Abstract
Alpha A and alpha B crystallins are key members of the small heat-shock protein family. In addition to being a major structural protein of the lens, they are constitutively found in many other cells, where their function to is not completely understood. Alpha B crystallin is also known to be over-expressed in many neurological diseases. To date, all efforts to crystallize alpha A or alpha B have failed. Thus, high-resolution data on the tertiary and quaternary structure of alpha crystallin is not available. The main reason for this failure seems to be the polydisperse nature of alpha crystallin. This review deals mainly with the polydisperse properties of alpha crystallin and the influence of post-translational modification, chemical modifications, truncations and mutation on its quaternary structure.
1. Introduction
In recent years alpha-crystallin as well as other members of the small heat-shock proteins family have been the subject of intense studies. Both alpha A and alpha B crystallin are constitutively expressed in many cells, are known to be involved in many diseases, and are known to be multi-functional. There are, at present, excellent reviews in the literature dealing with biochemical, biophysical, and physiological properties of alpha-crystallin and other members of the small heat-shock protein family (van Montfort et al., 2001; Narberhaus, 2002; Horwitz, 2003; Haslbeck et al., 2005; Andeley, 2007; Arrigo et al., 2007). In spite of many efforts by various laboratories to obtain high-resolution crystallographic information, at present, we still don’t have high-resolution data of the tertiary and quaternary structure of alpha-crystallin. The problem lies in the fact that alpha-crystallin always exist as a polydisperse population with a variable number of subunit that can range from 10 to more than 40 subunits (Horwitz, 2003; Aquilina et al., 2003). Crystallographic data is available for three other members of the small heat-shock protein family namely: for Hsp 16.5 from Methanococcus jannaschii (Kim et al., 1998), for Hsp 16.9 from wheat (van Montfort et al, 2001) and for Tsp 36 from a parasitic flatworm (Stamler et al., 2005). In all of the above-mentioned cases these proteins are highly symmetrical and are always obtained as a homogeneous multimeric assemblies hence, amenable to crystallization. On the other hand, alpha-crystallin can never be obtained as a homogeneous preparation with a set number of subunits. It suggests therefore, that the quaternary structure of small heat shock proteins can vary from being highly symmetrical and homogeneous to being asymmetrical and polydispersed (Haley et al., 2000; Shi et al., 2006).
The polydispersity of alpha crystallin was first recognized by Siezen et al thirty years ago (Siezen et al., 1978) and numerous papers were published dealing with the “exact” size of “native” alpha crystallin (for a review of the early work see Derham and Harding, 1999). There are several methods for determining the homogeneity or heterogeneity of a protein in solution. The classical method is using analytical ultracentrifugation and sedimentation velocity measurements (Cole et al., 2008). More recently, a multi-angle laser light scattering systems, quasielastic light scattering, and mass spectrometry systems are used to determine the homogeneity or polydispersity of a protein preparation (Wyatt PJ, 1993; Wen et al., 1996; Aquilina et al., 2003; Lomakin et al., 2005; Sharon and Robinson, 2007).
2. Native alpha crystallin
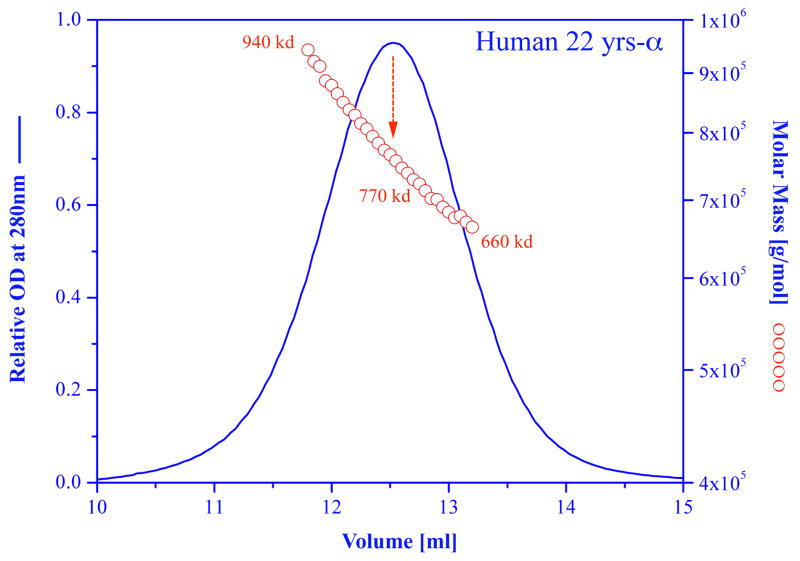
The polydisperse nature of native alpha-crystallin is shown as an example in (Fig. 1), where a preparation of alpha-crystallin from a normal human 22 years old lens was subjected to molecular weight determination using size-exclusion chromatography with online light scattering, absorbance and refractive index detectors (Horwitz, 2003). As shown, at the half band width of the chromatograph the molecular weight varies from 660,000 to 940,0000, with an average of 770,000 at the peak. Native alpha crystallin is composed of subunits of alpha A and alpha B with a monomeric molecular weight of approximately 20,000. Thus, this preparation contains molecular species containing from less 33 subunits to more than 47 subunits. Similar polydispersity is always observed for recombinant alpha A or alpha B crystallin using either light scattering or mass spectrometry (Aquilina et al., 2003, 2005; Ecroyd et al., 2007).
