Abstract
The novel technique of transcutaneous sampling of drugs by electroporation was developed to study the dermatokinetics of ciprofloxacin and 8-methoxypsoralen. The selected drugs differ in their aqueous solubility and also with respect to the extent of protein binding. Ciprofloxacin (15mg/kg) was administered i.v. through tail vein whereas 8-methoxypsoralen (5mg/kg) was given by oral administration, in hairless rats and the time course of drug concentration in the plasma was determined. Drug concentration in the dermal ECF was determined by ETS and microdialysis sampling techniques. The extent of penetration into dermal ECF for ciprofloxacin and 8-methoxypsoralen was found to be ∼19-32% and ∼13-23% respectively. The drug concentration in the dermal ECF determined by ETS and microdialysis did not differ significantly from each other and so as were the pharmacokinetic parameters. The results show that ETS can be utilized as a potential technique for sampling of drugs from the dermal extracellular fluid.
Keywords: Electroporation, Transcutaneous sampling, Hairless rats, Microdialysis, Pharmacokinetics, Extracellular fluid
1. Introduction
Determination of drug levels in the dermal extracellular fluid (ECF) is extremely important for efficient treatment of skin infections and other disorders (Tegederer et al., 2002, Bielecka-Grzela et al., 2005,). Conventional methods of drug sampling in the skin include biopsy, skin blister fluid and cutaneous microdialysis sampling techniques (Wise et al., 1984, Ault et al., 1992, Muller et al., 1996, Alguire et al., 1998, Brunner et al., 2002). Skin biopsy sampling and skin blister fluid sampling are both invasive in nature and also have limitation with the number of samples that could be obtained. Presently, microdialysis is the most widely used method for sampling of unbound drug from dermal ECF. However, it is also an invasive technique which makes it unsuitable for implementation in routine therapeutic drug monitoring.
Electroporation is one of the promising electrically mediated techniques to enhance the transdermal drug delivery. It is a technique in which reversible permeabilization of skin is brought about by application of short electrical pulses (Lombry et al., 2000, Denet et al., 2003, Murthy et al., 2004). ETS involves sampling of drugs from dermal ECF by facilitating reverse diffusion of drug in the direction of dermis to stratum corneum. The applicability of the ETS technique in case of sampling acyclovir, salicylates and diagnostic analyte glucose has already been demonstrated (Murthy et al., 2005, Murthy et al., 2008, Srinivasa et al., 2008).
In the present study ciprofloxacin and 8-methoxypsoralen which are used in treatment of skin infections and pigmentation disorders respectively are selected as model drugs. Ciprofloxacin, a broad spectrum antibacterial agent belonging to the group of fluoroquinolones is effective against microbial infections localized in skin. The time course of ciprofloxacin in the skin needs to be monitored for determination of the frequency and dose from safety and efficacy perspectives (Brunner et al., 1999, Tsai et al., 2001, Brunner et al., 2002, Bielecka-Grzela et al., 2005).
8-methoxypsoralen, a furocoumarinic is used in conjuction with UV radiation (PUVA therapy) for the treatment of dermatoses like vitilgo and psoriasis (Mays et al., 1985, Santo et al., 1985, Ketchum et al., 1990). The effectiveness of 8-methoxypsoralen depends on ultraviolet A irradiation, and optimum response will be elicited only when the drug concentration in skin is over the effective concentration. For this reason, it is imperative to determine drug concentration in skin to achieve better PUVA therapy (Gazith et al., 1978, Tegederer et al., 2002, Brautigam et al., 2003).
The two drugs ciprofloxacin and 8-methoxypsoralen possess different physicochemical properties. They differ in their extents of protein binding. For the purpose of assessing the validity of the technique, the two drugs were administered by different routes in this project (Ciprofloxacin by intranveous bolus and 8-methoxypsoralen by oral route).
2. Materials and Methods
2.1. Chemicals
Ciprofloxacin Hydrochloride, 8-Methoxypsoralen were purchased from Sigma-Aldrich Inc (St.Louis, MO), Phosphate buffered saline (PBS, pH 7.4) premixed powder was obtained from EMD Chemicals (Gibbstown, NJ), and all other chemicals were obtained from Fischer Scientific (Fairway, NJ).
2.2. In Vitro transcutaneous sampling studies
The in vitro diffusion studies were carried out in Franz diffusion cells (FDC) (Logan Instruments Ltd, Somerset, NJ) using hairless rat skin excised from the abdomen region. Hairless rat skin is considered to be a good model for topical and transdermal drug delivery studies due to the similarity between the rat and human skin with respect to lipid content and water uptake properties (Morimoto et al., 1992). Moreover, a good correlation of permeation data between the hairless rat model and human skin models have been reported by several research groups in the past (Wester et al., 1993). The skin was mounted on the diffusion cell in such a way that the epidermis side of the skin was in contact with upper sampling compartment and dermal side with the lower reservoir compartment. The active diffusion area of FDC was 0.64 cm2. Ag/AgCl electrode wires of 2mm diameter (In Vivo Metric, CA) made in the form of circular rings were placed 2mm away from skin in both sampling and reservoir compartments. The sampling compartment and the reservoir compartment were filled with 0.4 and 5ml PBS respectively and the skin was allowed to equilibrate for an hour. The AC electrical resistance of the epidermis was measured by placing a load resistor RL (100 kΩ) in series with the epidermis. The voltage drop across the whole circuit (VO) and across the skin (VS) was measured using an electrical set up consisting of a wave form generator and a digital multimeter (Agilent Technologies, Santa Clara, CA). For measuring resistance, voltage of 100 mv was applied at 10 Hz and the skin resistance in kΩ was approximated from the formula:
| (1) |
Where RS is the skin resistance and RL is the load resistor in kΩ. The piece of skin, which had a resistance greater than 20 kΩ.cm2 was used for the experiment.
