Abstract
Polymer nanomaterials have sparked a considerable interest as vehicles used for diagnostic and therapeutic agents; research in nanomedicine has not only become a frontier movement but is also a revolutionizing drug delivery field. A common approach for building a drug delivery system is to incorporate the drug within the nanocarrier that results in increased solubility, metabolic stability, and improved circulation time. With this foundation, nanoparticles with stealth properties that can circumvent RES and other clearance and defense mechanisms are the most promising. However, recent developments indicate that select polymer nanomaterials can implement more than only inert carrier functions by being biological response modifiers. One representative of such materials is Pluronic block copolymers that cause various functional alterations in cells. The key attribute for the biological activity of Pluronics is their ability to incorporate into membranes followed by subsequent translocation into the cells and affecting various cellular functions, such as mitochondrial respiration, ATP synthesis, activity of drug efflux transporters, apoptotic signal transduction, and gene expression. As a result, Pluronics cause drastic sensitization of MDR tumors to various anticancer agents, enhance drug transport across the blood brain and intestinal barriers, and causes transcriptional activation of gene expression both in vitro and in vivo. Collectively, these studies suggest that Pluronics have a broad spectrum of biological response modifying activities which make it one of the most potent drug targeting systems available, resulting in a remarkable impact on the emergent field of nanomedicine.
Pluronic Block Copolymers as Micellar Nanocarriers for Drug Delivery
Pluronic unimers and micelles
Polymer-based nanotechnology became one of the most attractive and fast growing areas of pharmaceutical research. The materials that are currently being researched include polymer micelles, polymer-DNA complexes (“polyplexes”), nanogels, liposomes, and other nanoscale sized materials for medical use that are collectively called nanomedicines. Specific arrangements of polymeric molecules at the nanoscale achieved within such materials represent unique opportunities for safe and efficient delivery of drugs, genes, and imaging molecules [1-13].
One promising example of such polymer nanomaterials is represented by a class of Pluronic block copolymers (also known under non-proprietary name “poloxamers”). These block copolymers consist of hydrophilic poly(ethylene oxide) (PEO) and hydrophobic poly(propylene oxide) (PPO) blocks arranged in A-B-A tri-block structure: PEO-PPO-PEO (Figure 1A). The block copolymers with different numbers of hydrophilic ethylene oxide and hydrophobic propylene oxide units are characterized by different hydrophilic-lipophilic balance (HLB). Due to their amphiphilic character these copolymers display surfactant properties including ability to interact with hydrophobic surfaces and biological membranes. In aqueous solutions at concentrations above critical micelle concentration (CMC) these copolymers self-assemble into micelles. The diameters of Pluronic micelles usually vary from ca. 10 nm to 100 nm [14]. The core of the micelles consists of hydrophobic PPO blocks that are separated from the aqueous exterior by the shell hydrated of hydrophilic PEO chains. By itself the core represents a “cargo hold” for incorporation of various therapeutic or diagnostic reagents (Figure 1B). In selected cases the PPO core can incorporate considerable amounts (up to 20-30 % wt.) of water-insoluble drugs [15]. The PEO shell ensures that the micelles remain in a dispersed state and decreases undesirable drug interactions with cells and proteins. Pharmacokinetics and biodistribution of one of the micelles and single chains of Pluronic P85 was reported in [16]. Interestingly, half-life of P85 depends on the aggregation state of the block copolymer and varies from 60 hours for unimers to 90 hours for micelles, respectively. Formation of micelles decreased the uptake of the block copolymer in the liver, although it had no effect on the total clearance, suggesting that the elimination of P85 was controlled by the renal elimination of P85 unimers and not by the rate of micelle disposition or disintegration.
Figure 1.
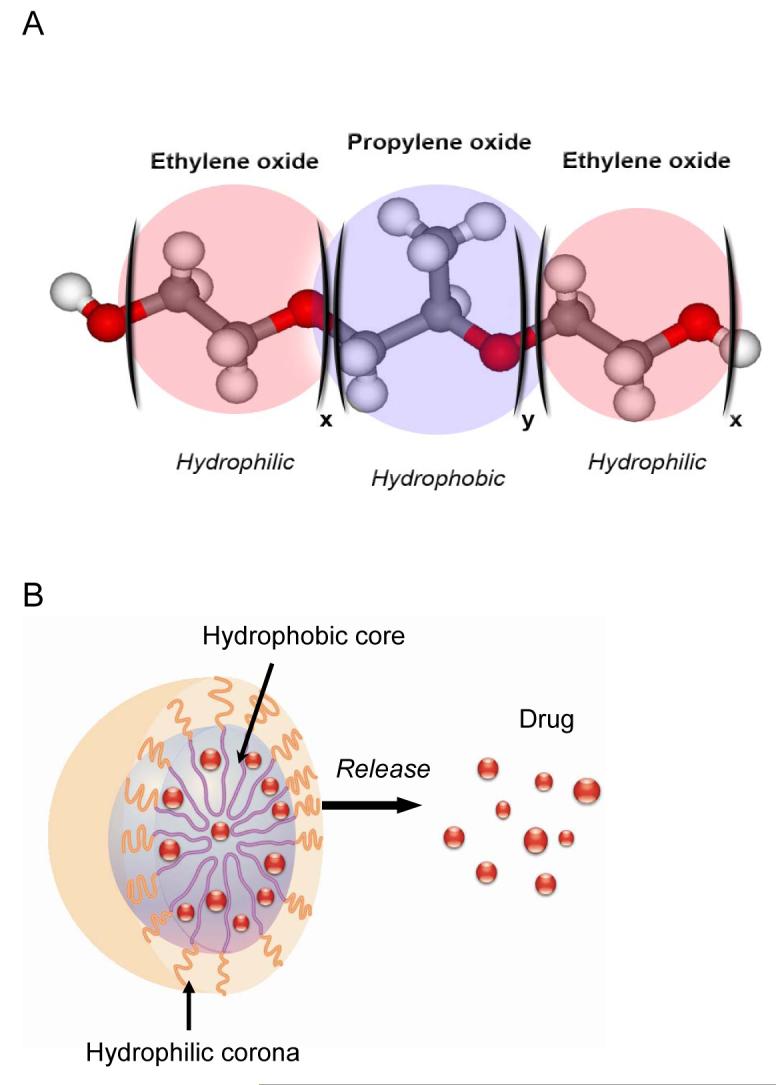
Pluronic block copolymer molecule (A) and micelle with a solubilized drug (B).
Pluronic micelles for drug delivery
Incorporation of low molecular mass drugs into Pluronic micelles can increase drug solubility and drug stability, and can improve drug pharmacokinetics and biodistribution. Polymeric micelles were utilized for delivery of CNS drugs across the blood brain barrier (BBB) [17, 18], oral delivery of drugs [19-21] and tumor-specific delivery of antineoplastic agents [22-24]. For example, in one early study a neuroleptic drug loaded Pluronic P85 micelles were targeted to the brain by conjugating the micelles with neurospecific antibodies or insulin as targeting moieties [25]. An improvement of oral bioavailability of a poorly water-soluble phytoestrogen, genistein, was achieved by incorporation of this drug into Pluronic F127 micelles [21]. Pluronic block copolymers were also reported to significantly enhance the bioavailability of various antibacterial and antifungal drugs and to enhance the activity of these drugs with respect to many microorganisms [26-29].
In application to anticancer chemotherapy, drug encapsulation in micelles can diminish drug extravasation into normal tissues and provide for a passive drug targeting to tumors via the enhanced permeability and retention (EPR) effect. The EPR is realized due to abnormally high permeability of tumor blood vessels combined with prolonged circulation of the micelles due to their decreased extravasation in normal vessels and lack of renal clearance. SP1049C, the first anti-cancer micellar formulation to reach clinical evaluation, contained doxorubicin (Dox) in the mixed micelles of Pluronics L61 and F127, [30]. Analysis of pharmacokinetics and biodistribution of Dox incorporated into these micelles demonstrated more efficient accumulation of micellar drug in the tumors compared to the free drug. Furthermore, this study indicated that the peak levels of Dox formulated with SP1049C in the tumor were delayed and the drug residence time was increased in comparison with the free Dox [22]. Formulation of Dox, paclitaxel or other drugs with Pluronics also resulted in enhancement of drug anticancer effect in in vivo tumor models [22, 31-33]. In these studies, mice bearing drug-sensitive and drug-resistant tumors were treated with Dox alone and with Dox in Pluronic formulations. The tumor panel included i.p. murine leukemias (P388, P388-Dox), s.c. murine myelomas (Sp2/0, Sp2/0-Dnr), i.v. and s.c. Lewis lung carcinoma (3LL-M27), s.c. human breast carcinomas (MCF7, MCF7/ADR), and s.c. human oral epidermoid carcinoma (KBv). The results showed that the tumors were more responsive to Pluronic/Dox than to Dox alone. Another study reported that micellar solutions of Pluronic P85 and L61 increased carboplatin (CPt) toxicity in the experimental colorectal carcinoma in vivo [24, 34]. A total of 120 subcutaneous tumors (4 per rat) were inoculated in 30 BDIX rats and were treated weekly for 4 weeks with intratumoral injection of CPt alone or with either P85 or L61. The results indicated that tumors treated with low-dose CPt (2.8 mg/kg) and P85 or L61 exhibited significant reductions in tumor volume compared with tumors treated with the free drug.
