Abstract
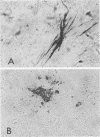
Peroxidase-labeled monoclonal antibodies against respiratory syncytial virus (RSV) and influenza A virus were used for immunoperoxidase staining (IPS) of cell cultures inoculated with nasopharyngeal aspirates. Cells were grown in 24-well plates, and specimens were inoculated by low-speed centrifugation. Cultures were incubated for 2 days at 37 degrees C and then fixed, stained, and observed by light microscopy. IPS was compared with standard virus isolation by using cultures of human diploid fibroblasts and Vero, HEp-2, and HeLa cell lines for RSV and Madin-Darby canine kidney cells for influenza A virus; these cultures were inoculated with specimens that were previously stored at -70 degrees C. Of 40 known RSV-positive specimens, 30 were found to be positive on reinoculation by both methods, and an additional 5 specimens were found to be positive by IPS only. Of 190 specimens tested for influenza A virus, 14 were positive by IPS and in tubes, and a further 8 specimens were positive by IPS only. IPS was also compared with direct detection of viral antigens in nasopharyngeal aspirates by a time-resolved fluoroimmunoassay (TR-FIA). Fresh nasopharyngeal aspirates were inoculated into human diploid fibroblasts and Madin-Darby canine kidney cells and tested for RSV and influenza A virus, respectively, by IPS. Of 110 specimens tested for RSV, 37 were positive in total, 32 were positive by IPS, and 33 were positive by TR-FIA. Of 150 specimens tested for influenza A virus, 39 were positive in total, 35 were positive by IPS, and 34 were positive by TR-FIA. IPS of cultures inoculated by centrifugation and incubated for 2 days is a sensitive method for the diagnosis of respiratory virus infections, and 24-well plates allow for the easy processing of a large number of specimens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. J., Hierholzer J. C., Tsou C., Hendry R. M., Fernie B. F., Stone Y., McIntosh K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985 Apr;151(4):626–633. doi: 10.1093/infdis/151.4.626. [DOI] [PubMed] [Google Scholar]
- Darougar S., Gibson J. A., Thaker U. Effect of centrifugation on herpes simplex virus isolation. J Med Virol. 1981;8(4):231–235. doi: 10.1002/jmv.1890080403. [DOI] [PubMed] [Google Scholar]
- Espy M. J., Smith T. F., Harmon M. W., Kendal A. P. Rapid detection of influenza virus by shell vial assay with monoclonal antibodies. J Clin Microbiol. 1986 Oct;24(4):677–679. doi: 10.1128/jcm.24.4.677-679.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P. S., McQuillin J. Application of immunofluorescent antibody technique in rapid diagnosis of respiratory syncytial virus infection. Br Med J. 1968 Aug 10;3(5614):340–343. doi: 10.1136/bmj.3.5614.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleaves C. A., Smith T. F., Shuster E. A., Pearson G. R. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol. 1984 Jun;19(6):917–919. doi: 10.1128/jcm.19.6.917-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. B., McBride J. T., Gala C. L., Hildreth S. W., Schnabel K. C. Ribavirin treatment of respiratory syncytial viral infection in infants with underlying cardiopulmonary disease. JAMA. 1985 Dec 6;254(21):3047–3051. [PubMed] [Google Scholar]
- Harmon M. W., Pawlik K. M. Enzyme immunoassay for direct detection of influenza type A and adenovirus antigens in clinical specimens. J Clin Microbiol. 1982 Jan;15(1):5–11. doi: 10.1128/jcm.15.1.5-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. H., Mann D. R., Hamparian V. V. Detection of respiratory syncytial virus in clinical specimens by viral culture, direct and indirect immunofluorescence, and enzyme immunoassay. J Clin Microbiol. 1988 Mar;26(3):588–591. doi: 10.1128/jcm.26.3.588-591.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters H. B., Bate B. J., Wren C., Lauer B. A. Detection of respiratory syncytial virus antigen in nasopharyngeal secretions by Abbott Diagnostics enzyme immunoassay. J Clin Microbiol. 1988 Jun;26(6):1103–1105. doi: 10.1128/jcm.26.6.1103-1105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Kurachek S. C., Cairns L. M., Burns J. C., Goodspeed B. Treatment of respiratory viral infection in an immunodeficient infant with ribavirin aerosol. Am J Dis Child. 1984 Mar;138(3):305–308. doi: 10.1001/archpedi.1984.02140410083024. [DOI] [PubMed] [Google Scholar]