Later, the sampling compartment was replaced with fresh 0.4 ml of PBS and reservoir compartment was filled with 5ml of ciprofloxacin solution prepared in PBS (2.5-40 μg/ml). In case of 8-methoxypsoralen, the sampling compartment was replaced with PBS:Ethanol (50:50) and the reservoir compartment was filled with 5ml of 8-methoxypsoralen solution (5-40μg/ml) prepared in PBS:Ethanol (50:50). Thirty square electrical pulses each of 10ms duration at 120V/cm2, 1Hz was applied using ECM 830 Electro Square Porator (BTX Harvard apparatus, Holliston, USA). The electrical resistance of the skin was measured immediately after application of the electrical pulses to ensure skin permeabilization. The solution from the sampling compartment was withdrawn 15 min after application of electrical pulses and the amount of drug was analyzed by HPLC.
2.3. Ex Vivo plasma protein binding
The blood was collected by cardiac puncture in rats and the plasma was separated by centrifugation at 2000g at 4°C. Rat plasma was spiked with drug to prepare samples of concentration ranging from 1-40μg/ml. The spiked plasma samples were thoroughly mixed by vortexing and allowed to equilibrate for 12 h at 4°C. After equilibration, protein free plasma was obtained by carrying out ultra filtration (Millipore Centrifree® filtration units) by centrifugation of 0.5 ml of plasma at 2000g for 20 min (Schaefer et al., 1996, Murthy et al., 2005). The amount of unbound drug present in the filtrate was measured by HPLC after suitable dilution with PBS.
2.4. In Vivo studies
The in vivo experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi (Protocol # 07-004). The in vivo studies were carried out in hairless rats (Taconic, Hudson, Newyork) (250-300 g) under ketamine (80mg/kg) and xylazine (10mg/kg) anesthesia administered intraperitoneally.
Blood sampling, ETS and microdialysis sampling was carried out in the same group of rats (n=6). The samples by all three procedures were obtained at the same time points in each rat simultaneously. Ciprofloxacin solution (150-180μl) of 15 mg/kg prepared in sterile isotonic saline was administered by i.v. into tail vein as a bolus injection, whereas in case of 8-methoxypsoralen, solution (415-500μl) of 5mg/kg prepared in 50:50 of PBS:Ethanol was given by oral gavage using a ball ended feeding needle.
2.4.1. Cutaneous microdialysis
For cutaneous microdialysis, a 20G needle was inserted intradermally through a distance of 1cm and penetration depth of 2mm. A linear microdialysis probe of 5mm membrane length and 30kDa cutoff molecular weight (BASi, West Lafayette IN) was inserted through this needle and the needle was withdrawn leaving the probe implanted in the dermal tissue. The proper placement of probe in the dermis was confirmed by making an incision at the site of probe implantation after completion of the study. Any experiment, in which the probe was not placed horizontally at the intended depth was not considered.
The inlet tube was connected to an injection pump (BASi, West Lafayette, IN) and perfusion was set to a flow rate of 2μl/min. The probe was equilibrated for 30 minutes (Groth et al., 1998a, 1998b, Schnetz et al., 2001). The perfusate was PBS for studies involving ciprofloxacin and PBS:Ethanol (50:50) for that of 8-methoxypsoralen. Two samples were collected before drug administration. The drug was administered after equilibration of the probe. Subsequently the microdialysis samples were collected continuously at every 15 minutes interval including at time points corresponding to ETS and plasma sampling at 30, 60, 120,180,240,300 and 360min.
The microdialysis probe recovery was determined in vitro by placing microdialysis probe in PBS containing known drug concentration and perfusing the probe with PBS (for ciprofloxacin) and 50:50 PBS:Ethanol (for 8-methoxypsoralen) at flow rate of 2μl/min. The dialysate coming out of the probe is collected every 15min and analyzed for drug content (Dempsey et al., 1997, Leveque et al., 2001). The in vitro recovery rate was calculated using the formula:
| (2) |
2.4.2. Transcutaneous sampling by electroporation
A custom made sampling cell was fixed using an adhesive (Krazy glue, Elmers products Inc, Ohio) on the back of the rats. The sampling cell was fitted with a Ag/AgCl electrode and the counter electrode was secured just adjacent to the cell on the surface of the skin using a micropore surgical tape (3M Healthcare, MN). The skin was hydrated with 100μl of saline for 5 minutes before each sampling and was replaced with 100μl of sampling buffer (PBS for ciprofloxacin, PBS:Ethanol(50:50) for 8-methoxypsoralen). One blank sample was collected before drug administration, and subsequent samples were collected at (30, 60,120,180,240,300 and 360min). For ETS procedure, thirty electrical pulses each of 10ms duration at 120V/cm2, 1Hz was applied and the sampling fluid remained in the chamber for 15 minutes after pulsing.
