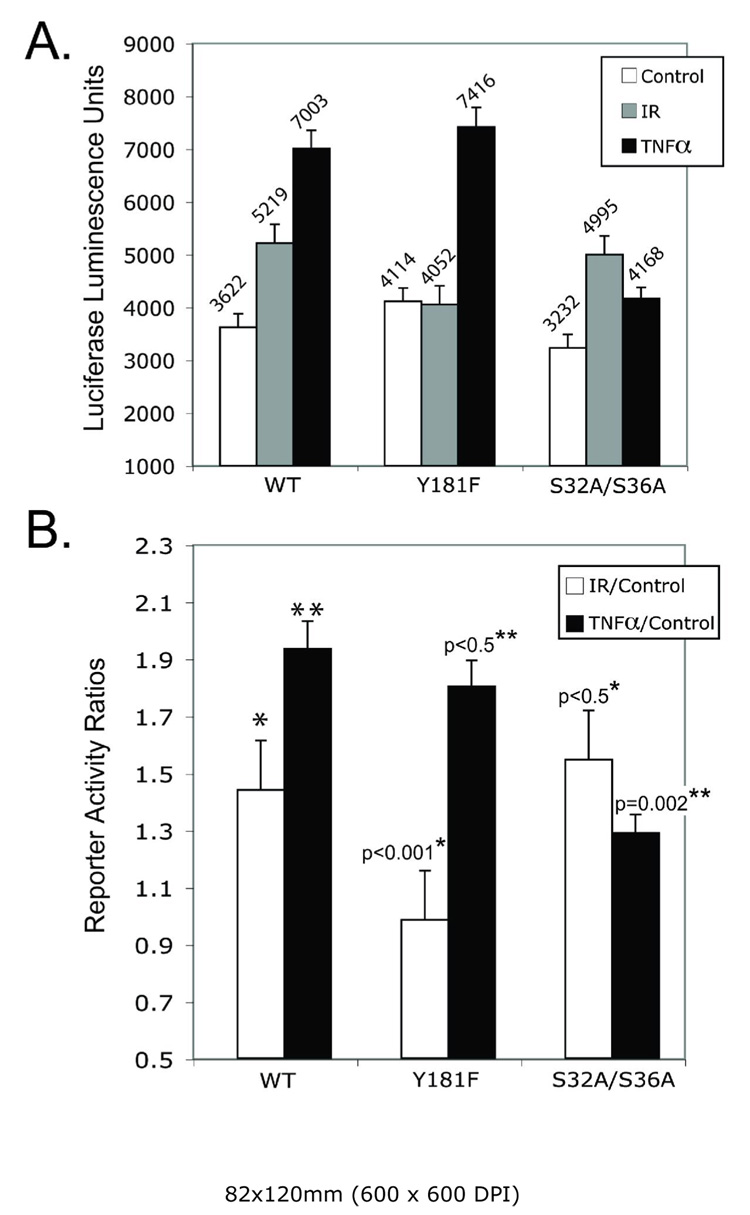
IκBα Y181F can play role of super-mutant in the radiation-dependent NFκB activation. A. MCF-7 cells were co-transfected by pNF-κB-luc with IκBα wild type, or IκBα Y181F mutant, or IκBα S32A/S36A mutant. Cells were radiated (5 Gy) or incubated with TNF-α (10nM) 48 h after transfection and luciferase activity was measured in cell lysates 24 h later. B. Absolute luminescence values are provided in Fig.5A converted into reporter activity ratios of treated versus non-treated cells. Data are means ±SD for quadruplicate samples and representative for experiments performed in triplicate. *, ** – The p values were determined by Student’s t-test relative to the IκBα wild type transfected cells for radiated/non-radiated ratios. ** –The p values were determined by Student’s t-test relative to the IκBα wild type transfected cells for TNF-α-treated/non-treated ratios.
Tyrosine-42 was also examined because previous reports indicated that its phosphorylation was important for oxidative activation of NF-κB without degradation of IκBα (41, 45). However, mutation of Y42 to phenylalanine was without effect on either the stability of the NF-κB/IκBα complex after ONOO− treatment (Fig. 4E) or IR-induced NF-B promoter activity (Fig. 4F).
Mutation of tyrosine-181 blocks IR-but not TNFα induced NF-κB activity
The effects of overexpressing wild type IκBα or the Y181F and S32A/S36A mutants of IκBα on IR and TNFα stimulated NF-κB activities were compared in MCF-7 cells. Serine-32 and -36 are phosphorylated by an IKK-dependent mechanism and thus the double mutant at these sites is a super-repressor for those activation mechanisms that proceed solely through IKK (1, 39). MCF-7 cells demonstrate a relatively weak NF-κB response to TNFα compared to other cell types reflecting the variable amount of TNF receptor in these cells (46). Nonetheless the results in Figure 5 and in Fig. 1G demonstrate that as with other cell types, the TNFα response proceeds through a mechanism involving IKK and serines-32 and 36. The experimental results are presented in absolute amounts of reporter luciferase activity (Fig. 5A) or as ratios of treated versus control activities (Fig. 5B). Expression of the S32A/S36A double mutant was significantly more effective than wild type IκBα in suppressing TNFα-stimulated NF-κB activity. The Y181F mutant and wild type IκBα, in contrast, were no different in their effectiveness as inhibitors of TNFα-stimulated NF-κB activity. Different results were obtained with IR as the activating mechanism. The Y181F mutant was significantly more effective at suppressing IR induced NF-κB activity but was without any super-repressor activity with TNFα as the inducing agent. The results with the S32A/S36A mutant and IR parallel the findings provided above demonstrating that IR at the doses used here did not stimulate IκBα phosphorylation and degradation and that blocking expression of IKK with shRNA only partially inhibited IR stimulated NF-κB activation (Fig. 1G)
A structural analysis of the effects of Y181 nitration on the IκBα/NF-κB complex
The experimental results with IR and exogenous ONOO− coupled with the site-directed mutagenesis studies strongly support a mechanism of Y181 nitration in NF-κB activation by IR. Other oxidative modifications of IκBα and nitration of either p50 or p65 after IR or ONOO− treatments were not detected. To further substantiate a role for Y181 nitration in IR-induced dissociation of the IκBα/NF-κB complex an assessment of hydropathic interactions of Y181 with neighboring residues was made and molecular dynamics simulations were performed.