In another study multiple Dox molecules were chemically conjugated via pH-cleavable hydrazone linkages to the repeating units of PPO chains of a Pluronic-mimicking triblock copolymer [35]. The Dox-copolymer conjugates spontaneously formed polymeric micelles. The conjugated Dox was released from the micelles due to the cleavage of hydrazone linkages, which was accelerated at acidic pH 5 compared to pH 7.4. Dox-copolymer conjugate exhibited a different intracellular distribution profile and enhanced cytotoxicity with respect to MCF7 cells compared to free Dox. It was suggested that Dox-copolymer conjugate transported into cells via endocytosis as opposed to transmembrane diffusion realized in the case of free Dox. The endocytosis mechanism of entry of Dox-copolymer conjugate may have advantage for overcoming multidrug resistance (MDR) in cancer cells [35]. A pH-dependent non-covalent incorporation and release of Dox in Pluronic P85-poly(acrylic acid) block copolymer was reported in [36]. In this case at the extracellular pH the Dox molecules were apparently bound to the micelles due combination of hydrophobic interactions with PPO chains and electrostatic interactions with carboxylic groups of poly(acrylic acid). Acidification at pH5.0 resulted in protonation of the carboxylic groups and release of the drug.
Recently, to enhance stability of micelles in the blood stream upon dilution, Pluronic L121 micelles were cross-linked through their hydrophilic shells [37]. To form the crosslinks, the end hydroxyl groups of Pluronic L121 were first chemically converted to aldehydes and then bridged via Schiff bases. This greatly reduced the CMC of the micelles and enhanced the micelle stability.
A series of studies used Pluronic P105 micelles for the delivery of Dox into solid tumors in mice [38-41]. In these studies the localized ultrasonic irradiation of the tumor was applied upon accumulation of the micelles in the tumor interstitium to facilitate the drug release into the tumor cells [40]. Furthermore, the ultrasound-enhanced intracellular uptake of Dox administered with the Pluronic P105 micelles was demonstrated in vitro [38]. It was suggested that the enhanced uptake was caused by either micelle disintegration that released free Dox or cell membrane perturbations that facilitated the cellular uptake of the micelles as a whole. Overall, micellar delivery combined with ultrasonic irradiation resulted in a substantial decrease of the s.c. tumor growth rates compared to a positive control (Figure 2).
Figure 2.
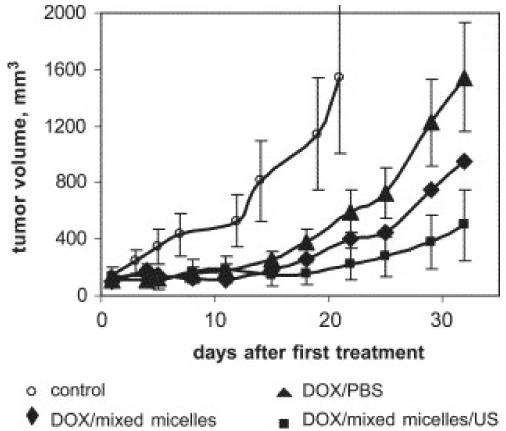
Growth curves of s.c. A2780 ovarian carcinoma tumors inoculated in female nu/nu mice; DOX (3 mg/kg) was either dissolved in PBS (positive control) or encapsulated in mixed micelles; drug injections were combined or not combined with tumor sonication. Treatments were initiated when the tumor volume reached 75–125 mm3; three consecutive treatments were applied on days 1, 3, and 5. Ultrasound parameters: frequency-1 MHz; power density—3.4 W/cm2, duty cycle 50%, duration 30 s, time of application—4 h after the i.v. injection of the drug. Based on Ref. [39].
Pluronic Block Copolymers as Biological Response Modifiers
Biological activity of Pluronic molecules
The polymers used for drug delivery were considered as biologically inert components that protect drugs from degradation, prolong exposure of drugs to tissues, and enhance transport of drugs into cells. However, this paradigm is undergoing substantial evolution due to growing evidence that select synthetic polymers can drastically alter specific cellular responses [42-44]. Thus, besides their ability to self-assemble into micelles, Pluronic block copolymers were shown to be potent biological response modifiers capable of sensitizing multidrug resistant (MDR) cancer cells and enhancing drug transport across cellular barriers, such as polarized intestinal epithelial cells, Caco-2, and brain endothelium [45, 46].
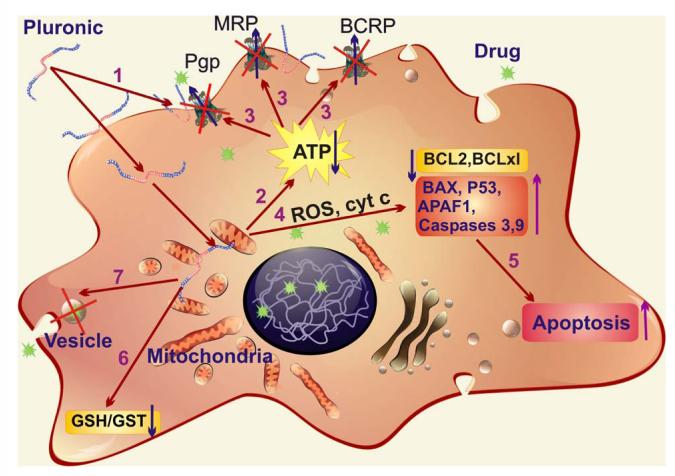
The complex mechanisms of Pluronic effects in MDR cells were thoroughly studied. It was demonstrated that Pluronic block copolymers 1) incorporate into membranes changing its microviscosity; 2) induce a dramatic reduction in ATP levels in cancer and barrier cells; 3) inhibit drug efflux transporters, such as Pgp [45, 47-50], multidrug resistance proteins (MRPs) [51], and breast cancer resistance protein (BCRP) [52, 53]; 4) induce release of cytochrome C and increase of reactive oxygen species (ROS) levels in the cytoplasm; 5) enhance pro-apoptotic signaling and decreasing anti-apoptotic defense in MDR cells [54]; 6) inhibit the glutathione/glutathione S-transferase detoxification system [48, 51]; and 7) abolish drug sequestration within cytoplasmic vesicles [32] (Figure 3). Remarkably, these effects were most apparent at polymer concentrations below the critical micellization concentration CMC [55, 56], suggesting that unimers, i.e. single block copolymer molecules, are responsible for biological modifying properties (CMC for Pluronic P85 is 0.03%wt). It was suggested that a crucial role of unimers (but not micelles) is determined by their ability to incorporate and translocate across the cellular membranes [48, 57]. The hydrophobic PPO chains of Pluronic immerse into the membrane hydrophobic areas, resulting in alterations of the membrane structure, and decrease its microviscosity (“membrane fluidization”). In contrast, formation of micelles at high concentrations of the block copolymer results in hiding these hydrophobic PPO chains in the micellar core and diminishes Pluronics availability to affect the cellular membranes.
Figure 3.
Multiple effects of Pluronic block copolymers in MDR cells: 1) incorporation of Pluronic molecules into membranes and decrease of the membrane microviscosity; 2) induction of ATP depletion; 3) inhibition of drug efflux transporters; 4) release of cytochrome C from mitochondria and increase in ROS levels in cytoplasm; 5) increase of pro-apoptotic signaling and decrease of anti-apoptotic defense in MDR cells; 6) inhibition of the glutathione/glutathione S-transferase detoxification system; and 7) abolishment of drug sequestration within cytoplasmic vesicles.
Effect on membrane microviscosity and ATPase activity of drug efflux transporters
Incorporation of Pluronic unimers into the plasma membrane decreases its microviscosity and accompanied by the inhibition of Pgp or MRP ATPase activity in the cells overexpressing these transporters [48]. Indeed, strong evidence indicates that lipid microenvironment of biological membranes is closely related to function of drug efflux transporters [58, 59]. It was suggested that inhibition of drug efflux transporters occurs due to conformational changes in the efflux proteins and/or sterically hindrance of protein-drug interaction in the appropriate binding sites induced by the immersed copolymer chains in the plasma membranes [60]. In particular, Pluronic P85 unimers displayed the effects characteristic of a mixed type enzyme inhibitor - decreasing maximal reaction rate, Vmax and increasing Michaelis constant, Km for ATP as well as Pgp-specific substrates such as vinblastine [60]. The magnitude of these effects for vinblasine was as high as over 200-fold Vmax/Km change (interestingly, MRP1 ATPase activity was affected less, which could explain somewhat smaller effects of Pluronic on this transporter). In contrast, at the high (micellar) concentrations, binding of Pluronic to the membrane actually results in restoration of Pgp ATPase activity.
Our recent data indicate that Pluronic unimers can selectively localize in particular domains of cellular membrane called “lipid rafts”. The co-localization of FITCPluronic P85 with caveolin-1 that is known to associate with these membrane domains was shown in Mardin-Derby canine kidney (MDCK) cells and brain microvessel endothelial cells by confocal microscopy (G. Sahay, in preparation). Furthermore, fluorescence resonance energy transfer (FRET) studies showed that energy transfer occurs between donor attached to Pluronic molecule (FITC) and acceptor on Cholera toxin B (Alexa Flour 555) localized in the lipid rafts, i.e. donor and acceptor are in close proximity of 10 nm (D. Alakhova, in preparation). This may explain complete inhibition of Pgp drug efflux system and only partial effect on MRP activity, since Pgp is believed to associate with lipid rafts [61, 62], while MRP may have different localization.