2.4.3. Plasma pharmacokinetics of drugs
For plasma pharmacokinetic studies, one hundred micro liters of blood was collected by retro orbital bleeding before injection of drug and before each episode of transcutaneous sampling and cutaneous microdialysis. The blood samples were diluted with 200μl of PBS and plasma was separated by centrifugation at 2000g. In case of ciprofloxacin, plasma samples were subjected to protein precipitation with equal volume of 0.5M perchloric acid, followed by centrifugation at 10000g for 10min and drug content was analyzed by HPLC (Bielecka-Grzela et al., 2005). 8-methoxypsoralen in the plasma was extracted with methylene chloride for 30min followed by centrifugation at 5000g at 4°C for 10min, the organic phase was separated and evaporated to dryness at room temperature under nitrogen and the residue was dissolved in PBS:Ethanol (50:50) and analyzed by HPLC (Monbaliu et al., 1981).
2.5. Analytical method
The amounts of ciprofloxacin and 8-methoxypsoralen present in plasma, ETS and microdialysis samples were analyzed by high performance liquid chromatography. The HPLC system (Waters, MA) consisted of a chromatographic pump (Waters 1525) and an autosampler (Waters 717 plus).
Analysis of ciprofloxacin samples were carried out using a fluorescence detector (Waters 2475) at an excitation wavelength of 278nm and emission wavelength of 445nm. Symmetry® C18 column (4.6 × 150mm) was used and the mobile phase consisted of a mixture of acetonitrile and 0.1M aqueous monopotassium phosphate solution adjusted to pH 2.5 with orthophosphoric acid (15:85 v/v) with flow rate of 2 ml/min (Bielecka-Grzela et al., 2005). The linearity range was between 1-1000ng/ml (R2= 0.99).
Analysis of 8-methoxypsoralen samples were carried out using a UV detector (Waters 2487) at 248nm using Symmetry® C18 column (4.6 × 150mm). The mobile phase consisted of a mixture of methanol, acetonitrile and water (2:30:68) and the flow rate was 1ml/min (Monbaliu et al., 1981, Kappes et al., 2003). The linearity range was between 10-1000ng/ml (R2= 0.99). The recovery for ciprofloxacin was 96.90 ± 1.98% and that of 8-methoxypsoralen was 95.81 ± 1.57%. HPLC method of ciprofloxacin had an intra and inter day precision of <6% with an accuracy >95% and the limit of detection was 1ng/ml. 8-methoxypsoralen HPLC method had an intra and inter day precision of <8% and accuracy of >94% with limit of detection being 10ng/ml.
2.6. Data analysis
The pharmacokinetic parameters were calculated using non compartmental pharmacokinetic model. The terminal elimination rate constant (λz) was determined from the slope of terminal exponential phase of the logarithmic plasma concentration-time curve. The elimination half life (t1/2) was calculated using 0.693/ λz. The area under the curve (AUC0-t) was caclulated using the trapezoidal rule and AUC0-∞ was obtained by adding Clast/ λz to AUC0-t. In case of 8-methoxypsoralen, the maximum plasma concentration (C max) and the time to reach maximum plasma concentration (T max) were determined from the concentration-time curves.
The statistical analysis was carried out using GraphPad Instat 3 software and the t-test was selected for comparing the parameters obtained from ETS and microdialysis techniques. p < 0.05 was considered as level of significance. The data points shown in graphs are an average of 6 trials with error bars representing standard deviation.
3. Results and discussion
Solubility of drug in the sampling fluid is one of the key determinants of recovery of drug in case of both ETS and microdialysis. PBS was found to be an appropriate solvent for sampling ciprofloxacin. However, in the case of 8-methoxypsoralen, the the recovery was poor with PBS which was likely due to the low water solubility of 8-methoxypsoralen (Water solubility of 8 methoxy psoralen is ∼55.8μg/ml). The recovery of 8-methoxypsoralen was improved with 50:50 PBS:Ethanol system in both ETS and microdialysis techniques.
Electroporation leading to permeabilization of the skin is indicated by the drastic drop in electrical resistance of the skin. The drop in electrical resistance has been reported to be due to formation of transient aqueous pathways in the lipid domain of the stratum corneum. The aqueous pathways reseal and the skin will regain its barrier property (thus its original electrical resistance). The extent of drop in electrical resistance and the duration required for the skin to recover is determined by the applied electrical protocol. With the protocol of 120 V, 10ms, 30 pulses applied in this project, the drop in skin resistance was ∼70 ± 5%. There was no significant recovery in skin resistance during the sampling time of 15min. However, the skin resistance recovered within few hours reflecting the fact that the electrical protocol was not too aggressive to bring about irreversible impairment of the skin barrier. This was in agreement with the observations in our previous studies carried out across the rat skin and porcine epidermis (Murthy et al., 2005, Murthy et al., 2008, Srinivasa et al., 2008).