A computational chemistry program HINT (Hydropathic INTeractions) permits the quantitative analysis of all possible noncovalent atom-atom interactions including hydrogen bonding, coulombic, acid-base, and hydrophobic using the crystal structure of proteins (47–49). HINT uses empirically derived constants based on thermodynamic hydropathy values from solvent partition measurements. The more positive the HINT value the more energetically favorable the change in free energy. HINT calculations have accurately estimated changes in free energy resulting from site-specific mutations and their effect on hemoglobin dimer-tetramer assembly (48, 49). Using HINT calculations, the interactions of Y181 or nitro-Y181 with the remainder of the NF-κB/IκBα complex were compared on an atom-by-atom basis with an 8Å cutoff.
The initial calculations required a quantum-mechanical comparison of the unmodified tyrosine residue with the nitrated form to generate topology files for tyrosine and nitro-tyrosine (Fig. S4, Supplemental data). These calculations demonstrated an altered side-chain electron distribution that accounts for the measured decrease in pK of the phenolic group from ~10 to ~7 (50, 51). The portion of nitro-tyrosine containing the NO2 group was highly electronegative with the most electropositive portion localized over the Cα and Cβ atoms. This results in a dipole moment vector oriented almost parallel with the Cε−NO2 bond (Fig. S4, Supplemental Data). The magnitude of the calculated dipole moment of nitro-tyrosine was 5.78 Debye, compared with 3.63 Debye for tyrosine. Without nitration, the tyrosine dipole moment vector was oriented along the long axis of the side chain aligning the hydroxyl group of the phenol ring with Cβ and Cα atoms.
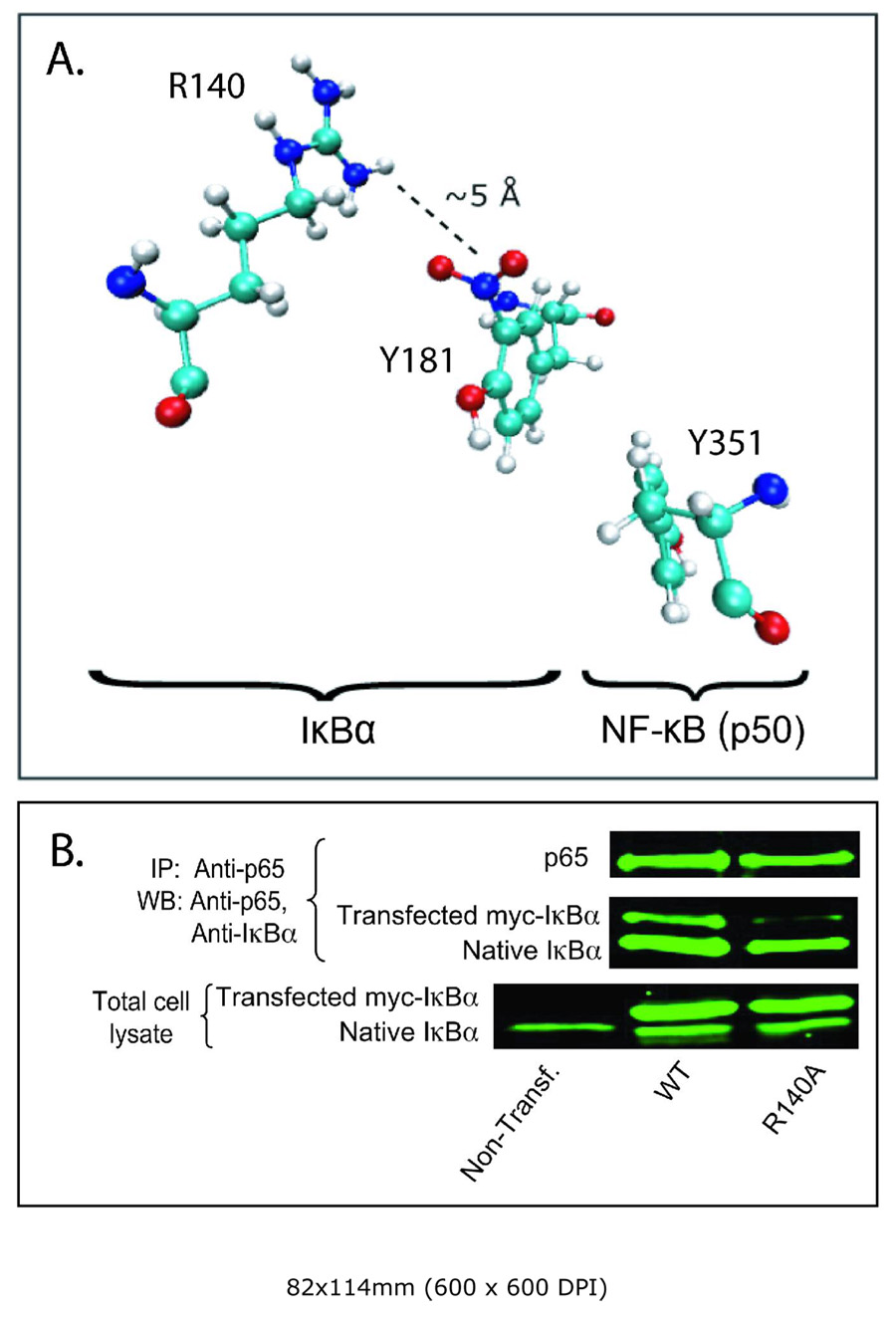
Crystallographic studies of the NF-κB/IκBα complex show that IκBα is orientated such that fingers 3/4, 4/5, and 5/6 of IκBα contact the p50 subunit (28, 29). Y181 and N182 extending from finger 3/4 have multiple contacts with p50. Y181, in particular, has an important role in these interactions since it forms hydrogen bonds with p50 K252 and R258, π-stacks with Y351, and makes multiple van der Waals contacts with A260, P327, and L349. Table 1 shows results on an atom-by-atom basis of HINT calculations using a cut-off distance between atoms of 8 Å. ‘Nitration’ of Y181 using the above generated topology file cause significant destabilizing changes