Figure 1.
Molar mass distribution plot and absorption at 280 nm for a sample of human (22 years) alpha crystallin. Size exclusion chromatography was carried out on a GE Healthcare Superose HR6 column connected in line with an absorption detector, a multi-angle laser light scattering detector, and a refractive index detector (DAWN-EOS; Optilab-DSP system, Wyatt Technology Corp, Santa Barbara, California USA). The blue curve represents the absorption of the sample as it is being eluted from the column. The red circles represent the molar mass of each fraction. For more details see Wen et al., 1996, Horwitz, 2003.
3. Effects of mutations on the polydispersity of alpha crystallin
Numerous point mutations were produced by various investigators in alpha A and alpha B, however, none of the mutations produced, thus far, have resulted in improving the polydispersity of alpha crystallin. The human mutation R120G in alpha B crystallin, that leads to cataract and desmin-related myopathy (Vicart et al., 1998) was extensively studied. A recombinant R120G alpha B crystallin exists as a significantly larger oligomer than the wild type protein. However like the wild type the R120G protein is also polydisperse (Bova et al., 1999; Kumar et al., 1999; Treweek, 2005). Recent work has established that arginine 116 in alpha A, and the equivalent arginine 120 in alpha B are essential for their quaternary structure and functional integrity. Various mutations of these residues may cause an increase in the average molar mass and polydispersity (Berengian et al., 1997 and Simon et al., 2007). Two recent mouse alpha A mutations that cause cataract, Arginine 54 to cystenine and tyrosine 118 to aspartic acid (Xia et al., 2006) also result in a higher molecular weight polydisperse complexes. Phosphorylation of alpha B crystallin affects its chaperone-like function and its polydisperse properties. These studies were done by producing recombinant phosphorylation mimics (Aquilina et al., 2004 and Ecroyd et al., 2007).
4. Effects of truncation on the polydispersity of alpha crystallin
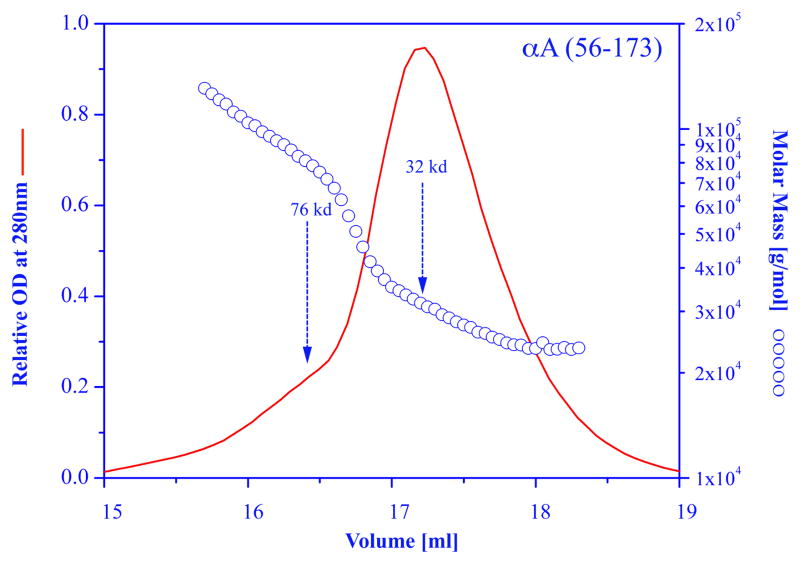
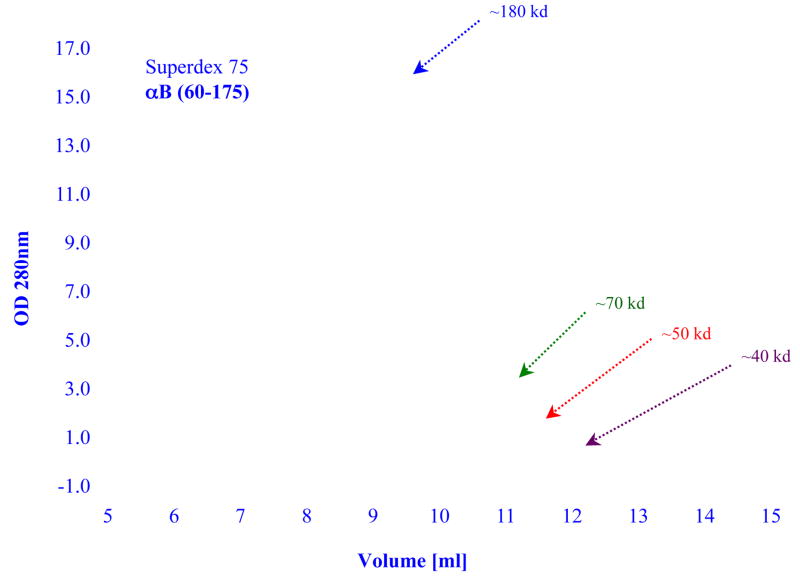