Transcutaneous sampling was carried out across the untreated skin (did not apply electrical pulses) which served as control set of experiment. In case of untreated skin (control samples), detectable level of drugs could not be sampled within the sampling duration of 15 minutes. In case of ETS, detectable amounts of drug was sampled even at low reservoir drug concentrations. The amount of drug sampled in 15min following application of electrical pulses was plotted against the corresponding reservoir drug concentrations (Figure 1 & 2). A linear relationship (R2=0.96 and 0.98 for ciprofloxacin and 8-methoxypsoralen respectively) was observed between the amount of drug sampled and the reservoir drug concentration, indicating that the subdermal drug concentration can be represented by ETS samples. The percentage recovery by ETS (slope × 100) was 4.8 ± 0.8% and 7.2 ± 1.3% for ciprofloxacin and 8-methoxypsoralen respectively. The recovery of 8-methoxypsoralen was less (∼ 0.5%) when ETS was carried out with PBS alone.
Fig.1.
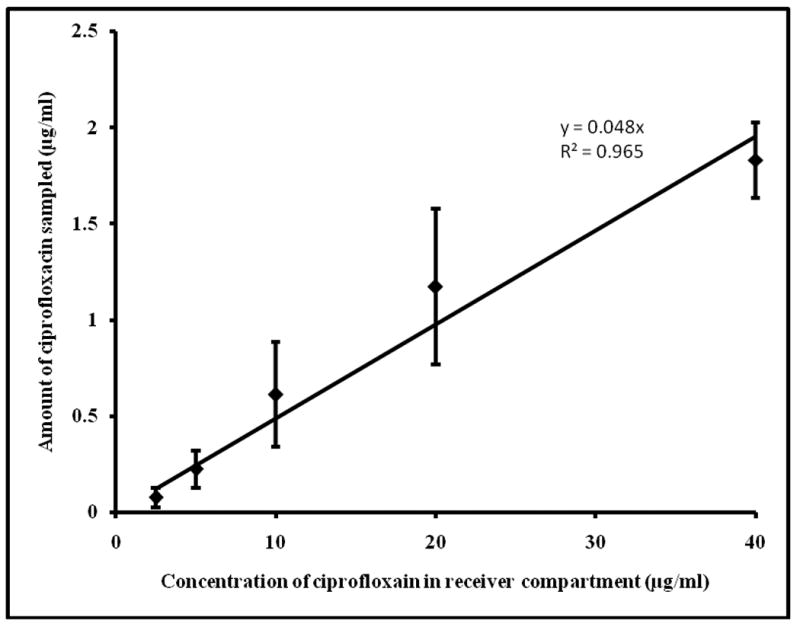
Correlation between ciprofloxacin concentration (2.5-40μg/ml) in the reservoir compartment and amount sampled by ETS across hairless rat skin in vitro. The data points represent an averages of n=6 ± s.d.
Fig.2.
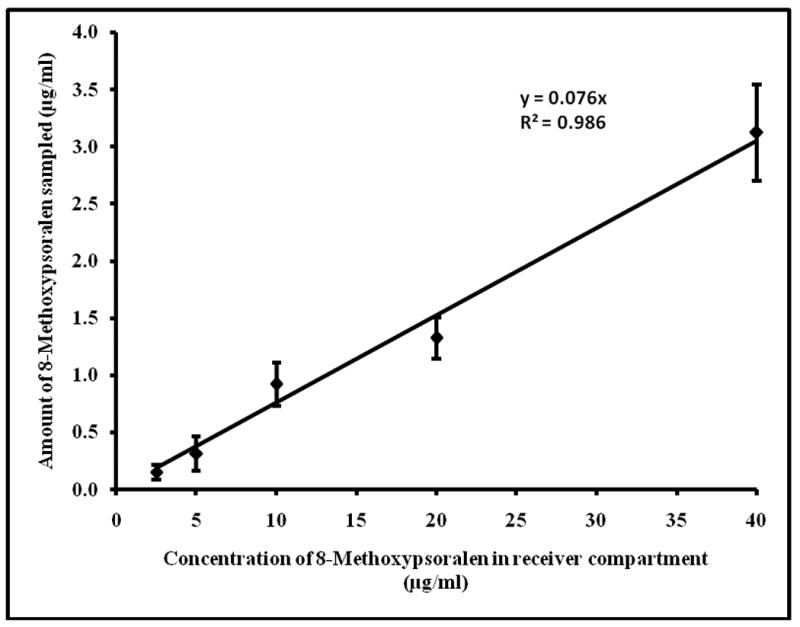
Correlation between 8-methoxypsoralen concentration (2.5-40μg/ml) in the reservoir compartment and amount sampled by ETS across hairless rat skin in vitro. The data points represent an averages of n=6 ± s.d.
The free drug present in the blood plasma equilibrates rapidly with the skin ECF. In otherwords, the extent of distribution of drug into peripheral tissues is known to be determined predominantly by the plasma protein binding. Generally, the drugs that show high protein binding penetrate less into the peripheral compartment. Ex vivo plasma protein binding studies were carried out for both ciprofloxacin and 8-methoxypsoralen to assess the relationship between the protein binding and tissue penetration. The plasma protein binding of ciprofloxacin was found to be 20.65 ± 3.93% at concentrations between 1-40 μg/ml which was in aggreement with 16-40% reported by other research groups previously (Nouaille-Degorce et al., 1998, Zhu et al., 1999, Singh et al., 2006). The fraction of 8-methoxypsoralen bound to plasma was 80.97 ± 6.85%. This is comparable to 91.4% reported by Pibouin et al (Pibouin et al., 1987). The two drugs chosen for this project differ significantly with respect to their protien binding property. One of the major objectives of this project was to assess the workability of the ETS technique in case of drugs which exhibit different extents of protein binding.