in these interactions as seen by the net negative increase in total HINT score. A more instructive presentation of the HINT scores is shown in the Summary inset to Table 1 where the total HINT scores for IκBα Y181 interactions with p50 and with other residues of IκBα are separated. Nitration results in a significant destabilization of p50-IκBα interactions as indicated by a net negative increase in HINT score from −218 to −1970. Assuming approximately 1 kcal/mole per 500 HINT, this represents a change in free energy of approximately 3 kcal/mol. In contrast, the ‘nitration’ induced change in HINT scores for Y181 interactions with other IκBα residues within 8 Å is consistent with a net stabilizing effect. Major contributions to this positive interaction are the acid/base and hydrogen bond interactions of the nitro-group with R140 of IκBα (Table 1, Fig. 6A).
Table 1.
HINT score calculations (Y181 before and after nitration with p50 and IκBα).
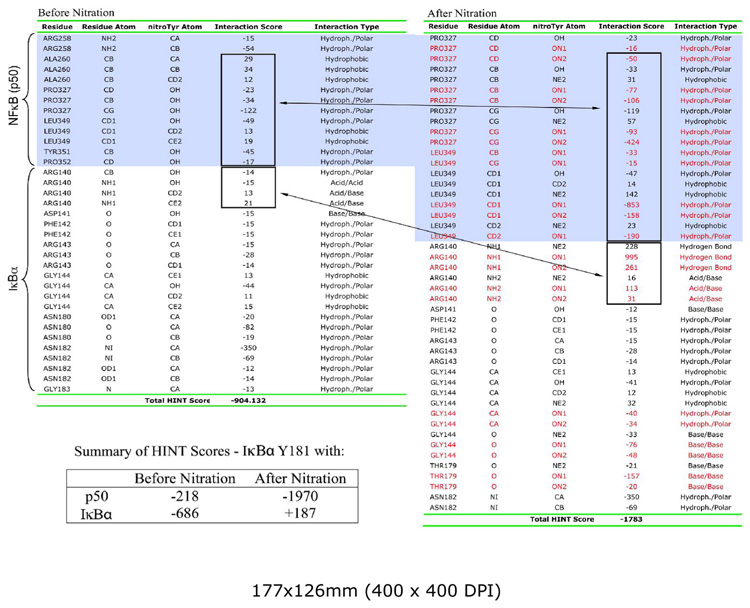 |
Figure 6.
The spatial relationship between NO2 group of Y181 and R140 of IκBα. A. R140, an inherently electropositive residue, buffers the electronegative charge of nitrated Y181 via long-range electrostatic interactions as evidenced by out-of-plane rotation of the NO2 group. Optimized structure of nitrated tyrosine indicates that the most optimal structure is when the NO2 group is in the plane of the phenol ring. B. R140 of IκBα is responsible for stability of NF-κB/IκBα complex. CHO cells were transfected by wild type and R140A mutant myc-IκBα. Cell lysates were prepared 24 hours after transfection and immunoprecipitated by anti-p65 antibodies. Immunoprecipitates were analyzed by blotting with anti-p65 and anti-IκBα antibodies. Equal amount of cell lysates were used as a transfection control (bottom panel).
To experimentally test for this, the R140A IκBα mutant was constructed, and the relative affinities of wild type and R140A myc-tagged IκBα to NF-κB compared by co-immunoprecipitation with anti-p65 antibody (Fig. 6B). The affinity of the R140A mutant for NF-κB is considerably reduced relative to wild type even in the absence of nitration. These results do not allow for any statement on the role of this particular interaction on the effect nitration on IκBα/NF-κB stability. However, they underline the importance of this surface area of IκBα in its interactions with p50/p65 and the potential for their disruption by tyrosine nitration.