Many papers were published on the effects of various truncations that occur naturally as a consequence of aging, or that were produced by recombinant technology. The bottom line is that to date, none of the truncation produced a homogeneous population of alpha crystallin. Merck and her co-investigators were the first to construct truncated rat alpha A (residues 64–173) and truncated bovine alpha B (residues 70–175) (Merck et al., 1992, 1993). These truncations contain the conserved C-terminal domain known also as the “alpha-crystallin domain” which is common to all small heat-shock proteins. The alpha A construct forms mainly dimers and tetramers, the alpha B construct forms large aggregate. While these truncations caused major changes in the oligomerization, the end result was always a heterogeneous population of the modified alpha crystallin. A gel filtration profile and the molecular weight distributions of a truncated bovine alpha A crystallin (αA 56–173) is shown in Figure 2. The data clearly shows the existence of a mixture of dimers, trimers, tetramers, etc. Similar results were obtained with a truncated human alpha B constructed (αB 60–175), a gel filtration profile of this construct is shown in Figure 3. Interestingly, the alpha B truncation exhibited strong protein concentration dependency. At low concentration the molecular weight of the main peak is at about 40,000 indicating a mixture of dimers and trimers; at higher concentration the main peak shifts to higher molecular weight. Note however, the asymmetric elution profile obtained at each concentration indicating the polydisperse nature of this truncated alpha crystallin.
Figure 2.
Molar mass distribution plot and absorption at 280 nm for a sample of truncated recombinant alpha A crystallin (alpha A 56–173). Size exclusion chromatography was carried out on a GE Healthcare Superose HR6 column connected to the multi-angle laser light scattering system as described in figure 1.
Figure 3.
Elution profiles of samples of truncated recombinant human alpha B crystallin (alpha B 60–175) as a function of protein concentration. Size exclusion chromatography was carried out on a GE Healthcare Superdex 75 column. Blue line ~3.3 mg; green line ~0.33 mg/ml; red line ~0.14 mg/ml; brown line ~0.07 mg/ml. In each experiment 100 ul of protein was injected. The elution buffer was 50 mM sodium phosphate and 0.1 M NaCl, pH7.