Effect on cell respiration
It has been shown that Pluronic unimers translocate across plasma membrane into cells and reach intracellular compartments [63]. More recently we have demonstrated that the initial stages of cellular entry of the unimers involve caveolae-mediated endocytosis (G. Sahay, in preparation). It was demonstrated that caveolae trafficking leads to the edoplasmatic reticulum [64] and further to mitochondria [65, 66]. This may explain the ultimate delivery of Pluronic unimers to the cell mitochondria that was previously reported [54]. Incorporation of Pluronic into the mitochondria membranes is accompanied by efficient inhibition of cellular respiration. The “molecular” targets for Pluronic in mitochondria include respiratory chain complexes I and IV (D. Alakhova, in preparation). In particular, we demonstrated that Pluronic P85 caused significant inhibition of these respiratory proteins in isolated mitochondria of MDR MCF7/ADR cells at relatively low concentrations of the block copolymer (0.01% and 0.001% for complexes I and IV, respectively). Consequently, release of cytochrome C and increase of reactive oxygen species (ROS) levels in the cytoplasm was also detected (Figure 3). In contrast, activity of respiratory complexes II and III was not altered. Furthermore, Pluronic decreased accumulation of a potential-dependent dye, JC-1, in resistant MCF7/ADR cells indicating the decrease in mitochondrial membrane potential (Ψm). As a result, within 15 minutes after exposure of various MDR cell to select Pluronics intracellular ATP levels were drastically decreased [48, 57, 67]. Our recent data obtained by Localized 31P Spectroscopy ISIS (image guided volume selective in-vivo spectroscopy) in collaboration with Dr. M. Boska (UNMC) suggest that Pluronic P85 can also cause ATP depletion in MDR tumors in vivo. Thus, i.v. injections of Dox formulated with 0.2% Pluronic P85 in tumor bearing mice resulted in the decreased ATP levels, increased inorganic phosphate (Pi), and decreased pH in P388/ADR solid tumors (paper in preparation).
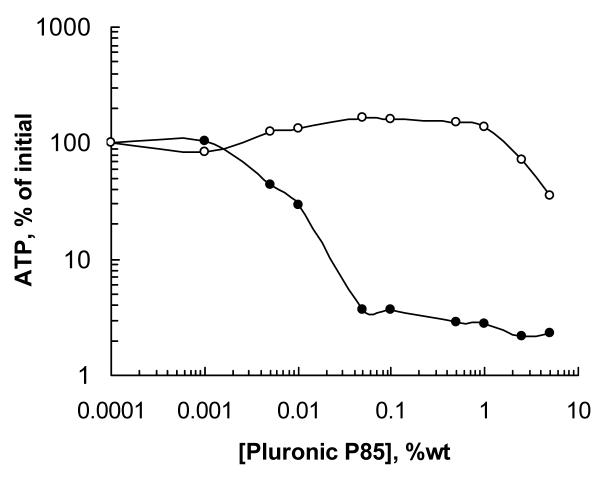
Remarkably, both in in vitro and in vivo models Pluronic P85 was shown to induce reduction in ATP levels selectively in MDR cells, while non-MDR cells were not responsive in this manner. This suggests that Pluronics are “selective” with respect to the MDR phenotype (Figure 4). Overall, the responses of the cells to Pluronic in ATP depletion studies correlated with the expression levels of drug efflux transporters (Pgp or MRP); in particular, for all the related pairs of MDR and non-MDR cells the overexpression of Pgp was accompanied by a significant increase in responsiveness to the Pluronic [57, 68]. One of the reasons for this selectivity may be the differences in the respiration rates related to the differences in the major fuel sources used by MDR and sensitive cells (fatty acids vs. glucose, respectively). Thus, it is known that drug-sensitive cells predominantly use glucose metabolism as a source of electrons entering the respiration chain, while MDR cells predominantly utilize fatty acids [69]. Therefore, since the central hydrophobic blocks of Pluronic molecules may interact with fatty acids even at low (unimers) concentrations and effectively immobilize fatty molecules inside micelle cores at higher concentrations, it may also affect this respiration mechanism at higher extent. Furthermore, it is known that mitochondria of MDR cells express high levels of mitochondrial uncoupling protein 2 (UCP2) resulting in inefficient ATP synthesis [69]. This should make the mitochondria of the MDR cells even more vulnerable to the Pluronic depolarization effects than the mitochondria of the sensitive cells. As a result, Pluronic P85 inhibited respiratory chain complexes I and IV as well as decreased Ψm in resistant MCF7/ADR cells more efficiently than in their sensitive counterparts (paper in preparation). For example, effective concentrations of block copolymer necessary for 50% decrease of mitochondria membrane potential (ECΨm50) differed by about an order of magnitude for MDR and non-MDR cells. In general, mitochondria in MDR cells exhibited much higher sensitivity to Pluronic depolarization effects than in their sensitive counterparts.
Figure 4.
Effects of Pluronic P85 on intracellular ATP levels in resistant MCF7/ADR (filled circles) and sensitive MCF7 (empty circles) cells. Cell monolayers were exposed to various concentrations of Pluronic P85 for two hours. Following treatment, the cells were washed with ice-cold PBS, solubilized in Triton X-100 (1%), and frozen immediately for subsequent ATP quantification by a luciferin/luciferase assay. Based on Ref. [57].
Overall, various drug resistance mechanisms including drug transport and detoxification systems, require consumption of energy to sustain their function in the barrier cells. Targeting this metabolic “Achilles’ heel” of MDR cancer cells by reducing fuel supply to mitochondria may open a new strategy for designing chemotherapeutic agents for MDR cancers.
Inhibition of drug efflux transporters
Selective suppression of energy production along with the membrane fluidization resulted in inhibition of drug-efflux pumps and strong sensitization of MDR cancer cells to the action of chemotherapeutic drugs in vitro [57]. Furthermore, enhancement of the drug accumulation in MDR tumors in the presence of Pluronic P85 was demonstrated in vivo. Transport of 99Tc-sestamibi was studied in Lewis Lung Carcinoma cells (3LL-M27) tumor bearing mice by animal single-photon emission computerized tomography (ASPECT). 99Tc-sestamibi is a widely used tracer for tumor detection in patients [70, 71]. Recently it was demonstrated that it also is a substrate for Pgp drug efflux transporter [72-74]. Obtained images indicated that indeed, co-administration of Pluronic P85 significantly (about 20 times) increased accumulation of 99Tc-sestamibi in MDR solid tumors in mice (paper in preparation). There was a clear indication that the higher dose of Pluronic P85 was used, the more the tracer was accumulated in the tumor. This was consistent with the fact that the amount of the block copolymer accumulated in the tumor linearly increased as the injected dose of Pluronic P85 increased. In other words, the more Pluronic accumulates in the tumor tissue, the higher the inhibition of the drug efflux transporter in MDR cancer cells is.
Inhibition of drug efflux transporters by Pluronic block copolymers enhanced transport of wide range of therapeutic agents across polarized brain microvessel endothelial cells and intestinal barrier cells in vitro [75] and in vivo [47]. Thus, brain delivery of a Pgp substrate, digoxin, administered intravenously in the wild-type mice expressing functional Pgp, was greatly enhanced in the presence of Pluronic P85 [47]. It was found that the digoxin brain/plasma ratios in the Pluronic-treated animals were practically the same as those in the knockout mice, an animal model that is deficient in both mdr1a and mdr1b isoforms of Pgp (Figure 5). This suggests that co-administration of Pluronic with the drug in mice resulted in complete inhibition of Pgp in the BBB of the wild-type animals. Notably, in was demonstrated that either component; membrane fluidization or ATP depletion alone, was insufficient for effective inhibition of Pgp drug efflux system in brain microvessel cells [48]. The Pgp remained functionally active when 1) ATP was restored using an ATP supplementation system in the presence of Pluronic, or 2) when ATP was depleted, but there was no direct contact between the Pluronic and Pgp (and no ATPase inhibition).
Figure 5.
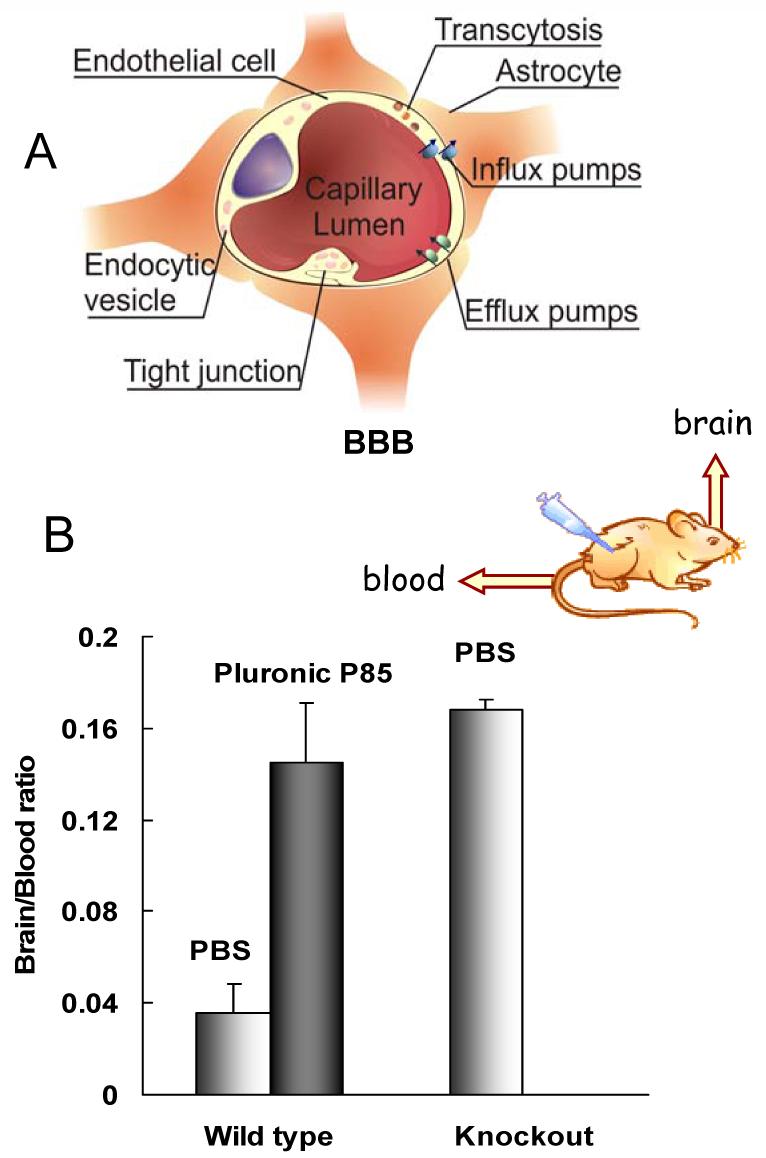
A) Schematic representation of the BBB; B) Effect of Pluronic P85 on the digoxin brain/plasma ratio in the wild-type mice at five hours postdose; digoxin brain/plasma ratio in mdr 1a/b (-/-) knockout mouse is shown for comparison. The brain/plasma ratio in the wild-type mouse group was significantly less than in Pluronic P85-treated wild-type or the knockout groups, p < 0.001; no statistical difference was seen between Pluronic P85-treated wild-type mouse and the knockout groups. Based on Ref. [47] with permission.