Discussion
The above experimentation indicates a new mechanism for NF-κB activation. IR is shown to stimulate NF-κB activity by a mechanism in which IκBα Y181 is nitrated as a consequence of NOS-1 activation, leading to dissociation of intact IκBα from NF-κB. Hence, this mechanism of NF-κB activation does not depend on IKK dependent phosphorylation and proteolytic degradation of IκBα. Modeling of free energy changes is consistent with the experimental findings that IR-induced Y181 nitration disrupts the noncovalent interactions of Y181 with p50 dissociating the IκBα/NF-κB complex. The apparent lack of IκBα degradation following treatment of cells with low IR doses is also in accord with findings that IR at doses > 0.2 Gy significantly inhibits proteosome activities (4, 6). The IR dose response analysis demonstrates a progressive increase in NF-κB activation up to the highest dose tested, 32 Gy. However, only at IR doses below 8 Gy is substantial inhibition observed with the NOS inhibitor L-NNA. Similar dose responses are observed for IR-induced IκBα nitration and NOS-1 activation.
The experimental results suggest that NF-κB is activated by IR through both, IKKβ-independent and IKKβ-dependent mechanisms. The IKKα-independent pathway involving tyrosine nitration of IκBα is prominent at IR doses ≤ 8 Gy whereas IKKα-dependent pathway involving IκBα phosphorylation/proteolysis becomes more prominent at IR doses > 8 Gy. Dose dependent mechanisms are also indicated in the IKK-β knockdown experiments. Whereas shRNA treatment completely abrogates TNFα-induced NF-κB activity, it only partially decreases IR-induced activity. The remaining activity is inhibited by NOS inhibitor L-NNA.
Analysis of the kinetics of NF-κB activation following low or high dose IR is also indicative of different mechanisms. A previous study using electrophoretic mobility shift analysis monitored activation of NF-κB in HeLa cells after 20 Gy (52). No activation was observed at 30 min, a slight increase at one hour and maximal activation at two hours. This contrasts with the responses obtained within minutes of exposure to lower doses of IR (Fig 1C, 2) or to TNFα (39). IR-stimulated nitration of IκBα is observed at 5 min post-irradiation at 5 Gy, the earliest time point tested (Figure 2). In contrast IκBα Ser-32/36 phosphorylation is first observed at 45 min post-irradiation with 20 Gy (39).
The IKKβ dependent and independent pathways are not mutually exclusive. This is suggested by the partial inhibition of NF-κB signaling at the lower doses of IR by shRNA knockdown of IKKβ. However, the IKKβ-mechanisms may differ depending on IR dose. For example, different mechanisms may reflect the cellular localization of the oxidative/nitrosative events: DNA damage in the nucleus and oxidative/nitrosative events in the cytoplasm. Further experiments are necessary to test this hypothesis. IKKβ can also modulate NF-κB transcriptional activity in the cytoplasm through an IκBα-independent mechanism: phosphorylation of the p65 subunit in its transactivation domain at S536 (7–10).
NF-κB activation by reactive nitrogen species has been previously investigated using a ONOO− donor, SIN-1 (11). NF-κB activity measured with a luciferase-based reporter assay was stimulated by a mechanism not blocked by the proteosome inhibitor, MG132, nor was IκBα degradation associated with the stimulation. These experimental findings directly relate to the results reported here with IR. Thus, our results provide a mechanism for how SIN-1 and other cellular ONOO− generating processes can activate NF-κB signaling.
A previous analysis of several IκBα mutants demonstrated that the Y181A mutation was most defective for complex formation with NF-κB (44). However, all IκBα mutants examined including Y181A still bound NF-κB with nanomolar affinities suggesting that several binding elements contribute to the overall stability of the NF-κB/IκBα complex. This conclusion would appear to be in conflict with the present findings that mutation of Y181 alone completely blocks IR stimulated NF-κB DNA binding activity and ONOO− induced disruption of the NF-κB/IκBα complex. A likely explanation is that nitration of tyrosine is a much more disruptive protein modification than substitution of an alanine for tyrosine. Besides introducing a bulky substituent, tyrosine nitration significantly alters the dipole moment of tyrosine and reduces the phenolic pK by 2–3 units effectively introducing a net negative charge in a relatively non-polar restricted space. Other studies have demonstrated that nitration of a specific tyrosine in proteins can have significant structural and functional consequences for proteins, e.g. (53–57). It is also important to note that the studies by Huxford et al (44) used a bacterial expression system with a truncated IκBα lacking both N-and C-terminal elements, including Y305. Nitration of Y305 may have an important role in modulating the stability of the NF-κB/IκBα complex.