The in vitro recovery of ciprofloxacin and 8-methoxypsoralen by microdialysis probe were found to 3.47 ± 0.35% and 16.41 ± 3.32% respectively. Generally microdialysis is regarded as less suitable for sampling lipophilic drugs because, microdialysis method of drug sampling involves perfusion of aqueous sampling fluid through the dialysis probe during which the exchange of ingredients between the perfusion fluid and tissue fluid takes place. The perfusate liquids are preferably aqueous and therefore when the drug to be sampled is highly lipophilic, the recovery of the drug with the aqueous perfusion liquids would be relatively very less (Malonne 1999, Schnetz et al., 2001, Kreilgaard 2002). It is not recommended to use organic solvents as the perfusate could alter the dialysis membrane properties and may also cause potential toxicity to the tissue. However, in this study, we found that the permeability of the dialysis membrane did not change significantly even after immersing the membrane for over 24 hours in the 50:50 alcohol: water system.
In case of ETS, as the technique is a noninvasive technique and the sampling fluid is in contact with the tissue for a short period of time during drug sampling, use of hydroalcoholic systems pose relatively less severe risk as compared to microdialysis. This shows that ETS technique could be used to sample even lipophilic drugs, by changing the the sampling fluid constitution appropriately. This is considered as one of the the advantages of ETS over microdialysis technique.
In the current experiments, as the animals were anesthetized, there were no notable irritation symptoms due to electroporation or microdialysis. There was no inflammation due to application of electrical pulses or insertion of probe. However, there was slight bleeding during the implantation of the probe which stopped within one or two minutes completely.
From both microdialysis and ETS techniques, the amount of ciprofloxacin and 8-methoxypsoralen present in the dermal ECF in rats was calculated using the amount sampled and the corresponding recovery values as follows:
| (3) |
Figure 3 represents the concentration time profile of ciprofloxacin (15mg/kg) in plasma and dermal ECF (determined by ETS and cutaneous microdialysis) following i.v. bolus administration. The pharmacokinetic parametes calculated from non-compartmental analysis are given in Table 1.
Fig.3.
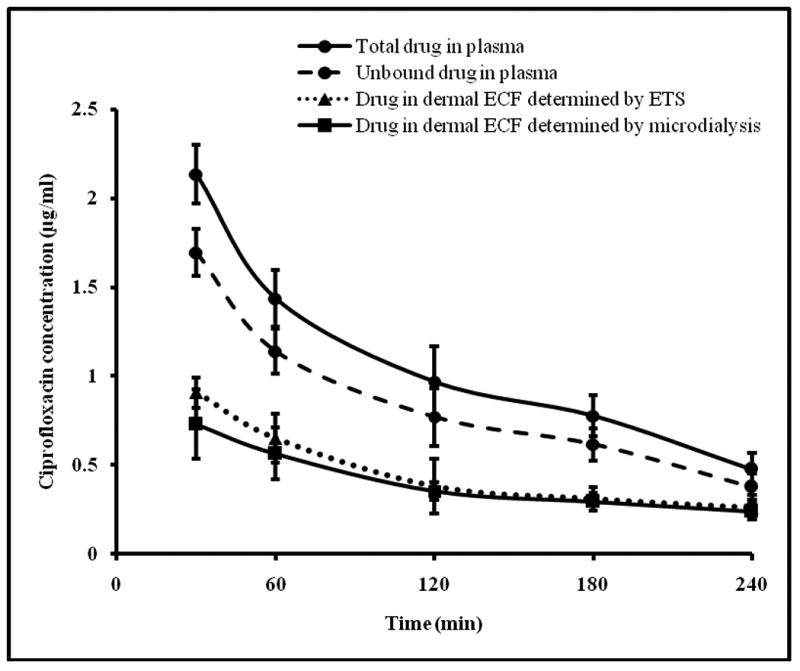
Time course of ciprofloxacin in rat plasma determined by blood sampling and dermal ECF determined by microdialysis and ETS technique following administration of 15mg/kg ciprofloxacin i.v. bolus. The data points represent an average of n=6 ± sd. Blood sampling, ETS and microdialysis were carried out simultaneously on each rat at the same time points.
Table 1.
Mean pharmacokinetic parameters of ciprofloxacin (unbound) in plasma and dermal ECF (determined by ETS and microdialysis) following administration of 15mg/kg i.v bolus in hairless rats (n=6 ± s.d).
| Parameter | ETS | Microdialysis | Plasma |
|---|---|---|---|
| Tmax (min) | 30 | 30 | 30 |
| Cmax (μg/ml) | 0.906 ± 0.046 | 0.729 ± 0.196 | 1.695 ± 0.131 |
| AUC0-∞ (min*μg/ml) | 88.29 ± 20.41 | 89.71 ± 25.18 | 240.33 ± 29.59 |
| t1/2 (min) | 55.84 ± 13.44 | 82.36 ± 22.54 | 124.12 ±25.99 |
The plasma elimination half life of ciprofloxacin in the present study was found to be 124.12±25.99 min. This was comparable to 102.3 ± 26.2 min and 91 ± 8.6 min reported by Nouaille-Degorce et al and Tsai et al respectively (Nouaille-Degorce et al., 1998, Tsai et al., 2001).