Recent studies, suggested that by Pluronic F68 is also able to inhibit Pgp-mediated transport of celiprolol (CEL) and CYP3A4-mediated formation of midazolam (MDZ) metabolite 1′-hydroxymidazolam in Caco-2 cell monolayers was also reported in [76]. It was suggested that drug formulations of Pluronic F68 have a potential for improving the pharmacokinetics of orally administered drugs that are Pgp and/or CYP3A4 substrates in vivo. Interestingly, our studies with the same cell monolayers suggest that Pluronic F68 is much less efficient biological modifier compared to Pluronics P85 and L81 [20].
Effect on apoptotic signal transduction
One could suggest that incorporation of Pluronic molecules into mitochondria membranes followed by alteration of the membrane structure; induction of cytochrome C release and increases in ROS levels in the cytoplasm (as discussed above) can also enhance mitochondria-related apoptosis. Indeed, we found that Pluronic P85 exhibits very peculiar effects on drug-induced apoptosis in MDR cells in vitro [54] (Figure 3). Evaluation of apoptosis-related gene levels (BCL2, BCLXL, BAX, P53, APAF1, Caspase 3, 9) was performed by reverse transcriptase PCR in MDR human breast carcinoma resistant cells (MCF7/ADR). It was demonstrated that treatment of cancer cells with Dox alone simultaneously activated a proapoptotic signaling and an antiapoptotic cellular defense. Therefore, the apoptosis induction by Dox was essentially limited. In contrast, treatment of MDR cells with Dox/Pluronic P85 significantly enhanced the proapoptotic activity and prevented the activation of the antiapoptotic cellular defense in vitro [54] (Figure 3). This resulted in the stronger cytotoxic response of the resistant cells to the Dox/Pluronic P85 formulation compared to the free drug. Furthermore, the effect of Dox/Pluronic P85 formulations on apoptosis was evaluated in vivo. Data indicated that i.v. co-administration of Dox/Pluronic P85 into tumor-bearing mice significantly (at least 6 times) increased the levels of pro-apoptotic genes, caspase 8 and caspase 9 in the drug-resistant solid tumors (3LL-M27) compared to injections of Dox alone. Activation of apoptosis in solid resistant tumors in mice by Pluronic/Dox formulation was also proved by TUNEL method (paper in preparation).
Similar effect of up-regulation proapoptotic genes (p53, p21, and Bax) and down-regulation of untiapoptotic gene (Bcl-2) by conjugates of linoleic acid (CLA) and Pluronic F127 was reported in [77]. In this study carboxyl group of CLA was coupled to Pluronic F127 terminal hydroxyl groups through an ester linkage. The conjugates exhibited enhanced anticancer efficacy in MCF7 breast cancer cells compared to the CLA alone, which was attributed to enhanced pro-apoptotic signaling.
Effect on GSH/GST detoxification system
Many biochemical functions including modulation of apoptosis depend on cellular redox potential that is largely determined by glutathione (GSH) content [78]. Generation of oxidative intermediates has been proposed to be a critical event in the process of programmed cell death induced by various agents. GSH is a ubiquitous tripeptide that protects cells against ROS as well as many toxins, mutagens, and drugs. Thus, GSH plays an important role in MDR either through its spontaneous reactions or through its function as a coenzyme in glutathione S-transferases (GST). Consequently, depletion of GSH has been found to either precede the onset of apoptosis or render the cells more sensitive to cell death.
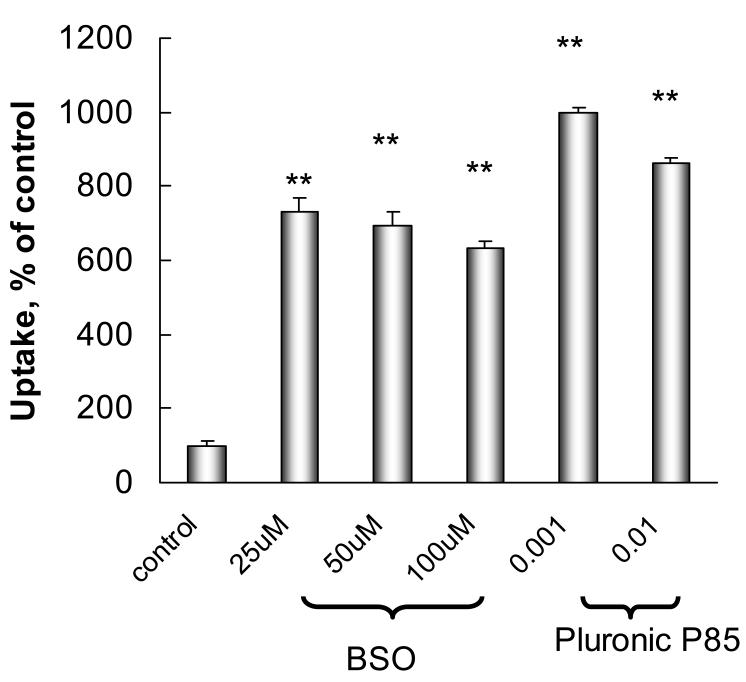
Pluronic P85 was shown to inhibit the GSH/GST detoxification system by depleting levels of GSH and inactivating GST in various MDR cancer cells (Figure 3). Thus, exposure to Pluronic P85 caused drastic reductions in GSH levels in Mardin Darby Canine Kidney (MDCKII) and human lung carcinoma epithelial cells (COR-L23/R) overexpressing MRP1 or MRP2 drug efflux transporters [51]. Furthermore, exposure of the cells to Pluronic P85 resulted in significant decreases of GST activity in each of these cell lines. These effects of the block copolymer were dose-dependent, as they increased when the concentration of the copolymer was elevated [51]. Noteworthy, the GSH depletion effects of Pluronic were shown to correlate linearly with the effects of the copolymer on the ATP levels in MDR cells. All together, these effects can decrease GST conjugation of MRP substrates, such as vinblastine (Vin), and elimination of these substrates from the cells through the MRP-mediated drug efflux pathway. Notably effect of Pluronic P85 in MRP cells was similar that of a well-known GSH depleting agent, buthionine sulfoximine (BSO) (Figure 6). Inhibition of GSH/GST detoxification system by Pluronic P85 similar to BSO increased accumulation of Vin in MDR cells.
Figure 6.
Effect of Pluronic P85 and BSO on [3H]-Vin accumulation in COR-L23/R cells. Cells were pre-treated for 24 hours with various concentrations of BSO or Pluronic P85 and then incubated for 90 min with [3H]-Vin in the presence of BSO or Pluronic P85. Control cells were incubated with [3H]-Vin in assay buffer.
Inhibition of drug sequestration within cytoplasmic vesicles
It was reported that some MDR cells exhibited an ability to sequester anticancer drugs (in particular, vinca alkaloids and anthracyclines) in cytoplasmic perinuclear vesicles [79, 80]. These drugs are believed to be trapped in the protonated form the within the acidic vesicles thus providing for an addition protective mechanism by decreasing nuclear entry of the drugs. This sequestration is usually followed by the drug deactivation or extrusion out of the cells. Pluronic L61 was shown to alter subcellular distribution of Dox in MDR CHRC5 cells [32] shifting drug accumulation from cytoplasmic vesicles to the nucleus. Similar effect of pluronic P85 was also observed for fluorescein in MRP overexpressing Panc-1 cells [81]. It was suggested that Pluronic block copolymers reduce vesicular drug accumulation by altering pH gradients in the intracellular compartments due to the copolymer ionophoric effect or/and energy depletion (Figure 3). It is known that elevated pH gradients across organelle membrane are maintained by the activity of H+ ATPase, an ATP-dependent pump [82]. Depletion of ATP may shut down this pump and abolish acidification of the vesicles. This may additionally contribute to sensitization of MDR cells to select chemotherapeutic agents and improvement of the outcome of the chemotherapy.