Tyrosine-305 of IκBα is also nitrated following radiation or an acute ONOO− treatment. We were unable to model the effects of Y305 nitration on the IκBα/NF-κB complex stability because both available crystal structures of IκBα/NF-κB lack the C-terminal regions containing Y305. From the 1IKN structure, it is clear that a turn in secondary structure occurs around P215 and the C-terminal portion of IκBα wraps on itself and runs along the interface with p65 and p50 subunits of NF-κB. Tyrosine-305 is part of this C-terminal sequence and its nitration can presumably contribute to disruption of non-covalent interactions stabilizing the IκBα/NF-κB complex. Is Y181 and Y305 nitration cooperative and is nitration of both residues required for rapid dissociation of the complex? From results of our work, it appears that nitration of Y181 is alone sufficient to destabilize the signaling complex, but the kinetics of dissociation of IκBα from NF-κB may be affected by additional nitration of Y305. A previous report also provides evidence that phosphorylation of Y305 increased the in vivo stability of IκBα (58). It is possible that its nitration serves a similar purpose. Some investigations have suggested that nitro-tyrosine may mimic phospho-tyrosine binding sites, e.g. (59). Further experimentation will be necessary to test these mechanisms involving Y305.
Experiments described here and in other published work demonstrate a high degree of selectivity in what tyrosines are nitrated by an acute ONOO− treatment (60). If complexed with p50/p65, IκBα is nitrated on only 2 of 8 tyrosines following an IR exposure of intact cells or treatment of cell lysates with ONOO−. With the same in vitro conditions attempts to nitrate purified IκBα with ONOO− have proven unsuccessful (C.S. Rabender, unpublished data). This suggests that the tertiary or quaternary structures of IκBα are important in determing susceptibiblity to nitration by ONOO−. An analysis of nitrated proteins suggests that no specific structural feature alone determines nitration (60, 61). Tyrosines located in loop structures have increased probability for nitration (60, 61) and Y181 is located in the β-turn loop between the 3rd and 4th ankyrin repeats.
No amino acid consensus sequence has been identified although it has been speculated that nitrated tyrosines are generally but not always near acidic amino acid residues (60). This is true for Y305 with one glutamate in close proximity (KPFLY305EIK), but this is not the case for Y181 (KATNY181NGHT). There are also no acidic amino acids from p50 or p65 at the interface near Y181 of IκBα that can contribute to this possible electrostatic environment. A more recent report on endogenously nitrated proteins of the brain argues for the importance of a positively charged amino acid near sites of nitration (61). This criterion is fulfilled by both Y181 and Y305 of IκBα.
The short half-life of ONOO− predicts that proximity of the protein to ONOO− source is also important in selectivity (19, 60). The primary source of IR-stimulated cellular ONOO− is the mitochondrion either by activity of a mitochondrial NOS-1 isoform or as a consequence of respiration generated superoxide anion that reacts with relatively stable NO• produced elsewhere in the cell (16, 19, 60, 62). A reversible mitochondrial protein tyrosine nitration initiated by hypoxia/reoxygenation has been described (62) and there are reports of mitochondrial localization of IκBα/NF-κB (63, 64).
Transient and localized generation of ONOO− may also explain why the results presented here differ from those obtained in some but not all studies on the effects of reactive nitrogen species on NF-κB activity (13–15, 65). High concentrations of NO•/ONOO− donors and/or prolonged exposure times inhibit NF-κB activity, e.g. 6–24 hr (15). This contrasts with the transient generation of ONOO− achieved by a short IR exposure of cells or a single bolus addition of ONOO− (half life <1 sec) to cell lysates. The transient nature of these treatments would be predictive of a higher degree of specificity in the nitration process. This is not only seen in terms of what tyrosines are nitrated but also in terms of whether other ONOO− induced oxidative events have occurred. Under the conditions used in the present experiments there is no evidence for nitration of either p65 or p50. There is also no evidence for the oxidation of either a cysteine or methionine. On the other hand a single Y181F mutation was sufficient to block IR stimulated NF-κB DNA binding activity and ONOO− induced dissociation of the NF-κB/IκBα complex. Combined with the effects of NOS inhibition and structural analysis, this is compelling evidence for a key role for Y181 nitration in IR stimulation of NF-κB activation.
An important characteristic of signal transduction pathways is their reversibility. The transient and relatively mild oxidative treatments used here may also have facilitated the apparent reversibility of the IR-stimulated IκBα tyrosine nitration. Although the mechanism of reversibility is not known, proteolytic degradation followed by resynthesis does not appear to be involved. This also is the case with the reversible nitration of mitochondrial proteins after hypoxia-reoxygenation (62). There are reports of denitrase activities in eukaryotic cells but underlying denitration mechanisms of eukaryotic cells remain undefined (66–68).
Because tyrosine-nitration is not commonly studied in the context of signal transduction, it may be an undiscovered but important component for NF-κB activation by stimuli other than IR. A previous report that mitochondria-generated superoxide anion and ONOO− are important for TNFα-stimulated NF-κB activation is interesting in this regard (69). Nitration and disruption of the interactions between Y181 of IκBα with p50 may be representative of a post-translational modification and structural motif critical for the stability of other protein complexes.
Supplementary Material
Abbreviations
- NOS-1
constitutive NO• synthase
- TNF
tumor necrosis factor
- IKK
IκB kinase
- IR
ionizing radiation
- L-NNA
NG-Nitro-L-arginine
Footnotes
Research was supported by National Institutes of Health Grants, CA65896, CA72955, CA89055 (RBM) and HD39110 and HL070061 (SMB) and an ASTRO Resident Research Grant (IJB). We thank Dr. Neal Scarsdale for the 13C NMR studies.
Research Collaboratory for Structural Bioinformatics Protein Databank = PDB # 1IKN; Research Collaboratory for Structural Bioinformatics Protein Databank = PDB # 1NFI.