5. Effects of detergent, chemical modification and concentration
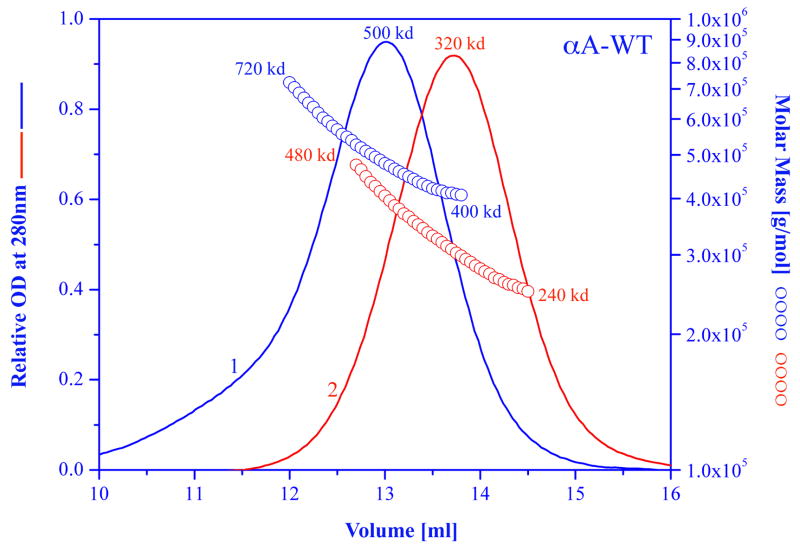
Nonionic detergents in general don’t have a significant effect on the quaternary structure of alpha crystallin. Anionic or cationic detergents such as sodium dodecyl sulfate (SDS) or dodecylethyldimethylammonium bromide (DTAB) will dissociate the alpha crystallin complex into monomers. However the use of these detergents will have a drastic effect on the secondary structure properties of alpha crystallin (Aerts et al., 1997; Biswas and Das, 2004). We have used the milder ionic detergent deoxycholate to show that alpha A crystallin can be converted to mostly tetramers with little or no change in the secondary and tertiary structures. However, the preparation was still polydisperse as is evident from the non-symmetrical gel filtration profile that was obtained (Kantorow et al., 1995). The results of dissociating alpha B crystallin into monomers with an ionic detergent such as SDS and then removing most of the detergent is shown in Figure 4. The average molecular weight dropped from 580,000 to 340,000. The gel filtration profile after the treatment with SDS is more symmetrical, with a smaller band-width elution volume (1.3 ml vs. 1.7 ml) indicating a less polydisperse population. However the molecular weight distribution shows that the polydispersity did not improve.
Figure 4.
Elution profile and molar mass distributions of recombinant bovine alpha A crystallin, in 50mM sodium phosphate buffer, 0.1 m NaCl, pH 7 (blue chromatogram) and after complete dissociation into monomers in 1% SDS, and removal of SDS prior to chromatography (red chromatogram). For the SDS experiment, alpha A crystallin sample was dissolved in 1% SDS solution, after 30 minute the SDS was removed by a “SDS-Out” precipitation kit (cat. #20308, Pierce, Rockford Il 61105). The sample then was eluted in the same buffer as the untreated sample.
There are not too many studies dealing with chemical modifications of alpha crystallin, Bindels et al (1985) showed that reaction of citraconic anhydride with bovine alpha crystallin lysine residues will dissociate the native oligomeric structure. More recently we have repeated this approach using recombinant alpha A and alpha B. While we reduced the native oligomeric proteins to dimers, trimers, tetramers, etc., we were unsuccessful in producing a homogeneous population of any of the oligomers. Interestingly, the dissociation into smaller oligomers did not affect the ability of the modified alpha crystallin to suppress non-specific aggregation of several target proteins (Horwitz et al., 2004).