Prevention of MDR development
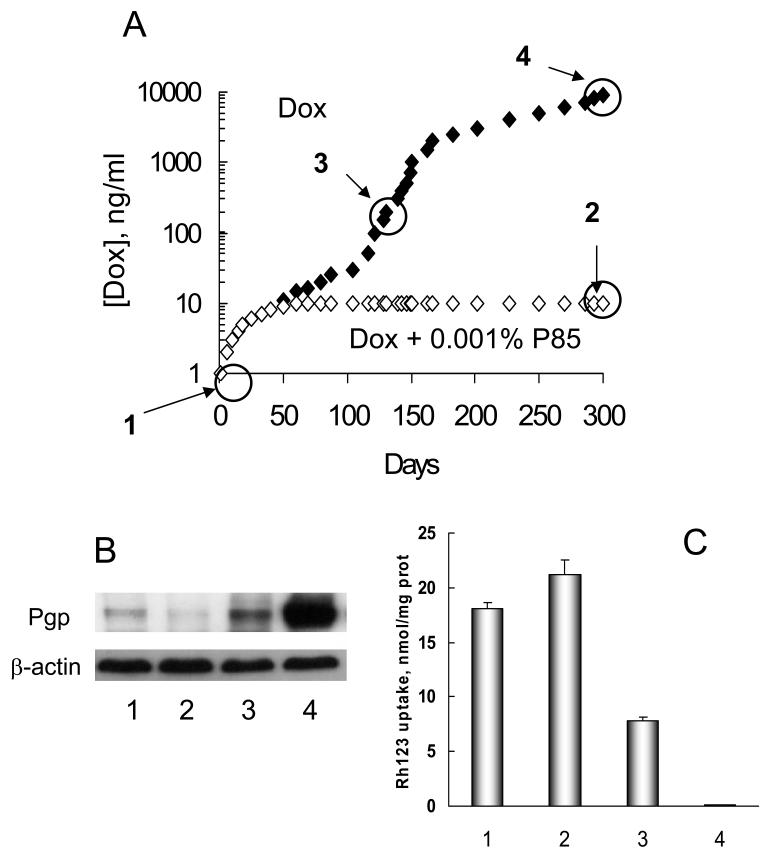
Pluronic block copolymers were recently shown to prevent the development of MDR in the cancer cells exposed to the drug [83]. Specifically, sensitive MCF7 cells cultured in the presence of Pluronic P85 were unable to stably grow in concentrations of Dox that exceeded 10 ng Dox/ml in the culture media. In sharp contrast, MCF7 cells cultured in the absence of the block copolymer developed MDR and eventually exhibited stable growth at 1000 times higher concentration of the drug (10,000ng Dox/ml culture media) compared to the initial parental cells (Figure 7A). Detailed characterization of the isolated sublines demonstrated that cells selected with Dox/Pluronic P85 did not show overexpression of the MDR1 gene, likely resulting in their high sensitivity to the drug (Figure 7 B,C). Conversely, the cells selected with Dox alone showed an elevated level in the expression of the MDR1 gene along with a corresponding increase in the expression level of the drug efflux transporter, Pgp, which contributed to high resistance of these cells to Dox [83]. This work provides an indication of the mechanism by which Pluronic P85 prevents development of MDR. Initially, during the process of cell selection at low drug concentrations, e.g. 10 ng/ml Dox, the MDR phenotype does not develop. Subsequently, as the cells are selected with higher concentrations of the drug, e.g. 1000 ng/ml Dox alone, the cells display amplification of MDR1, overexpression of Pgp, decreased uptake of a Pgp-specific probe and elevated resistance to Dox. Pluronic P85 “re-sensitizes” these resistant cells to the level observed for parental cells, suggesting that in the presence of the copolymer these cells have no selective advantage for growth. As a result, when selection is carried out in the presence of Pluronic, the resistant cells do not develop and the cells are able to grow only at a maximal dose of 10 ng/ml Dox. Global analysis of the expression profiles of 20K genes by DNA microarray revealed that the use of Pluronic in combination with Dox drastically changed the direction and magnitude of the genetic response of the tumor cells to Dox. Subsequent studies demonstrated that Pluronic P85 prevented development of MDR1 phenotype in leukemia cells in vitro and in vivo as determined by Pgp expression and functional assays of the selected cells (A. Sharma, paper in preparation). Cells selected with Dox in the presence of Dox/Pluronic P85 in vitro and in vivo exhibited some increases in IC50 values compared to parental cells, but these values were much less than IC50 in respective cells selected with the drug alone. In addition to mdr1 Pluronic P85 abolished alterations of genes implicated in apoptosis, drug metabolism, stress response, molecular transport and tumorigenesis. This further reinforces the potential benefits of using such formulations for chemotherapy of cancer tumors; if resistance is intrinsic, Pluronic sensitizes the tumor, whereas if resistance is acquired, MDR cells no longer have a selective advantage.
Figure 7.
Development of MDR in cancer cells. A: Time course of the development of resistance in MCF7 cell lines cultured with Dox either alone (filled diamonds) or in combination with 0.001% Pluronic P85 (open diamonds). [Dox] is the concentration of Dox in the growth medium. B: Western blot data for expression of Pgp in: MCF7 parental cells (lane 1), and selected MCF7 cells tolerating: 10ng/ml Dox with 0.001% Pluronic P85 (lane 2); 200ng/ml Dox (lane 3); 10,000ng/ml Dox (lane 4); and C: Rhodamine 123 (R123) accumulation in the developed cells tolerating various concentrations of Dox in the culture media (same lane assignments as designated above). Based on Ref. [83] with permission.
Transcriptional activation of gene expression
Finally, Pluronic block copolymers demonstrated exciting opportunities for development of novel gene therapies and vaccination strategies. It is well known that plasmid DNA can be promising modality for generation therapeutically meaningful levels of gene expression. However, in many cases, the immunogenecity of plasmid DNA has been limited by problems associated with inability to generate efficient and prolonged DNA expression or failure to stimulate immune system. It was discovered that certain Pluronic block copolymers significantly increase expression of plasmid DNA in skeletal muscle, spleen, and lymph nodes and stimulates plasmid DNA uptake and expression in antigen presenting cells in mice [84-87]. In the presence of Pluronic P85 high levels of expression of a reporter gene (luciferase) were sustained for at least 40 days and the area under the gene expression curve increased by at least 10 times compared to the DNA alone in mice [87]. The effect of the copolymer depended on the strain of the mouse and the type of the promoter used. Thus, Pluronic P85 enhanced luciferase expression by 17 to 19-fold in immunocompetent C57Bl/6 and Balb/c mice, while no enhancement was observed with athymic Balb/c nu/nu mice [86].
The most compelling result was the promoter selectivity of Pluronic P85 effect on the luciferase expression in the muscle (Figure 8) [86]. A very similar promoter selectivity of mixed micelles of Pluronics L61 and F127, SP1017 was also reported in [85]. Pluronic P85 activated the expression of luciferase gene driven by CMV promoter, NFκB and p53 response elements. There was much less or no effect on the gene driven by SV40 promoter or AP1 and CRE response elements. Thus, the differential effects of Pluronic on these two promoters and the selectivity with respect to the NF-κB response element are consistent with the activation of the NF-κB signaling pathway (Figure 9), which plays a central role in the regulation of the cellular defense and immunological responses [88]. The promoter selectivity study further concluded that Pluronic block copolymers activated gene driven by p53 response element, which was not present in either of the promoters used [87]. However, NFκB and p53 are engaged in a transcriptional cross talk with each other and display related regulatory activities. Therefore it is possible that Pluronic may activate both transcription factors by affecting same or related signaling components. In addition Pluronic increased the number of DNA copies and thus affected initial stages of gene transfer in a promoter selective manner. In the range of concentrations used in these studies Pluronics did not bind with and did not condensed the plasmid DNA and therefore could not protect DNA from degradation or promote its transport into the cell like cationic molecules do. It is therefore likely that the mechanism(s) by which Pluronics enhance gene expression is very different from that of cationic lipids or polycations.
Figure 8.
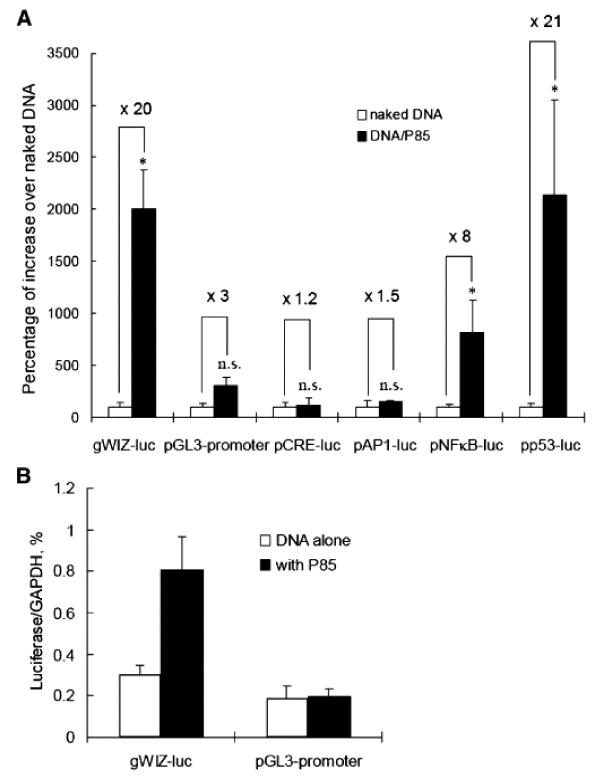
Promoter-dependent effects of Pluronic P85 on gene expression (A) and number of DNA copies following intramuscular injections of plasmid DNA in the muscle. (A) Groups of five Balb/c mice were injected i.m. with 0.3% Pluronic P85-formulated (black bars) or non-formulated (white bars) luciferase-encoding plasmids driven by CMV, SV40, CRE, AP1, NFκB or p53. Luciferase activity in the muscle was determined 24 h after injections. Data are the percentage of gene expression with Pluronic P85-formulated DNA over the naked DNA, mean ± s.e.m (n = 10). * p < 0.05, n.s. not significant (p = 0.06). (B) Balb/c mice were injected i.m. with luciferase-expressing vectors controlled by CMV or SV40 promoters with or without 0.3% Pluronic P85. The relative level of luciferase DNA in the muscles was measured 24 h after injection of the DNA by the real-time PCR. The bars show luciferase expression normalized for GAPDH (n = 6). Statistical comparisons were made for DNA/P85 versus DNA alone: * p < 0.05. Based on Ref. [86].
Figure 9.