References
- 1.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 2.Beg AA, Baldwin AS., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 3.Criswell T, Leskov K, Miyamoto S, Luo G, Boothman DA. Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene. 2003;22:5813–5827. doi: 10.1038/sj.onc.1206680. [DOI] [PubMed] [Google Scholar]
- 4.Pajonk F, McBride WH. The proteasome in cancer biology and treatment. Radiat Res. 2001;156:447–459. doi: 10.1667/0033-7587(2001)156[0447:tpicba]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Raju U, Gumin GJ, Noel F, Tofilon PJ. IkappaBalpha degradation is not a requirement for the X-ray-induced activation of nuclear factor kappaB in normal rat astrocytes and human brain tumour cells. Int J Radiat Biol. 1998;74:617–624. doi: 10.1080/095530098141195. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Huang W, Li C, Li P, Yuan J, Li X, Qiu XB, Ma Q, Cao C. Interaction between c-Abl and Arg tyrosine kinases and proteasome subunit PSMA7 regulates proteasome degradation. Mol Cell. 2006;22:317–327. doi: 10.1016/j.molcel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Hall G, Singh IS, Hester L, Hasday JD, Rogers TB. Inhibitor-kappaB kinase-beta regulates LPS-induced TNF-alpha production in cardiac myocytes through modulation of NF-kappaB p65 subunit phosphorylation. Am J Physiol Heart Circ Physiol. 2005;289:H2103–H2111. doi: 10.1152/ajpheart.00393.2005. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki CY, Barberi TJ, Ghosh P, Longo DL. Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}-independent NF-{kappa}B pathway. J Biol Chem. 2005;280:34538–34547. doi: 10.1074/jbc.M504943200. [DOI] [PubMed] [Google Scholar]
- 10.Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 11.Janssen-Heininger YM, Macara I, Mossman BT. Cooperativity between oxidants and tumor necrosis factor in the activation of nuclear factor (NF)-kappaB: requirement of Ras/mitogen-activated protein kinases in the activation of NF-kappaB by oxidants. Am J Respir Cell Mol Biol. 1999;20:942–952. doi: 10.1165/ajrcmb.20.5.3452. [DOI] [PubMed] [Google Scholar]
- 12.Levrand S, Pesse B, Feihl F, Waeber B, Pacher P, Rolli J, Schaller MD, Liaudet L. Peroxynitrite Is a Potent Inhibitor of NF-{kappa}B Activation Triggered by Inflammatory Stimuli in Cardiac and Endothelial Cell Lines. J Biol Chem. 2005;280:34878–34887. doi: 10.1074/jbc.M501977200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–1693. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 14.Matata BM, Galinanes M. Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem. 2002;277:2330–2335. doi: 10.1074/jbc.M106393200. [DOI] [PubMed] [Google Scholar]
- 15.Park SW, Huq MD, Hu X, Wei LN. Tyrosine nitration on p65: a novel mechanism to rapidly inactivate nuclear factor-kappaB. Mol Cell Proteomics. 2005;4:300–309. doi: 10.1074/mcp.M400195-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Kanai A, Epperly M, Pearce L, Birder L, Zeidel M, Meyers S, Greenberger J, de Groat W, Apodaca G, Peterson J. Differing roles of mitochondrial nitric oxide synthase in cardiomyocytes and urothelial cells. Am J Physiol Heart Circ Physiol. 2004;286:H13–H21. doi: 10.1152/ajpheart.00737.2003. [DOI] [PubMed] [Google Scholar]
- 17.Leach JK, Black SM, Schmidt-Ullrich RK, Mikkelsen RB. Activation of constitutive nitric-oxide synthase activity is an early signaling event induced by ionizing radiation. J Biol Chem. 2002;277:15400–15406. doi: 10.1074/jbc.M110309200. [DOI] [PubMed] [Google Scholar]
- 18.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer research. 2001;61:3894–3901. [PubMed] [Google Scholar]
- 19.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 20.Barrett DM, Black SM, Todor H, Schmidt-Ullrich RK, Dawson KS, Mikkelsen RB. Inhibition of protein-tyrosine phosphatases by mild oxidative stresses is dependent on S-nitrosylation. J Biol Chem. 2005;280:14453–14461. doi: 10.1074/jbc.M411523200. [DOI] [PubMed] [Google Scholar]
- 21.Phung YT, Black SM. Use of chimeric forms of neuronal nitric-oxide synthase as dominant negative mutants. IUBMB Life. 1999;48:333–338. doi: 10.1080/713803520. [DOI] [PubMed] [Google Scholar]
- 22.Amorino GP, Hamilton VM, Valerie K, Dent P, Lammering G, Schmidt-Ullrich RK. Epidermal growth factor receptor dependence of radiation-induced transcription factor activation in human breast carcinoma cells. Mol Biol Cell. 2002;13:2233–2244. doi: 10.1091/mbc.01-12-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA. General atomic and molecular electronic structure system. Journal of Computational Chemistry. 1993;14:1347–1363. [Google Scholar]
- 25.MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 26.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 27.Kale L, Skeel R, Bhandarkar M, Brunner R, Gursoy A, Krawetz N, Phillips J, Shinozaki A, Varadarajan K, Schulten K. NAMD2: Greater Scalability for Parallel Molecular Dynamics. Journal of Computational Physics. 1999;151:283–312. [Google Scholar]