Figure 4 represents the concentration time profile of 8-methoxypsoralen in plasma and dermal ECF (determined by ETS and cutaneous microdialysis) following oral administration of 5mg/kg of 8-methoxypsoralen. The pharmacokinetic parametes calculated from non-compartmental analysis are given in Table 2.
Fig.4.
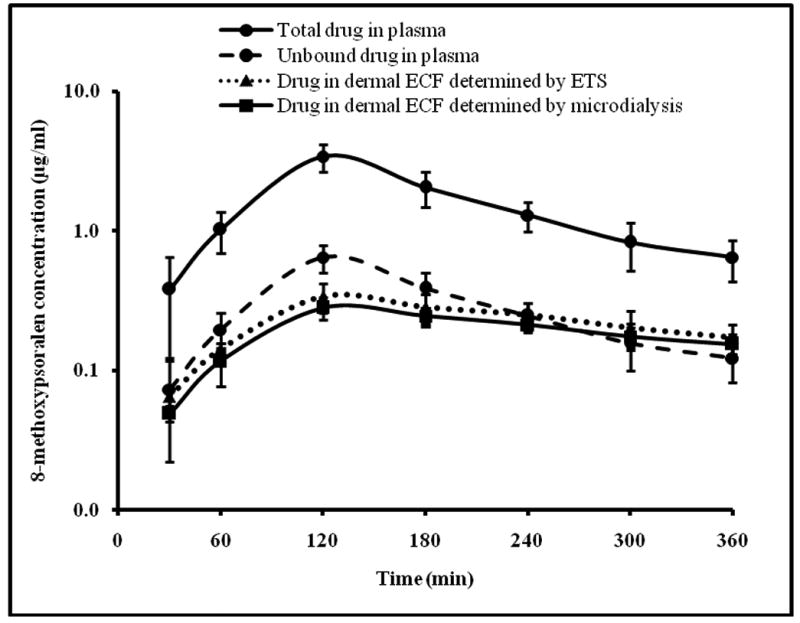
Time course of 8-methoxypsoralen in rat plasma determined by blood sampling and dermal ECF determined by microdialysis and ETS technique following oral administration of 5mg/kg 8-methoxypsoralen. The data points represent an average of n=6 ± sd. Blood sampling, ETS and microdialysis were carried out simultaneously on each rat at the same time points.
Table 2.
Mean pharmacokinetic parameters of 8-methoxypsoralen (unbound) in plasma and dermal ECF (determined by ETS and microdialysis) following oral administration of 5mg/kg in hairless rats (n=6 ± s.d).
| Parameter | ETS | Microdialysis | Plasma |
|---|---|---|---|
| Tmax (min) | 120 | 120 | 120 |
| Cmax (μg/ml) | 0.338 ± 0.080 | 0.280 ± 0.051 | 0.644 ± 0.142 |
| AUC0-∞ (min*μg/ml) | 116.08 ± 29.22 | 116.10 ± 22.85 | 120.72 ± 36.25 |
| t1/2 (min) | 243.83 ± 18.43 | 237.76 ± 15.83 | 97.15 ± 6.72 |
The Cmax and Tmax following oral administration of 5mg/kg of 8-methoxypsoralen to hairless rats was found to be 0.64 ± 0.14 μg/ml and 120 min respectively which is comparable to that reported by Roelandts et al. Roelandts et al have reported that following oral adminstration of 10mg/kg of 8-methoxypsoralen to rats, Cmax and Tmax were ∼1.2μg/ml and 150min respectively (Roelandts et al., 1983).
The tissue penetration was determined from the ratio of AUC0-∞ of unbound drug in dermal ECF to the AUC0-∞ of total drug (bound +unbound) in plasma. The extent of tissue penetration of 8-methoxypsoralen from ETS and cutaneous microdialysis was found be 18.41±3.76 and 18.47±3.30% respectively, which very well agrees with the percentage protein binding (80.97 ± 6.85%). In contrast, ciprofloxacin, penetration into skin tissue was was found to be 26.31±4.54 and 26.99±7.60% with ETS and cutaneous microdialysis respectively which did not correlate with the protein binding of 20.65 ± 3.93%. It is likely that in case of ciprofloxacin, there are additional factors other than protein binding that determine the extent of penetration of drug into peripheral tissues. In agreement with our data, despite a less extent of protein binding (∼30%), ciprofloxacin has been reported to penetrate only ∼30-60% into the interstitial fluid in humans. (Brunner et al., 2002, Singh et al., 2006).
The point to point comparison of the drug concentration in the dermal extracellular fluid and the dermatokinetic parameters i.e. Cmax, Tmax, AUC0-œ and t1/2 determined by ETS and microdialysis technique for ciprofloxacin and 8-methoxypsoralen did not differ significantly (t-test, p<0.05) (Table 1 and 2). This provides validity to the ETS technique for sampling drugs like ciprofloxacin and 8-methoxypsoralen. The study also demonstrates that ETS could be implemented in dermatokinetic investigation regardless of route of administration of drugs.
Successful development of a sampling device based on the ETS concept would be very useful in carrying out dermatokinetic investigations of drugs. The noninvasiveness of the technique permits frequent sampling of drugs which helps in relatively more precise calculation of the kinetic parameters. In addition the technique could be used to sample drugs in routine therapeutic drug monitoring. ETS is a potential technique for cutaneous sampling of drugs and analytes and could replace the invasive blood sampling procedure in future.