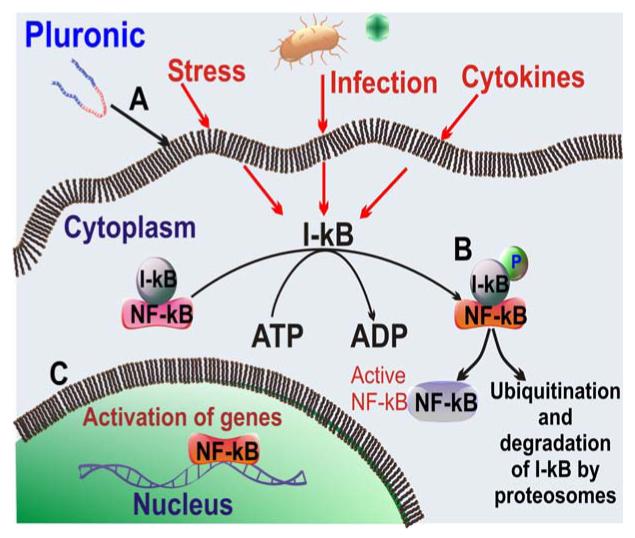
Pluronic block copolymers activate NF-κB signaling in cells: A) Pluronic affects NF-κB signaling presumably by interacting with cell plasma membrane; B) this leads to rapid phosphorylation of I-kB accompanied by the release of the NF-kB; c) the released NF-kB translocates to the nucleus and activates transcription of the corresponding genes.
Our recent studies also indicate that Pluronics enhance nuclear import of plasmid DNA (pDNA) through the activation of cellular trafficking machinery (Z. Yang, paper in preparation). Thus, addition of Pluronic P123 or Pluronic P85 to pDNA/polycation complexes (“polyplexes”) significantly enhanced nuclear import and gene expression of pDNA containing NF-κB–binding sites in vitro. Interestingly, Pluronic internalized during the same period into the cells exhibited little if any co-localization with the DNA. All together, our studies suggest that Pluronic block copolymers rapidly activate NF-κB, which binds cytosolic pDNA that possesses promoters containing NF-κB binding sites and consequently increases nuclear import of pDNA through NF-κB nuclear translocation.
This provides further evidence of the effects of Pluronic block copolymers on gene expression suggesting that these copolymers act as biological response modifying agents by 1) enhancing the gene transfer and 2) activating the transcription of the genes. These studies demonstrated that Pluronic block copolymers are promising agents for nonviral gene therapy. This may provide a method for a simple and efficient gene transfer, which is potentially applicable in multiple gene therapy protocols involving intramuscular injection of plasmid DNA in order to synthesize therapeutic proteins or to vaccinate by expression of a particular gene product.
Pluronic P85 was also shown to enhance gene transfection in HepG2 cells under ultrasound irradiation [89] in vitro. Plasmid encoding green fluorescent protein (EGFP) was used as a report gene. The results showed that the transfection efficacy in ulstrasound/Pluronic P85 group was three times higher than in the absence of Pluronic P85. It was concluded that ultrasound in combination with P85 could mediate the gene transfection of HepG2 cells, although P85 could somewhat increase the damage to cells caused by ultrasound. Overall, combining Pluronic block copolymers with existing nonviral vectors can significantly and safely increase the efficiency of gene delivery [43].
Structure requirements for Pluronic effects
It was demonstrated that a fine balance between hydrophilic (EO) and lipophilic (PO) components in the Pluronic molecule should be accomplished to potent enable inhibition of the drug efflux systems in MDR cells [63]. Overall, the most efficacious block copolymers are those with intermediate lengths of PPO block and relatively hydrophobic structure (HLB < 20), such as Pluronic P85 or Pluronic L61. Hydrophilic block copolymers, which have an extended PEO block, do not incorporate into lipid bilayers and practically do not transport into the cells. As a result, they have little effect on either Pgp ATPase activity or cellular ATP levels, which explains their negligible effect on Pgp efflux pump in the resistant cells. Very lipophilic block copolymers with long PPO blocks anchor in the plasma membranes and remain there for an extended period of time. As a result, although they are potent inhibitors of Pgp ATPase, they are not efficiently transported into the cell, do not cause ATP depletion and have little net effect on Pgp efflux system. In contrast, the block copolymers displaying intermediate lipophilicity transport across the membrane, spread throughout the cytoplasm and reach mitochondria and nuclei. They inhibit Pgp ATPase activity and decrease ATP intracellular levels, which combined results in effective inhibition of the drug efflux transport systems and enhanced drug transport to the brain [63].
Similar dependencies were found for gene delivery. Basically, the most potent block copolymers inhibiting drug efflux transporters are the best for DNA and oligonucleotide delivery [88]. Overall the ability of block copolymers to affect the membrane structure and to transport into the cells may is essential for their diverse biological modifying properties.
All together formulations of drugs and genes with Pluronic block copolymers represent novel and promising strategy for therapy of cancers, and other diseases. We believe that both the uses of Pluronic micellar formulations and unimer-associated biological response modifying effects of Pluronic provide for exciting and unique opportunities in pharmaceutics and nanopharmacology. Administered at micellar concentrations (above CMC) Pluronic/drug formulations disintegrate upon dilution in the blood stream and exert their unimer-associated properties. Furthermore, ability of Pluronic formulations to target multiple pathways of resistance provides additional advantages in treatment of drug resistant tumors. Both the mechanistic studies and clinical evaluations of these systems are in progress and we expect fascinating new developments in this area in the near future.
Acknowledgments
This study has been supported by a National Institutes of Health grants CA89225, CA116591, NS36229, NS051335 awarded to AVK. We acknowledge the graduate students in the UNMC Pharmaceutical Sciences Graduate Program, Daria Alakhova, Zagit Gaimalov, Gaurav Sahay and Amit Sharma, as well as Dr. Zhihui Yang for their scientific contributions in a number of recent studies in press or in preparation.
References
- [1].Kabanov AV, Vinogradov SV, Suzdaltseva YG, Alakhov V. Water-soluble block polycations as carriers for oligonucleotide delivery. Bioconjug Chem. 1995;6:639–643. doi: 10.1021/bc00036a001. [DOI] [PubMed] [Google Scholar]
- [2].Savic R, Luo L, Eisenberg A, Maysinger D. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science. 2003;300:615–618. doi: 10.1126/science.1078192. [DOI] [PubMed] [Google Scholar]
- [3].Kwon GS. Polymeric micelles for delivery of poorly water-soluble compounds. Crit Rev Ther Drug Carrier Syst. 2003;20:357–403. doi: 10.1615/critrevtherdrugcarriersyst.v20.i5.20. [DOI] [PubMed] [Google Scholar]
- [4].Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci U S A. 2003;100:6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Trentin D, Hubbell J, Hall H. Non-viral gene delivery for local and controlled DNA release. J Control Release. 2005;102:263–275. doi: 10.1016/j.jconrel.2004.09.029. [DOI] [PubMed] [Google Scholar]
- [6].Missirlis D, Tirelli N, Hubbell JA. Amphiphilic hydrogel nanoparticles. Preparation, characterization, and preliminary assessment as new colloidal drug carriers. Langmuir. 2005;21:2605–2613. doi: 10.1021/la047367s. [DOI] [PubMed] [Google Scholar]
- [7].Kabanov AV, Batrakova EV. New technologies for drug delivery across the blood brain barrier. Curr Pharm Des. 2004;10:1355–1363. doi: 10.2174/1381612043384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Salem AK, Searson PC, Leong KW. Multifunctional nanorods for gene delivery. Nat Mater. 2003;2:668–671. doi: 10.1038/nmat974. [DOI] [PubMed] [Google Scholar]
- [9].Nayak S, Lyon LA. Soft nanotechnology with soft nanoparticles. Angew Chem Int Ed Engl. 2005;44:7686–7708. doi: 10.1002/anie.200501321. [DOI] [PubMed] [Google Scholar]
- [10].Croy SR, Kwon GS. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12:4669–4684. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- [11].Escorcia FE, McDevitt MR, Villa CH, Scheinberg DA. Targeted nanomaterials for radiotherapy. Nanomed. 2007;2:805–815. doi: 10.2217/17435889.2.6.805. [DOI] [PubMed] [Google Scholar]
- [12].Hall JB, Dobrovolskaia MA, Patri AK, McNeil SE. Characterization of nanoparticles for therapeutics. Nanomed. 2007;2:789–803. doi: 10.2217/17435889.2.6.789. [DOI] [PubMed] [Google Scholar]
- [13].Suri SS, Fenniri H, Singh B. Nanotechnology-based drug delivery systems. J Occup Med Toxicol. 2007;2:16. doi: 10.1186/1745-6673-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kabanov A, Nazarova I, Astafieva I, Batrakova E, Alakhov V, Yaroslavov A, Kabanov V. Micelle formation and solubilization of fluorescent probes in poly(oxyethylene-b-oxypropilene-b-oxyethylene) solutions. Macromolecules. 1995;28:2303–2314. [Google Scholar]
- [15].Kozlov M, Melik-Nubarov N, Batrakova E, Kabanov A. Relationship between Pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes. Macromolecules. 2000;33:3305–3313. [Google Scholar]
- [16].Batrakova EV, Li S, Li Y, Alakhov VY, Elmquist WF, Kabanov AV. Distribution kinetics of a micelle-forming block copolymer Pluronic P85. J Control Release. 2004;100:389–397. doi: 10.1016/j.jconrel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- [17].Kabanov AV, Batrakova EV, Miller DW. Pluronic block copolymers as modulators of drug efflux transporter activity in the blood-brain barrier. Adv Drug Deliv Rev. 2003;55:151–164. doi: 10.1016/s0169-409x(02)00176-x. [DOI] [PubMed] [Google Scholar]