- 28.Jacobs MD, Harrison SC. Structure of an IkappaBalpha/NF-kappaB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- 29.Huxford T, Huang DB, Malek S, Ghosh G. The crystal structure of the IkappaBalpha/NF-kappaB complex reveals mechanisms of NF-kappaB inactivation. Cell. 1998;95:759–770. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- 30.Amadasi A, Spyrakis F, Cozzini P, Abraham DJ, Kellogg GE, Mozzarelli A. Mapping the energetics of water-protein and water-ligand interactions with the "natural" HINT forcefield: predictive tools for characterizing the roles of water in biomolecules. J Mol Biol. 2006;358:289–309. doi: 10.1016/j.jmb.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 31.Kellogg GE, Fornabaio M, Chen DL, Abraham DJ, Spyrakis F, Cozzini P, Mozzarelli A. Tools for building a comprehensive modeling system for virtual screening under real biological conditions: The Computational Titration algorithm. J Mol Graph Model. 2006;24:434–439. doi: 10.1016/j.jmgm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Cozzini P, Fornabaio M, Marabotti A, Abraham DJ, Kellogg GE, Mozzarelli A. Free energy of ligand binding to protein: evaluation of the contribution of water molecules by computational methods. Curr Med Chem. 2004;11:3093–3118. doi: 10.2174/0929867043363929. [DOI] [PubMed] [Google Scholar]
- 33.Cozzini P, Fornabaio M, Marabotti A, Abraham DJ, Kellogg GE, Mozzarelli A. Simple, intuitive calculations of free energy of binding for protein-ligand complexes. 1. Models without explicit constrained water. J Med Chem. 2002;45:2469–2483. doi: 10.1021/jm0200299. [DOI] [PubMed] [Google Scholar]
- 34.Kellogg GE, Burnett JC, Abraham DJ. Very empirical treatment of solvation and entropy: a force field derived from log Po/w. J Comput Aided Mol Des. 2001;15:381–393. doi: 10.1023/a:1011136228678. [DOI] [PubMed] [Google Scholar]
- 35.Marabotti A, Balestreri L, Cozzini P, Mozzarelli A, Kellogg GE, Abraham DJ. HINT predictive analysis of binding between retinol binding protein and hydrophobic ligands. Bioorg Med Chem Lett. 2000;10:2129–2132. doi: 10.1016/s0960-894x(00)00414-5. [DOI] [PubMed] [Google Scholar]
- 36.Rodel F, Hantschel M, Hildebrandt G, Schultze-Mosgau S, Rodel C, Herrmann M, Sauer R, Voll RE. Dose-dependent biphasic induction and transcriptional activity of nuclear factor kappa B (NF-kappaB) in EA.hy.926 endothelial cells after low-dose X-irradiation. Int J Radiat Biol. 2004;80:115–123. doi: 10.1080/09553000310001654701. [DOI] [PubMed] [Google Scholar]
- 37.Ueda T, Akiyama N, Sai H, Oya N, Noda M, Hiraoka M, Kizaka-Kondoh S. c-IAP2 is induced by ionizing radiation through NF-kappaB binding sites. FEBS letters. 2001;491:40–44. doi: 10.1016/s0014-5793(01)02145-7. [DOI] [PubMed] [Google Scholar]
- 38.Wang T, Hu YC, Dong S, Fan M, Tamae D, Ozeki M, Gao Q, Gius D, Li JJ. Co-activation of ERK, NF-kappaB, and GADD45beta in response to ionizing radiation. J Biol Chem. 2005;280:12593–12601. doi: 10.1074/jbc.M410982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 40.Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, Edwards SW, McDowell HP, Unitt JF, Sullivan E, Grimley R, Benson N, Broomhead D, Kell DB, White MR. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 41.Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 42.Bagnasco P, MacMillan-Crow LA, Greendorfer JS, Young CJ, Andrews L, Thompson JA. Peroxynitrite modulates acidic fibroblast growth factor (FGF-1) activity. Arch Biochem Biophys. 2003;419:178–189. doi: 10.1016/j.abb.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 43.Batthyany C, Souza JM, Duran R, Cassina A, Cervenansky C, Radi R. Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry. 2005;44:8038–8046. doi: 10.1021/bi0474620. [DOI] [PubMed] [Google Scholar]
- 44.Huxford T, Mishler D, Phelps CB, Huang DB, Sengchanthalangsy LL, Reeves R, Hughes CA, Komives EA, Ghosh G. Solvent exposed noncontacting amino acids play a critical role in NF-kappaB/IkappaBalpha complex formation. J Mol Biol. 2002;324:587–597. doi: 10.1016/s0022-2836(02)01149-x. [DOI] [PubMed] [Google Scholar]
- 45.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 46.Burow ME, Weldon CB, Tang Y, Navar GL, Krajewski S, Reed JC, Hammond TG, Clejan S, Beckman BS. Differences in susceptibility to tumor necrosis factor alpha-induced apoptosis among MCF-7 breast cancer cell variants. Cancer research. 1998;58:4940–4946. [PubMed] [Google Scholar]
- 47.Abraham DJ, Kellogg GE, Holt JM, Ackers GK. Hydropathic analysis of the non-covalent interactions between molecular subunits of structurally characterized hemoglobins. J Mol Biol. 1997;272:613–632. doi: 10.1006/jmbi.1997.1249. [DOI] [PubMed] [Google Scholar]