- Minnich L. L., Ray C. G. Early testing of cell cultures for detection of hemadsorbing viruses. J Clin Microbiol. 1987 Feb;25(2):421–422. doi: 10.1128/jcm.25.2.421-422.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A., Orvell C., Rafnar B., Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985 Oct;66(Pt 10):2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- Nikkari S., Halonen P., Kharitonenkov I., Kivivirta M., Khristova M., Waris M., Kendal A. One-incubation time-resolved fluoroimmunoassay based on monoclonal antibodies in detection of influenza A and B viruses directly in clinical specimens. J Virol Methods. 1989 Jan;23(1):29–40. doi: 10.1016/0166-0934(89)90086-4. [DOI] [PubMed] [Google Scholar]
- Shalit I., McKee P. A., Beauchamp H., Waner J. L. Comparison of polyclonal antiserum versus monoclonal antibodies for the rapid diagnosis of influenza A virus infections by immunofluorescence in clinical specimens. J Clin Microbiol. 1985 Nov;22(5):877–879. doi: 10.1128/jcm.22.5.877-879.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C. E., Bernstein J. M., Kyger S. A., Hayden F. G. Rapid diagnosis of influenza A and B by 24-h fluorescent focus assays. J Clin Microbiol. 1988 Jul;26(7):1263–1266. doi: 10.1128/jcm.26.7.1263-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson P. D., Kaplan M. H. Rapid detection of influenza virus in cell culture by indirect immunoperoxidase staining with type-specific monoclonal antibodies. Diagn Microbiol Infect Dis. 1987 Aug;7(4):265–268. doi: 10.1016/0732-8893(87)90142-8. [DOI] [PubMed] [Google Scholar]
- Tominack R. L., Hayden F. G. Rimantadine hydrochloride and amantadine hydrochloride use in influenza A virus infections. Infect Dis Clin North Am. 1987 Jun;1(2):459–478. [PubMed] [Google Scholar]
- Vainionpä R., Meurman O., Sarkkinen H. Antibody response to respiratory syncytial virus structural proteins in children with acute respiratory syncytial virus infection. J Virol. 1985 Mar;53(3):976–979. doi: 10.1128/jvi.53.3.976-979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls H. H., Harmon M. W., Slagle J. J., Stocksdale C., Kendal A. P. Characterization and evaluation of monoclonal antibodies developed for typing influenza A and influenza B viruses. J Clin Microbiol. 1986 Feb;23(2):240–245. doi: 10.1128/jcm.23.2.240-245.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls H. H., Johansson K. H., Harmon M. W., Halonen P. E., Kendal A. P. Time-resolved fluoroimmunoassay with monoclonal antibodies for rapid diagnosis of influenza infections. J Clin Microbiol. 1986 Dec;24(6):907–912. doi: 10.1128/jcm.24.6.907-912.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris M., Halonen P., Ziegler T., Nikkari S., Obert G. Time-resolved fluoroimmunoassay compared with virus isolation for rapid detection of respiratory syncytial virus in nasopharyngeal aspirates. J Clin Microbiol. 1988 Dec;26(12):2581–2585. doi: 10.1128/jcm.26.12.2581-2585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler T., Waris M., Rautiainen M., Arstila P. Herpes simplex virus detection by macroscopic reading after overnight incubation and immunoperoxidase staining. J Clin Microbiol. 1988 Oct;26(10):2013–2017. doi: 10.1128/jcm.26.10.2013-2017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]