- [18].Spitzenberger TJ, Heilman D, Diekmann C, Batrakova EV, Kabanov AV, Gendelman HE, Elmquist WF, Persidsky Y. Novel delivery system enhances efficacy of antiretroviral therapy in animal model for HIV-1 encephalitis. J Cereb Blood Flow Metab. 2007;27:1033–1042. doi: 10.1038/sj.jcbfm.9600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Batrakova E, Han H, Miller D, Kabanov A. Effects of pluronic P85 unimers and micelles on drug permeability in polarized BBMEC and Caco-2 cells. Pharm Res. 1998;15:1525–1532. doi: 10.1023/a:1011942814300. [DOI] [PubMed] [Google Scholar]
- [20].Batrakova E, Han H, Alakhov V, Miller D, Kabanov A. Effects of pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm Res. 1998;15:850–855. doi: 10.1023/a:1011964213024. [DOI] [PubMed] [Google Scholar]
- [21].Kwon SH, Kim SY, Ha KW, Kang MJ, Huh JS, Im TJ, Kim YM, Park YM, Kang KH, Lee S, Chang JY, Lee J, Choi YW. Pharmaceutical evaluation of genistein-loaded pluronic micelles for oral delivery. Arch Pharm Res. 2007;30:1138–1143. doi: 10.1007/BF02980249. [DOI] [PubMed] [Google Scholar]
- [22].Alakhov V, Klinski E, Li S, Pietrzynski G, Venne A, Batrakova EV, Bronitch T, Kabanov A. Block copolymer-based formulation of Doxorubicin. From cell screen to clinical trials. Colloids Surf. B: Biointerfaces. 1999;16:113–134. [Google Scholar]
- [23].Kabanov A, Alakhov V. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 2002;19:1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- [24].Krupka TM, Weinberg BD, Wu H, Ziats NP, Exner AA. Effect of intratumoral injection of carboplatin combined with pluronic P85 or L61 on experimental colorectal carcinoma in rats. Exp Biol Med (Maywood) 2007;232:950–957. [PubMed] [Google Scholar]
- [25].Kabanov AV, Chekhonin VP, Alakhov VY, Batrakova EV, Lebedev AS, Melik-Nubarov NS, Arzhakov SA, Levashov AV, Morozov GV, Severin ES, Kabanov VA. The neuroleptic activity of haloperidol increases after its solubilization in surfactant micelles. Micelles as microcontainers for drug targeting. FEBS Lett. 1989;258:343–345. doi: 10.1016/0014-5793(89)81689-8. [DOI] [PubMed] [Google Scholar]
- [26].Saski W, Shah SG. Availability Of Drugs In The Presence Of Surface-Active Agents. Ii. Effects Of Some Oxyethylene Oxypropylene Polymers On The Biological Activity Of Hexetidine. J Pharm Sci. 1965;54:277–280. doi: 10.1002/jps.2600540224. [DOI] [PubMed] [Google Scholar]
- [27].Saski W, Shah SG. Availability Of Drugs In The Presence Of Surface-Active Agents. I. Critical Micelle Concentrations Of Some Oxyethylene Oxypropylene Polymers. J Pharm Sci. 1965;54:71–74. doi: 10.1002/jps.2600540117. [DOI] [PubMed] [Google Scholar]
- [28].Croy SR, Kwon GS. The effects of Pluronic block copolymers on the aggregation state of nystatin. J Control Release. 2004;95:161–171. doi: 10.1016/j.jconrel.2003.11.003. [DOI] [PubMed] [Google Scholar]
- [29].Croy SR, Kwon GS. Polysorbate 80 and Cremophor EL micelles deaggregate and solubilize nystatin at the core-corona interface. J Pharm Sci. 2005;94:2345–2354. doi: 10.1002/jps.20301. [DOI] [PubMed] [Google Scholar]
- [30].Valle JW, Lawrance J, Brewer J, Clayton A, Corrie P, Alakhov V, Ranson M. A phase II, window study of SP1049C as first-line therapy in inoperable metastatic adenocarcinoma of the oesophagus. J Clin Oncol; ASCO Annual Meeting Proceedings (Post-Meeting Edition).2004. p. 4195. [Google Scholar]
- [31].Alakhov V, Moskaleva E, Batrakova E, Kabanov A. Hypersensitization of multidrug resistant human ovarian carcinoma cells by Pluronic P85 block copolymer. Bioconjug Chem. 1996;7:209–216. doi: 10.1021/bc950093n. [DOI] [PubMed] [Google Scholar]
- [32].Venne A, Li S, Mandeville R, Kabanov A, Alakhov V. Hypersensitizing effect of Pluronic L61 on cytotoxic activity, transport, and subcellular distribution of doxorubicin in multiple drug-resistant cells. Cancer Res. 1996;56:3626–3629. [PubMed] [Google Scholar]
- [33].Batrakova EV, Dorodnych TY, Klinskii EY, Kliushnenkova EN, Shemchukova OB, Goncharova ON, Arjakov SA, Alakhov VY, Kabanov AV. Anthracycline antibiotics non-covalently incorporated into the block copolymer micelles: in vivo evaluation of anti-cancer activity. Br J Cancer. 1996;74:1545–1552. doi: 10.1038/bjc.1996.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Krupka TM, Weinberg BD, Ziats NP, Haaga JR, Exner AA. Injectable polymer depot combined with radiofrequency ablation for treatment of experimental carcinoma in rat. Invest Radiol. 2006;41:890–897. doi: 10.1097/01.rli.0000246102.56801.2f. [DOI] [PubMed] [Google Scholar]
- [35].Lee Y, Park SY, Mok H, Park TG. Synthesis, Characterization, Antitumor Activity of Pluronic Mimicking Copolymer Micelles Conjugated with Doxorubicin via Acid-Cleavable Linkage. Bioconjug Chem. 2008 doi: 10.1021/bc700382z. in press.
- [36].Tian Y, Bromberg L, Lin SN, Hatton TA, Tam KC. Complexation and release of doxorubicin from its complexes with pluronic P85-b-poly(acrylic acid) block copolymers. J Control Release. 2007;121:137–145. doi: 10.1016/j.jconrel.2007.05.010. [DOI] [PubMed] [Google Scholar]
- [37].Yang TF, Chen CN, Chen MC, Lai CH, Liang HF, Sung HW. Shell-crosslinked Pluronic L121 micelles as a drug delivery vehicle. Biomaterials. 2007;28:725–734. doi: 10.1016/j.biomaterials.2006.09.035. [DOI] [PubMed] [Google Scholar]
- [38].Gao Z, Fain HD, Rapoport N. Ultrasound-enhanced tumor targeting of polymeric micellar drug carriers. Mol Pharm. 2004;1:317–330. doi: 10.1021/mp049958h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Release. 2005;102:203–222. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- [40].Rapoport NY, Christensen DA, Fain HD, Barrows L, Gao Z. Ultrasound-triggered drug targeting of tumors in vitro and in vivo. Ultrasonics. 2004;42:943–950. doi: 10.1016/j.ultras.2004.01.087. [DOI] [PubMed] [Google Scholar]
- [41].Husseini GA, de la Rosa M.A. Diaz, Gabuji T, Zeng Y, Christensen DA, Pitt WG. Release of doxorubicin from unstabilized and stabilized micelles under the action of ultrasound. J Nanosci Nanotechnol. 2007;7:1028–1033. doi: 10.1166/jnn.2007.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kabanov AV, Batrakova EV, Sriadibhatla S, Yang Z, Kelly DL, Alakov VY. Polymer genomics: shifting the gene and drug delivery paradigms. J Control Release. 2005;101:259–271. doi: 10.1016/j.jconrel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- [43].Kabanov A, Zhu J, Alakhov V. Pluronic Block Copolymers for Gene Delivery. Adv Genet. 2005;53PA:231–261. doi: 10.1016/S0065-2660(05)53009-8. [DOI] [PubMed] [Google Scholar]
- [44].Kabanov AV. Polymer genomics: an insight into pharmacology and toxicology of nanomedicines. Adv Drug Deliv Rev. 2006;58:1597–1621. doi: 10.1016/j.addr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kabanov A, Batrakova E, Alakhov V. Pluronic block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;54:759–779. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]
- [46].Kabanov A, Batrakova E, Alakhov V. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82:189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- [47].Batrakova E, Miller D, Li S, Alakhov V, Kabanov A, Elmquist W. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther. 2001;296:551–557. [PubMed] [Google Scholar]
- [48].Batrakova E, Li S, Vinogradov S, Alakhov V, Miller D, Kabanov A. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299:483–493. [PubMed] [Google Scholar]
- [49].Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- [50].Regev R, Katzir H, Yeheskely-Hayon D, Eytan GD. Modulation of P-glycoprotein-mediated multidrug resistance by acceleration of passive drug permeation across the plasma membrane. Febs J. 2007;274:6204–6214. doi: 10.1111/j.1742-4658.2007.06140.x. [DOI] [PubMed] [Google Scholar]
- [51].Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug-resistant proteins by pluronic P85. Pharm Res. 2003;20:1581–1590. doi: 10.1023/a:1026179132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y. Effect of excipients on breast cancer resistance protein substrate uptake activity. J Control Release. 2007;124:1–5. doi: 10.1016/j.jconrel.2007.08.021. [DOI] [PubMed] [Google Scholar]
- [53].Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y. Improvement of the oral drug absorption of topotecan through the inhibition of intestinal xenobiotic efflux transporter, breast cancer resistance protein, by excipients. Drug Metab Dispos. 2007;35:1142–1148. doi: 10.1124/dmd.106.014217. [DOI] [PubMed] [Google Scholar]