4. Conclusion
ETS is a potential noninvasive technique for sampling of drugs from the dermal ECF. The results of this project have shown the workability of ETS in case of drugs with different solubility and different degree of protein binding. In addition the present work also demonstrates the workability of the ETS technique in case of intravenous and oral routes of drug administration.
Acknowledgments
This project described was supported by Grant Number AR053097 from National Institute of Arthritis and Musculoskeletal and skin Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alguire PC, Mathes BM. Skin biopsy techniques for internist. J Gen Intern Med. 1998;13:46–54. doi: 10.1046/j.1525-1497.1998.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault JM, Lunte CE, Meltzer NM, Riley CM. Microdialysis sampling for the investigation of dermal drug transport. Pharm Res. 1992;9:1256–1261. doi: 10.1023/a:1015892914649. [DOI] [PubMed] [Google Scholar]
- Bielecka-Grzela S, Klimowicz A. Penetration of ciprofloxacin and its desethylenemetabolite into skin in humans after a single oral dose of the parent drug assessed by cutaneous microdialysis. J Clin pharm Ther. 2005;30:383–390. doi: 10.1111/j.1365-2710.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- Brautigam L, Seegel M, Tegeder I, Schmidt H, Meier S, Podda M, Kaufmann R, Grundmann-Kollmann M, Geisslinger G. Determination of 8-methoxypsoralen in human plasma, and microdialysates using liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2003;798:223–229. doi: 10.1016/j.jchromb.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Brunner M, Hollenstein U, Delacher S, Jager D, Schmid R, Lackner E, Georgopoulus A, Eichler HG, Muller M. Distribution and antimicrobial activity of ciprofloxacin in human soft tissues. Antimicrob Agents Chemother. 1999;43:1307–1309. doi: 10.1128/aac.43.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner M, Staβ H, Moller JG, Schrolnberger C, Erovic B, Hollenstein U, Zeitlinger M, Eichler HG, Muller M. Target concentrations of ciprofloxacin after single intravenous and oral doses. Antimicrob Agents Chemother. 2002;46:3724–3730. doi: 10.1128/AAC.46.12.3724-3730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey E, Diamond D, Smyth MR, Malone MA, Rabenstein K, Mcshane A, MvKenna M, Keaveny TV, Freaney R. In Vitro optimisation of a microdialysis system with potential for online monitoring of lactate and glucose in biological samples. Analyst. 1997;122:185–189. doi: 10.1039/a606029c. [DOI] [PubMed] [Google Scholar]
- Denet AR, Preat V. Transdermal delivery of timolol by electroporation through human skin. J Control Release. 2003;88:253–262. doi: 10.1016/s0168-3659(03)00010-5. [DOI] [PubMed] [Google Scholar]
- Gazith J, Schalla W, Bauer E, Schaefer H. 8-Methoxypsoralen (8-MOP) in human skin: Penetration kinetics. J Invest Dermatol. 1978;71:126–130. doi: 10.1111/1523-1747.ep12546709. [DOI] [PubMed] [Google Scholar]
- Groth L, Serup J. Cutaneous microdialysis in man:Effects of needle insertion trauma and anaesthesia on skin perfusion, erythema and skin thickness. Acta Derm Venereol. 1998a;78:5–9. doi: 10.1080/00015559850135733. [DOI] [PubMed] [Google Scholar]
- Groth L, Serup J. Cutaneous microdialysis in the rat:Insertion trauma studied by ultrasound imaging. Acta Derm Venereol. 1998b;78:10–14. doi: 10.1080/00015559850135742. [DOI] [PubMed] [Google Scholar]
- Kappes UP, Barta U, Merkel U, Balogh A, Elsner High plasma levels of 8-methoxypsoralen following bath water delivery in dermatological patients. Skin Pharmacol Appl Skin Physiol. 2003;16:305–312. doi: 10.1159/000072070. [DOI] [PubMed] [Google Scholar]
- Ketchum C, Robinson CA, Huang ST. Analysis of 8-methoxypsoralen by high performance liquid chromatography. Clin Chem. 1990;36:1956–1957. [PubMed] [Google Scholar]
- Kreilgaard M. Assessment of cutaneous drug delivery using microdialysis. Adv Drug Deliv Rev. 2002;54:S99–S121. doi: 10.1016/s0169-409x(02)00117-5. [DOI] [PubMed] [Google Scholar]
- Leveque N, Robin S, Makki S, Muret P, Mary, Berthelot A, humbert P. Iron concentrations in human dermis assessed by microdialysis associated with atomic absorption spectroscopy. Biol Pharm Bull. 2001;24:10–13. doi: 10.1248/bpb.24.10. [DOI] [PubMed] [Google Scholar]
- Lombry C, Dujardin N, Preat V. Transdermal delivery of macromolecules using skin electroporation. Pharm Res. 2000;17:32–37. doi: 10.1023/a:1007510323344. [DOI] [PubMed] [Google Scholar]
- Malonne I Davis. A review of microdialysis sampling for pharmacokinetic applications. Anal Chim Acta. 1999;379:227–249. [Google Scholar]
- Mays DC, Rogers SL, Guiler RC, Sharp DE, Hecht SG, Staubus AE, Gerber N. Disposition of 8-Methoxypsoralen in the rat: methodology for measurement, dose-dependent pharmacokinetics, tissue distribution and identification of metabolites. J Pharmacol Exp Ther. 1985;236:364–373. [PubMed] [Google Scholar]