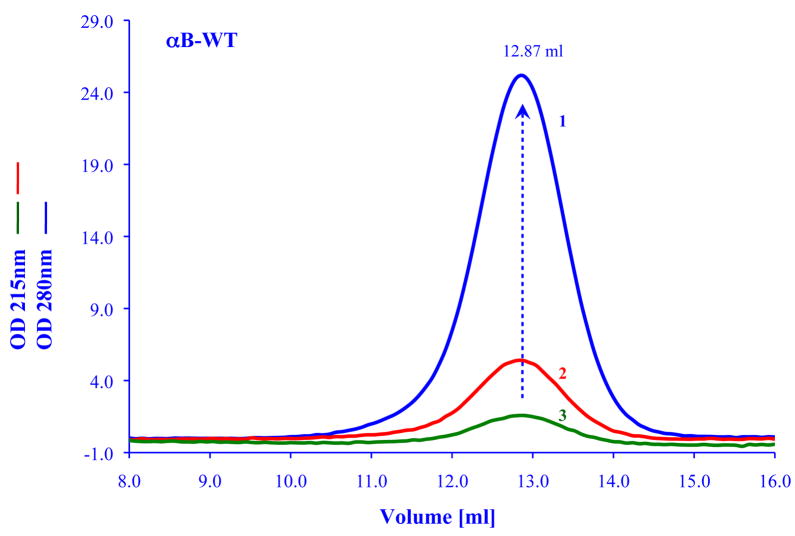
The oligomeric structure of many of the small heat shock proteins is highly sensitive to temperature and protein concentration (see for example, Lentze et al., 2004). In some cases the change in oligomerization is essential for its chaperone function (Giese and Vierling, 2002). Similarly for human Hsp27 which is highly homologous to alpha crystallin, it was shown that dissociation of its oligomeric state is required in order to bind the target protein. The dissociation was shown to be concentration dependent (Shashidharamurthy, 2005). A important finding was recently reported by Franzman et al (2008) who showed that Hsp 26 which is the principle small heat shock protein in the yeast S. cerevisiae can act as a “thermosensor”. These investigators showed that the temperature sensing occurs by conformational changes in the middle domain of Hsp 26. The conformational changes that switch Hsp 26 from an inactive chaperone to an active chaperone occur within a narrow temperature range. In contrast to the examples discussed above alpha crystallin does not exhibit drastic conformational changes upon variation in temperature or concentration. Earlier work by Vanhoudt et al (1998, 2000) showed that the average molecular weight of alpha crystallin would change with temperature. They found that the changes in quaternary structure are very slow, and it may take several hours to observe a change in the average molecular weight when the temperature increases from 4°C to 37°C. At a given temperature, changes in concentration will also have a minimal effect on alpha crystallin. This can be seen in Figure 5 where the elution profile of alpha B crystallin is shown for a sample with a concentration of 1.5 mg/ml to a sample with a concentration of 0.012 mg/ml. In all cases the elution volume remain the same with no evidence for any dissociation of the complex to smaller molecular species. We were able to monitor the effects of 2000 fold dilution of an alpha B crystallin sample (2.5 mg to 0.00125 mg/ml) by gel filtration chromatography and found no evidence of dissociation. The important point to emphasize is that even though these elution profiles in Figure 5 look relatively symmetrical, the alpha crystallin is always polydisperse when checked with the light scattering system described in Figure 1.
Figure 5.
Effects of protein concentrations on the elution profiles of recombinant human alpha B crystallin. Profiles are shown at 0.5 mg/ml (blue line); 0.005 mg/ml (red line); 0.0025 mg/ml (green line). In general, for concentrations >0.1 mg/ml, the absorption detector was set at 280 nm, for lower protein concentration the detector was set at 215 nm. All samples were run on a GE Healthcare Superose HR6 column connected to GE ACTA Basic Chromatography with UV-900 detector system. The flow rate was 0.5 ml/min, all samples were in 50 mM sodium phosphate and 0.1 M NaCl pH7, 100 ul of a given concentration was always injected. The peak of all the samples were always eluted at 12.87±0.02 ml over a concentration range from 2.5 mg/ml to 0.0013 mg/ml.
6. Using other members of the small heat shock protein family for explaining the polydispersivity of alpha crystallin
While alpha crystallin represents a member of the small heat shock protein that has a variable structure, is asymmetrical and highly polydispersed, Hsp 16.5 from Methanococcus jannaschii represents the other side of the spectrum being highly symmetrical, monodispersed and with a known crystallographic structure (Kim et al., 1998). In a recent seminal paper (Shi et al., 2006), the authors employed “rational insertion mutagenesis” to modify Hsp 16.5 and to drastically change its structural properties from being highly symmetrical and monodisperse to be polydisperse. The authors used spin labeling EPR and Cryoelectron microscopy (to a resolution of 10Å!) to derive a model that explain the interactions among N-terminal domain, the alpha crystallin domain, and the C-terminal extension that are common to all of the small heat shock family members. This study is highly relevant to our understanding of the structure and function of alpha crystallin and provides insight into the reason of its polydispersity. Similarly, from the high-resolution data available on Hsp 16.9 and Tsp 36 (Van Montfort et al., 2001 and Stamler et al., 2005), we can also learn about the structure of alpha crystallin. As more structures of other members of the small heat shock protein family are solved, we can expect that it will add to understanding of the structure of alpha crystallin.