- 48.Burnett JC, Kellogg GE, Abraham DJ. Computational methodology for estimating changes in free energies of biomolecular association upon mutation. The importance of bound water in dimer-tetramer assembly for beta 37 mutant hemoglobins. Biochemistry. 2000;39:1622–1633. doi: 10.1021/bi991724u. [DOI] [PubMed] [Google Scholar]
- 49.Burnett JC, Botti P, Abraham DJ, Kellogg GE. Computationally accessible method for estimating free energy changes resulting from site-specific mutations of biomolecules: systematic model building and structural/hydropathic analysis of deoxy and oxy hemoglobins. Proteins. 2001;42:355–377. doi: 10.1002/1097-0134(20010215)42:3<355::aid-prot60>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 50.Tawfik DS, Chap R, Eshhar Z, Green BS. pH on-off switching of antibody-hapten binding by site-specific chemical modification of tyrosine. Protein Eng. 1994;7:431–434. doi: 10.1093/protein/7.3.431. [DOI] [PubMed] [Google Scholar]
- 51.Oneda H, Inouye K. Effect of nitration on the activity of bovine erythrocyte Cu,Zn-superoxide dismutase (BESOD) and a kinetic analysis of its dimerizationdissociation reaction as examined by subunit exchange between the native and nitrated BESODs. J Biochem (Tokyo) 2003;134:683–690. doi: 10.1093/jb/mvg193. [DOI] [PubMed] [Google Scholar]
- 52.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hodara R, Norris EH, Giasson BI, Mishizen-Eberz AJ, Lynch DR, Lee VM, Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J Biol Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- 54.Ischiropoulos H, Gow A. Pathophysiological functions of nitric oxide-mediated protein modifications. Toxicology. 2005;208:299–303. doi: 10.1016/j.tox.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 55.Ji Y, Neverova I, Van Eyk JE, Bennett BM. Nitration of tyrosine 92 mediates the activation of rat microsomal glutathione s-transferase by peroxynitrite. J Biol Chem. 2006;281:1986–1991. doi: 10.1074/jbc.M509480200. [DOI] [PubMed] [Google Scholar]
- 56.Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44:13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- 57.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawai H, Nie L, Yuan ZM. Inactivation of NF-kappaB-dependent cell survival, a novel mechanism for the proapoptotic function of c-Abl. Mol Cell Biol. 2002;22:6079–6088. doi: 10.1128/MCB.22.17.6079-6088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mallozzi C, Di Stasi AM, Minetti M. Nitrotyrosine mimics phosphotyrosine binding to the SH2 domain of the src family tyrosine kinase lyn. FEBS letters. 2001;503:189–195. doi: 10.1016/s0014-5793(01)02726-0. [DOI] [PubMed] [Google Scholar]
- 60.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 61.Sacksteder CA, Qian WJ, Knyushko TV, Wang H, Chin MH, Lacan G, Melega WP, Camp DG, 2nd, Smith RD, Smith DJ, Squier TC, Bigelow DJ. Endogenously Nitrated Proteins in Mouse Brain: Links to Neurodegenerative Disease. Biochemistry. 2006;45:8009–8022. doi: 10.1021/bi060474w. [DOI] [PubMed] [Google Scholar]
- 62.Aulak KS, Koeck T, Crabb JW, Stuehr DJ. Dynamics of protein nitration in cells and mitochondria. Am J Physiol Heart Circ Physiol. 2004;286:H30–H38. doi: 10.1152/ajpheart.00743.2003. [DOI] [PubMed] [Google Scholar]
- 63.Bottero V, Rossi F, Samson M, Mari M, Hofman P, Peyron JF. Ikappa b-alpha, the NF-kappa B inhibitory subunit, interacts with ANT, the mitochondrial ATP/ADP translocator. J Biol Chem. 2001;276:21317–21324. doi: 10.1074/jbc.M005850200. [DOI] [PubMed] [Google Scholar]
- 64.Cogswell PC, Kashatus DF, Keifer JA, Guttridge DC, Reuther JY, Bristow C, Roy S, Nicholson DW, Baldwin AS., Jr NF-kappa B and I kappa B alpha are found in the mitochondria. Evidence for regulation of mitochondrial gene expression by NF-kappa B. J Biol Chem. 2003;278:2963–2968. doi: 10.1074/jbc.M209995200. [DOI] [PubMed] [Google Scholar]
- 65.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorg B, Qvartskhava N, Voss P, Grune T, Haussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS letters. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 67.Irie Y, Saeki M, Kamisaki Y, Martin E, Murad F. Histone H1.2 is a substrate for denitrase, an activity that reduces nitrotyrosine immunoreactivity in proteins. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5634–5639. doi: 10.1073/pnas.1131756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuo WN, Kanadia RN, Shanbhag VP, Toro R. Denitration of peroxynitrite-treated proteins by 'protein nitratases' from rat brain and heart. Mol Cell Biochem. 1999;201:11–16. doi: 10.1023/a:1007024126947. [DOI] [PubMed] [Google Scholar]
- 69.Higuchi M, Manna SK, Sasaki R, Aggarwal BB. Regulation of the activation of nuclear factor kappaB by mitochondrial respiratory function: evidence for the reactive oxygen species-dependent and -independent pathways. Antioxid Redox Signal. 2002;4:945–955. doi: 10.1089/152308602762197489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.