- Monbaliu JG, Rosseel MT, Bogaert MG. Analysis of methoxsalen in plasma by reversed-phase high performance liquid chromatography. J Pharm Sci. 1981;70:965–966. doi: 10.1002/jps.2600700840. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Hatanaka T, Sugibayashi K, Omiya H. Prediction of skin permeability of drugs: Comparison of human and hairless rat skin. J Pharm Pharmacol. 1992;44:634–639. doi: 10.1111/j.2042-7158.1992.tb05484.x. [DOI] [PubMed] [Google Scholar]
- Muller M, Haag O, Burgdorff T, Georgopoulos A, Weninger W, Jansen B, Stanek G, Pehamberger H, Agneter E, Eichler HG. Characterization of peripheral compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob Agents Chemother. 1996;40:2703–2709. doi: 10.1128/aac.40.12.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Staβ H, Brunner M, Moller JG, Lackner E, Eichler HG. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob Agents Chemother. 1999;43:2345–2349. doi: 10.1128/aac.43.10.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SN, Zhao YL, Sen A, Hui SW. Cyclodextrin enhanced transdermal delivery of piroxicam and carboxyfluorescein by electroporation. J Control Release. 2004;99:393–402. doi: 10.1016/j.jconrel.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Murthy SN, Zhao YL, Hui SW, Sen A. Electroporation and transcutaneous extraction (ETE) for pharmacokinetic studies of drugs. J Control Release. 2005;105:132–141. doi: 10.1016/j.jconrel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Murthy SN, Zhang S. Electroporation and transcutaneous sampling (ETS) of acyclovir. J Dermatol Sci. 2008;49:249–251. doi: 10.1016/j.jdermsci.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouaille-Degorce B, Veau C, Dautrey S, Tod M, Laouari D, Carbon C, Farinotti R. Influence of renal failure on ciprofloxacin pharmacokinetics in rats. Antimicrob Agents Chemother. 1998;42:289–292. doi: 10.1128/aac.42.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibouin M, Zini R, Nguyen P, Tillement JP. Binding of 8-methoxypsoralen to human serum proteins and red blood cells. Br J Dermatol. 1987;117:207–215. doi: 10.1111/j.1365-2133.1987.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Roelandts R, Boven MV, Adriaens P, Schryver FD, degree H. The relationship between 8-methoxypsoralen skin and blood levels. J Invest Dermatol. 1983;81:331–333. doi: 10.1111/1523-1747.ep12519792. [DOI] [PubMed] [Google Scholar]
- Schaefer HG, Stass H, Wedgwood J, Hampel B, Fischer C, Kuhlmann J, Schaad UB. Pharmacokinetics of ciprofloxacin in pediatric cystic fibrosis patients. Antimicrob Agents Chemother. 1996;40:29–34. doi: 10.1128/aac.40.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetz E, Fartasch M. Microdialysis for the evaluation of penetration through the human skin barrier- a promising tool for future research. Eur J Pharm Sci. 2001;12:165–174. doi: 10.1016/s0928-0987(00)00155-x. [DOI] [PubMed] [Google Scholar]
- Singh SS, Mehta J. Measurement of drug-protein binding by immobilized human serum albumin-HPLC and comparison with ultrafiltration. J Chromatogr B. 2006;834:108–116. doi: 10.1016/j.jchromb.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Srinivasa Murthy S, Siva Ram Kiran V, Mathur SK, Murthy SN. Noninvasive transcutaneous sampling of glucose by electroporation. J Diabetes Sci Technol. 2008;2:250–254. doi: 10.1177/193229680800200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susato F, Humfeld S, Reinauer H. High performance liquid chromatography measurement of 8-methoxypsoralen in plasma. Chromatographia. 1986;21:443–446. [Google Scholar]
- Tegeder I, Brautigam L, Podda M, Meier S, Kaufmann R, Geisslinger G, Kollmann MG. Time course of 8-methoxypsoralen concentrations in skin and plasma after topical (bath and cream) and oral administration of 8-methoxypsoralen. Clin Pharmacol Ther. 2002;71:153–161. doi: 10.1067/mcp.2002.121908. [DOI] [PubMed] [Google Scholar]
- Tsai TH, Wu JW. Pharmacokinetics of ciprofloxacin in the rat and its interaction with cyclosporine A: a microdialysis study. Analytica Chimica Acta. 2001;448:195–199. [Google Scholar]
- Wise R, Lockley RM, Webberly M, Dent J. Pharmacokinetics of intravenously administered ciprofloxacin. Antimicrob Agents Chemother. 1984;26:208–210. doi: 10.1128/aac.26.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester RC, Maibach HI. Animal Models for Percutaneous Absorption. In: Shah VP, Maibach HL, editors. Topical Drug Bioavailability, Bioequivalence, and Penetration. Plenum Press; NY: 1993. [Google Scholar]
- Zhu M, Wong PYK, Li RC. Influence of sanguisorba officinalis, a mineral rich plant drug, on the pharmacokinetics of ciprofloxacin in the rat. J Antimicrob Chemother. 1999;44:125–128. doi: 10.1093/jac/44.1.125. [DOI] [PubMed] [Google Scholar]