Conclusions
The vast amount of work on the physic-chemical properties of alpha crystallin shows it to be a very flexible and dynamic protein with well-characterized subunit exchange properties. All of the above mentioned properties in addition to its polydispersity make it to be a very unlikely candidate for crystallization in its native state. It is important to note that in recent years and at present attention is directed into the function of intrinsically disordered/unstructured proteins and within a protein structure into the so-called unstructured or unordered domains. These domains, which do not possess a well-defined 3-dimentional, are very common in many proteins. From circular dichroism measurement it is estimated that in alpha crystallin, about 30 percent of the protein possess an “unordered” structure. It was suggested recently that these unordered and flexible domains are crucial for chaperone function (Tompa and Csermely, 2004). The N-terminal and C-terminal extensions of alpha crystallin are thought to be unstructured, but are critical to its function.
Consequently, to get high-resolution information about the tertiary and quaternary structure of alpha crystallin we must look at other methodologies. Recent advances in electron paramagnetic resonance spectroscopy combined with site-directed spin labeling appear to be a powerful methodology for obtaining high- resolution structural information. This approach was recently tested on T4-lysozyme and alpha A crystallin (Alexander et al., 2008). The results obtained in this paper are very promising. Other promising new methodologies include ion-mobility mass spectrometry (Ruotolo et al., 2005) and TROSY-NMR techniques (Fernandez and Wilder, 2003), which are applicable to large proteins. The challenge for the structural biologists is to give us a complete high-resolution structure of alpha crystallin utilizing as many methodologies as needed so that we can understand its multiple functions in the cells that harbor it, and find the reasons for its innate polydisperse nature that seems to be essential for its function.
Acknowledgments
I wish to thank the reviewers for helpful and constructive comments. I thank Linlin Ding and Qingling Huang for helping with many of the experiments described in this review. Given the size limitation of this review, it is impossible to do justice and cite all of references that are relevant to this topic. I therefore apologize to my colleagues and all investigators in the field whose work was not cited. Supported by the National Eye Institute Grant: R-O1 EY03897. Dr. Horwitz is the Oppenheimer Brothers Chair in Ophthalmology at Jules Stein Eye Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aerts T, Clauwaert J, Haezebrouck P, Peeters E, Van Dael H. Interaction of detergents with bovine lens alpha-crystallin: evidence for an oligomeric structure based on amphiphilic interactions. Eur Biophys J. 1997;25:445–454. doi: 10.1007/s002490050059. [DOI] [PubMed] [Google Scholar]
- Alexander N, Bortolus M, Al-Mestarihi A, Mchaourab H, Meiler J. De novo high-resolution protein structure determination from sparse spin-labeling EPR data. Structure. 2008;16:181–195. doi: 10.1016/j.str.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Aquilina JA, Benesch JL, Bateman OA, Slingsby C, Robinson CV. Polydispersity of a mammalian chaperone: mass spectrometry reveals the population of oligomers in alphaB-crystallin. Proc Natl Acad Sci U S A. 2003;100:10611–10616. doi: 10.1073/pnas.1932958100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, Robinson CV. Subunit exchange of polydisperse proteins: mass spectrometry reveals consequences of alphaA-crystallin truncation. J Biol Chem. 2005;280:14485–14491. doi: 10.1074/jbc.M500135200. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Bindels JG, Misdom LW, Hoenders HJ. The reaction of citraconic anhydride with bovine alpha-crystallin lysine residues. Surface probing and dissociation-reassociation studies. Biochim Biophys Acta. 1985;828:255–260. doi: 10.1016/0167-4838(85)90305-x. [DOI] [PubMed] [Google Scholar]