- [54].Minko T, Batrakova EV, Li S, Li Y, Pakunlu RI, Alakhov VY, Kabanov AV. Pluronic block copolymers alter apoptotic signal transduction of doxorubicin in drug-resistant cancer cells. J Control Release. 2005;105:269–278. doi: 10.1016/j.jconrel.2005.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Batrakova E, Lee S, Li S, Venne A, Alakhov V, A. K. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells. Pharm Res. 1999;16:1373–1379. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- [56].Miller D, Batrakova E, Waltner T, Alakhov V, Kabanov A. Interactions of Pluronic block copolymers with brain microvessel endothelial cells: evidence of two potential pathways for drug absorption. Bioconjugate Chem. 1997;8:649–657. doi: 10.1021/bc970118d. [DOI] [PubMed] [Google Scholar]
- [57].Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br J Cancer. 2001;85:1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Romsicki Y, Sharom FJ. The ATPase and ATP-binding functions of P-glycoprotein--modulation by interaction with defined phospholipids. Eur J Biochem. 1998;256:170–178. doi: 10.1046/j.1432-1327.1998.2560170.x. [DOI] [PubMed] [Google Scholar]
- [59].Romsicki Y, Sharom FJ. The membrane lipid environment modulates drug interactions with the P-glycoprotein multidrug transporter. Biochemistry. 1999;38:6887–6896. doi: 10.1021/bi990064q. [DOI] [PubMed] [Google Scholar]
- [60].Batrakova EV, Li S, Li Y, Alakhov VY, Kabanov A. Effect of Pluronic P85 on ATPase Activity of Drug Efflux Transporters. Pharm Res. 2004;21:2226–2233. doi: 10.1007/s11095-004-7675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Luker GD, Pica CM, Kumar AS, Covey DF, Piwnica-Worms D. Effects of cholesterol and enantiomeric cholesterol on P-glycoprotein localization and function in low-density membrane domains. Biochemistry. 2000;39:7651–7661. doi: 10.1021/bi9928593. [DOI] [PubMed] [Google Scholar]
- [62].Jodoin J, Demeule M, Fenart L, Cecchelli R, Farmer S, Linton KJ, Higgins CF, Beliveau R. P-glycoprotein in blood-brain barrier endothelial cells: interaction and oligomerization with caveolins. J Neurochem. 2003;87:1010–1023. doi: 10.1046/j.1471-4159.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- [63].Batrakova E, Li S, Alakhov V, Miller D, Kabanov A. Optimal structure requirements for pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Ther. 2003;304:845–854. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
- [64].Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer. 2003;3:571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- [65].Bozidis P, Williamson CD, Colberg-Poley AM. Mitochondrial and secretory human cytomegalovirus UL37 proteins traffic into mitochondrion-associated membranes of human cells. J Virol. 2008;82:2715–2726. doi: 10.1128/JVI.02456-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McMahon KA, Zhu M, Kwon SW, Liu P, Zhao Y, Anderson RG. Detergent-free caveolae proteome suggests an interaction with ER and mitochondria. Proteomics. 2006;6:143–152. doi: 10.1002/pmic.200500208. [DOI] [PubMed] [Google Scholar]
- [67].Batrakova E, Li S, Alakhov V, Kabanov A. Selective energy depletion and sensitization of multiple drug resistant cancer cells by Pluronic block copolymers. Polym. Prepr. 2000;41:1639–1640. [Google Scholar]
- [68].Kabanov AV, Batrakova EV, Alakhov VY. An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers. J Control Release. 2003;91:75–83. doi: 10.1016/s0168-3659(03)00211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Harper ME, Antoniou A, Villalobos-Menuey E, Russo A, Trauger R, Vendemelio M, George A, Bartholomew R, Carlo D, Shaikh A, Kupperman J, Newell EW, Bespalov IA, Wallace SS, Liu Y, Rogers JR, Gibbs GL, Leahy JL, Camley RE, Melamede R, Newell MK. Characterization of a novel metabolic strategy used by drug-resistant tumor cells. Faseb J. 2002;16:1550–1557. doi: 10.1096/fj.02-0541com. [DOI] [PubMed] [Google Scholar]
- [70].Fukumoto M. Single-photon agents for tumor imaging: 201Tl, 99mTc-MIBI, and 99mTc-tetrofosmin. Ann Nucl Med. 2004;18:79–95. doi: 10.1007/BF02985098. [DOI] [PubMed] [Google Scholar]
- [71].Mele A, Offidani M, Visani G, Marconi M, Cambioli F, Nonni M, Catarini M, Brianzoni E, Berbellini A, Ascoli G, Brunori M, Agostini V, Corvatta L, Isidori A, Spinelli A, Gradari M, Leoni P. Technetium-99m sestamibi scintigraphy is sensitive and specific for the staging and the follow-up of patients with multiple myeloma: a multicentre study on 397 scans. Br J Haematol. 2007;136:729–735. doi: 10.1111/j.1365-2141.2006.06489.x. [DOI] [PubMed] [Google Scholar]
- [72].Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10:159–165. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- [73].Cayre A, Cachin F, Maublant J, Mestas D, Feillel V, Ferriere JP, Kwiaktowski F, Chevillard S, Finat-Duclos F, Verrelle P, Penault-Llorca F. Single static view 99mTc-sestamibi scintimammography predicts response to neoadjuvant chemotherapy and is related to MDR expression. Int J Oncol. 2002;20:1049–1055. [PubMed] [Google Scholar]
- [74].Cayre A, Cachin F, Maublant J, Mestas D, Penault-Llorca F. Does 99mTc-sestamibi uptake discriminate breast tumors? Cancer Invest. 2004;22:498–504. doi: 10.1081/cnv-200026388. [DOI] [PubMed] [Google Scholar]
- [75].Batrakova E, Li S, Miller D, Kabanov A. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res. 1999;16:1366–1372. doi: 10.1023/a:1018990706838. [DOI] [PubMed] [Google Scholar]
- [76].Huang J, Si L, Jiang L, Fan Z, Qiu J, Li G. Effect of pluronic F68 block copolymer on P-glycoprotein transport and CYP3A4 metabolism. Int J Pharm. 2008 doi: 10.1016/j.ijpharm.2007.12.028. in press.
- [77].Guo DD, Moon HS, Arote R, Seo JH, Quan JS, Choi YJ, Cho CS. Enhanced anticancer effect of conjugated linoleic acid by conjugation with Pluronic F127 on MCF-7 breast cancer cells. Cancer Lett. 2007;254:244–254. doi: 10.1016/j.canlet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- [78].Coffey RN, Watson RW, Hegarty NJ, O’Neill A, Gibbons N, Brady HR, Fitzpatrick JM. Thiol-mediated apoptosis in prostate carcinoma cells. Cancer. 2000;88:2092–2104. doi: 10.1002/(sici)1097-0142(20000501)88:9<2092::aid-cncr15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- [79].Simon S, Roy D, Schindler M. Intracellular pH and the control of multidrug resistance. Proc Natl Acad Sci U S A. 1994;91:1128–1132. doi: 10.1073/pnas.91.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Meschini S, Molinari A, Calcabrini A, Citro G, Arancia G. Intracellular localization of the antitumour drug adriamycin in living cultured cells: a confocal microscopy study. J Microsc. 1994;176:204–210. doi: 10.1111/j.1365-2818.1994.tb03516.x. [DOI] [PubMed] [Google Scholar]
- [81].Miller D, Batrakova E, Kabanov A. Inhibition of multidrug resistance-associated protein (MRP) functional activity with pluronic block copolymers. Pharm Res. 1999;16:396–401. doi: 10.1023/a:1018873702411. [DOI] [PubMed] [Google Scholar]
- [82].Benderra Z, Morjani H, Trussardi A, Manfait M. Role of the vacuolar H+ ATPase in daunorubicin distribution in etoposide-resistant MCF7 cells overexpressing the multidrug-resistance associated protein. Int. J. Oncol. 1998;12:711–715. doi: 10.3892/ijo.12.3.711. [DOI] [PubMed] [Google Scholar]
- [83].Batrakova EV, Kelly DL, Li S, Li Y, Yang Z, Xiao L, Alakhova DY, Sherman S, Alakhov VY, Kabanov AV. Alteration of genomic responses to doxorubicin and prevention of MDR in breast cancer cells by a polymer excipient: pluronic P85. Mol Pharm. 2006;3:113–123. doi: 10.1021/mp050050g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lemieux P, Guerin N, Paradis G, Proulx R, Chistyakova L, Kabanov A, Alakhov V. A combination of poloxamers increases gene expression of plasmid DNA in skeletal muscle. Gene Ther. 2000;7:986–991. doi: 10.1038/sj.gt.3301189. [DOI] [PubMed] [Google Scholar]
- [85].Alakhov V, Klinski E, Lemieux P, Pietrzynski G, Kabanov A. Block copolymeric biotransport carriers as versatile vehicles for drug delivery. Expert Opin Biol Ther. 2001;1:583–602. doi: 10.1517/14712598.1.4.583. [DOI] [PubMed] [Google Scholar]
- [86].Yang Z, Zhu J, Sriadibhatla S, Gebhart C, Alakhov V, Kabanov A. Promoter-and strain-selective enhancement of gene expression in a mouse skeletal muscle by a polymer excipient Pluronic P85. J Control Release. 2005;108:496–512. doi: 10.1016/j.jconrel.2005.08.015. [DOI] [PubMed] [Google Scholar]
- [87].Sriadibhatla S, Yang Z, Gebhart C, Alakhov VY, Kabanov A. Transcriptional activation of gene expression by pluronic block copolymers in stably and transiently transfected cells. Mol Ther. 2006;13:804–813. doi: 10.1016/j.ymthe.2005.07.701. [DOI] [PubMed] [Google Scholar]
- [88].Gilmore TD. The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene. 1999;18:6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- [89].Wang F, Li K, Chen Y, Deng Y, Hong K. Gene transfection mediated by ultrasound and Pluronic P85 in HepG2 cells. J Huazhong Univ Sci Technolog Med Sci. 2007;27:700–702. doi: 10.1007/s11596-007-0621-0. [DOI] [PubMed] [Google Scholar]