- Biswas A, Das KP. SDS induced structural changes in alpha-crystallin and it’s effect on refolding. Protein J. 2004;23:529–538. doi: 10.1007/s10930-004-7880-4. [DOI] [PubMed] [Google Scholar]
- Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci U S A. 1999;96:6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JL, Lary JWP, Moody T, Laue TM. Analytical ultracentrifugation: sedimentation velocity and sedimentation equilibrium. Methods Cell Biol. 2008;84:143–179. doi: 10.1016/S0091-679X(07)84006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derham BK, Harding JJ. Alpha-crystallin as a molecular chaperone. Prog Retin Eye Res. 1999;18:463–509. doi: 10.1016/s1350-9462(98)00030-5. [DOI] [PubMed] [Google Scholar]
- Ecroyd H, Meehan S, Horwitz J, Aquilina JA, Benesch JL, Robinson CV, Macphee CE, Carver JA. Mimicking phosphorylation of alphaB-crystallin affects its chaperone activity. Biochem J. 2007;401:129–141. doi: 10.1042/BJ20060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández C, Wider G. TROSY in NMR studies of the structure and function of large biological macromolecules. Curr Opin Struct Biol. 2003;13:570–580. doi: 10.1016/j.sbi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Franzmann TM, Menhorn P, Walter S, Buchner J. Activation of the chaperone Hsp26 is controlled by the rearrangement of its thermosensor domain. Mol Cell. 2008;29:207–216. doi: 10.1016/j.molcel.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Giese KC, Vierling E. Changes in oligomerization are essential for the chaperone activity of a small heat shock protein in vivo and in vitro. J Biol Chem. 2002;277:46310–46318. doi: 10.1074/jbc.M208926200. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Haley DA, Bova MP, Huang QL, Mchaourab HS, Stewart PL. Small heat-shock protein structures reveal a continuum from symmetric to variable assemblies. J Mol Biol. 2000;298:261–272. doi: 10.1006/jmbi.2000.3657. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Horwitz J, Huang Q, Ding L. The native oligomeric organization of alpha-crystallin, is it necessary for its chaperone function? Exp Eye Res. 2004;79:817–821. doi: 10.1016/j.exer.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kantorow M, Horwitz J, van Boekel MA, de Jong WW, Piatigorsky J. Conversion from oligomers to tetramers enhances autophosphorylation by lens alpha A-crystallin. Specificity between alpha A- and alpha B-crystallin subunits. J Biol Chem. 1995;270:17215–17220. doi: 10.1074/jbc.270.29.17215. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Kumar LV, Ramakrishna T, Rao CM. Structural and functional consequences of the mutation of a conserved arginine residue in alphaA and alphaB crystallins. J Biol Chem. 1999;274:24137–24141. doi: 10.1074/jbc.274.34.24137. [DOI] [PubMed] [Google Scholar]
- Lentze N, Aquilina JA, Lindbauer M, Robinson CV, Narberhaus F. Temperature and concentration-controlled dynamics of rhizobial small heat shock proteins. Eur J Biochem. 2004;271:2494–2503. doi: 10.1111/j.1432-1033.2004.04180.x. [DOI] [PubMed] [Google Scholar]
- Lomakin A, Teplow DB, Benedek GB. Quasielastic light scattering for protein assembly studies. Methods Mol Biol. 2005;299:153–174. doi: 10.1385/1-59259-874-9:153. [DOI] [PubMed] [Google Scholar]
- Merck KB, De Haard-Hoekman WA, Oude Essink BB, Bloemendal H, De Jong WW. Expression and aggregation of recombinant alpha A-crystallin and its two domains. Biochim Biophys Acta. 1992;1130:267–276. doi: 10.1016/0167-4781(92)90439-7. [DOI] [PubMed] [Google Scholar]
- Merck KB, Horwitz J, Kersten M, Overkamp P, Gaestel M, Bloemendal H, de Jong WW. Comparison of the homologous carboxy-terminal domain and tail of alpha-crystallin and small heat shock protein. Mol Biol Rep. 1993;18:209–215. doi: 10.1007/BF01674432. [DOI] [PubMed] [Google Scholar]
- Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66:64–93. doi: 10.1128/MMBR.66.1.64-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotolo BT, Giles K, Campuzano I, Sandercock AM, Bateman RH, Robinson CV. Evidence for macromolecular protein rings in the absence of bulk water. Science. 2005;310:1658–1661. doi: 10.1126/science.1120177. [DOI] [PubMed] [Google Scholar]
- Sharon M, Robinson CV. The role of mass spectrometry in structure elucidation of dynamic protein complexes. Annu Rev Biochem. 2007;76:167–193. doi: 10.1146/annurev.biochem.76.061005.090816. [DOI] [PubMed] [Google Scholar]
- Shashidharamurthy R, Koteiche HA, Dong J, McHaourab HS. Mechanism of chaperone function in small heat shock proteins: dissociation of the HSP27 oligomer is required for recognition and binding of destabilized T4 lysozyme. J Biol Chem. 2005;280:5281–5289. doi: 10.1074/jbc.M407236200. [DOI] [PubMed] [Google Scholar]
- Shi J, Koteiche HA, McHaourab HS, Stewart PL. Cryoelectron microscopy and EPR analysis of engineered symmetric and polydisperse Hsp16.5 assemblies reveals determinants of polydispersity and substrate binding. J Biol Chem. 2006;281:40420–40428. doi: 10.1074/jbc.M608322200. [DOI] [PubMed] [Google Scholar]
- Siezen RJ, Bindels JG, Hoenders HJ. The quaternary structure of bovine alpha-crystallin. Size and charge microheterogeneity: more than 1000 different hybrids? Eur J Biochem. 1978;91:387–396. doi: 10.1111/j.1432-1033.1978.tb12691.x. [DOI] [PubMed] [Google Scholar]
- Simon S, Michiel M, Skouri-Panet F, Lechaire JP, Vicart P, Tardieu A. Residue R120 is essential for the quaternary structure and functional integrity of human alphaB-crystallin. Biochemistry. 2007;46:9605–9614. doi: 10.1021/bi7003125. [DOI] [PubMed] [Google Scholar]
- Stamler R, Kappé G, Boelens W, Slingsby C. Wrapping the alpha-crystallin domain fold in a chaperone assembly. J Mol Biol. 2005;353:68–79. doi: 10.1016/j.jmb.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Tompa P, Csermely P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 2004;18:1169–1175. doi: 10.1096/fj.04-1584rev. [DOI] [PubMed] [Google Scholar]
- Treweek TM, Rekas A, Lindner RA, Walker MJ, Aquilina JA, Robinson CV, Horwitz J, Perng MD, Quinlan RA, Carver JA. R120G alphaB-crystallin promotes the unfolding of reduced alpha-lactalbumin and is inherently unstable. FEBS J. 2005;272:711–724. doi: 10.1111/j.1742-4658.2004.04507.x. [DOI] [PubMed] [Google Scholar]
- Vanhoudt J, Aerts T, Abgar S, Clauwaert J. Quaternary structure of bovine alpha-crystallin: influence of temperature. Int J Biol Macromol. 1998;22:229–237. doi: 10.1016/s0141-8130(98)00020-8. [DOI] [PubMed] [Google Scholar]
- Vanhoudt J, Abgar S, Aerts T, Clauwaert J. Native quaternary structure of bovine alpha-crystallin. Biochemistry. 2000;39:4483–4492. doi: 10.1021/bi990386u. [DOI] [PubMed] [Google Scholar]
- van Montfort RL, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- van Montfort R, Slingsby C, Vierling E. Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem. 2001;59:105–156. doi: 10.1016/s0065-3233(01)59004-x. [DOI] [PubMed] [Google Scholar]
- Vicart P, Caron A, Guicheney P, Li Z, Prévost MC, Faure A, Chateau D, Chapon F, Tomé F, Dupret JM, Paulin D, Fardeau M. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- Xia CH, Liu H, Chang B, Cheng C, Cheung D, Wang M, Huang Q, Horwitz J, Gong X. Arginine 54 and Tyrosine 118 residues of {alpha}A-crystallin are crucial for lens formation and transparency. Invest Ophthalmol Vis Sci. 2006;47:3004–3010. doi: 10.1167/iovs.06-0178. [DOI] [PubMed] [Google Scholar]
- Wen J, Arakawa T, Philo JS. Size-exclusion chromatography with on-line light-scattering, absorbance, and refractive index detectors for studying proteins and their interactions. Anal Biochem. 1996;240:155–166. doi: 10.1006/abio.1996.0345. [DOI] [PubMed] [Google Scholar]
- Wyatt PJ. Light scattering and the absolute characterization of macromolecules. Anal Chim Acta. 1993;272:1–40. [Google Scholar]