Abstract
The amygdala is involved in behavioral and physiological responses to fear, and the anxiolytic properties of several drugs are localized to this region. Activation of endogenous opioid systems is known to occur in response to stress and a growing body of literature suggests that opioid systems regulate the properties of anxiolytic drugs. These experiments sought to elucidate the role of opioid receptors in the central (CeA) and basolateral (BLA) nuclei of the amygdala in regulating the anxiolytic properties of ethanol and diazepam. Male rats fitted with cannula received bilateral microinjections of the non-selective opioid receptor antagonist naltrexone (NAL) immediately followed by systemic delivery of either ethanol (1 g/kg) or diazepam (2 mg/kg) in the elevated plus maze. Both diazepam and ethanol decreased anxiety-like behavior. Delivery of NAL into the CeA blocked the anxiolytic properties of diazepam. Delivery of NAL into the BLA slightly increased open arm avoidance, but had no effect on the anxiolytic properties of diazepam. Microinjection of NAL into either nucleus failed to block the effects of ethanol. These results were specific to the anxiolytic properties of diazepam, since baseline behaviors were unaffected by microinjection of naltrexone. Microinjection of lidocaine produced results distinct from NAL and failed to block the anxiolytic actions of diazepam. These studies indicate distinct roles for opioid receptor systems in the CeA and BLA in regulating the anxiolytic properties of diazepam in the elevated plus maze. Further, opioid receptor systems in the CeA and BLA do not regulate the anxiolytic properties of ethanol in this test.
Keywords: opioid, receptors, ethanol, benzodiazepine, anxiety, rat
Introduction
Benzodiazepines are a class of drugs commonly prescribed for the treatment of anxiety disorders, the prototypical compound being diazepam. This class of drugs enhances gamma aminobutyric acid (GABA) neurotransmission by binding to benzodiazepine receptors in the GABAA receptor complex and augmenting response to GABA (as reviewed by (Wilson 1996)). Another drug that enhances GABAA receptor function is ethanol (Nie et al. 2004; Nestoros 1980; Macdonald 1995), a drug commonly abused by individuals suffering from depression and anxiety (for reviews see (Eckardt et al. 1998; Pohorecky 1981)). Both these drugs are known to be anxiolytic in humans and produce reductions in anxiety-like behavior in several animal models of behavior (Wilson et al. 2004; Pellow et al. 1985; Eckardt et al. 1998).
The amygdala has been well documented as a region of the brain involved in learning, fear and anxiety, and is believed to play a critical role in anxiety disorders (for reviews see, (Davis et al. 1994; LeDoux 2000; Maren 2003; Cardinal et al. 2002)). Further, the anxiolytic properties of some drugs are localized to the amygdala (for reviews see (File 2000; Menard and Treit 1999)). Lesioning the amygdala, however, fails to alter baseline behavior in some tests (McHugh et al. 2004) or block the properties of anxiolytic drugs (Treit et al. 1993b; Treit et al. 1993a) in tests that rely on novelty, suggesting that this region may not be critically involved in regulating anxiety-like behavior. Contrary to these reports the effects of chlordiazepoxide were blocked by microinjection of GABAA receptor antagonists into the BLA (Sanders and Shekhar 1995). At present, the role of the amygdala in regulating anxiolytic processes is unclear, and these discrepancies may be due to drug injection and lesion techniques that are not selective for individual amygdalar nuclei.
Activation of the endogenous opioid system is one of the physiological responses evoked during exposure to stressful stimuli (Vaccarino and Kastin 2001), and a growing body of evidence suggests an essential interaction between endogenous opioid systems and the effects of anxiolytic compounds. Systemic delivery of naloxone inhibits the anticonflict effects of diazepam (Agmo et al. 1995). In contrast, naloxone has also been shown to potentiate the anxiolytic properties of subeffective doses of benzodiazepines and the 5HT1A receptor agonist buspirone (Belzung and Agmo 1997), suggesting that the modulatory role of opioid systems may be different in distinct paradigms. Specific opioid receptor subtypes appear to play distinct roles in regulating the anxiolytic properties of benzodiazepines, since systemic administration of the μ-receptor antagonist β-funaltrexamine and the κ-receptor antagonist nor-binaltorphimine blocked the anxiolytic properties of chlordiazepoxide, while the δ-receptor antagonist naltrindole had no effect on elevated plus maze behavior (Agmo and Belzung 1998). Opioid antagonists also block the anxiolytic effects of diazepam in humans (Duka et al. 1982). Although the role of opioid receptor systems in regulating the anxiolytic properties of ethanol has received less attention, there is evidence that this system mediates some of the motivational responses associated with ethanol administration (Wilson et al. 2003; Moller et al. 1997). Both high doses of morphine (Volpicelli et al. 1991) and intraventricular infusion of met-enkephalin (Ho and Rossi 1982) decrease ethanol consumption in rats. Further, naltrexone decreases operant responding for ethanol in rats, and decreases ethanol intake in rodents and humans (O’Malley et al. 2000; Hyytia and Sinclair 1993; Froehlich et al. 1990). Selective receptor antagonists for both μ- (Hyytia and Kiianmaa 2001) and δ-opioid receptors (Krishnan-Sarin et al. 1995) are also efficacious in decreasing ethanol intake.
Additional studies suggest a role for amygdalar opioid systems in regulating anxiety-related responses or the anxiolytic properties of benzodiazepines and ethanol. Microinjection of morphine into the amygdala decreases social interaction (File and Rodgers 1979) suggesting that opioid systems in this region play a modulatory role in anxiety-like behavior. Overexpression of enkephalin in the amygdala potentiates a sub-effective dose of diazepam in the elevated plus maze (Kang et al. 2000), while large injections of naltrexone into the amygdala block the anxiolytic properties of diazepam (Kang et al. 2000) and ethanol (Wilson et al. 2003) in the same paradigm. These previous studies used injection parameters that could not localize effects to select amygdalar nuclei, Therefore, the present experiments evaluated the role of opioid receptor systems in the central or basolateral nuclei of the amygdala in regulating the anxiolytic properties of diazepam and ethanol in the elevated plus maze. In these studies the non-selective opioid receptor antagonist naltrexone was injected into either the BLA or CeA, in combination with systemic injections of diazepam or ethanol. Lidocaine microinjection into these regions was also investigated to determine how inactivation of the BLA or CeA affected behavioral responses to diazepam in the elevated plus maze. Based on previous work we hypothesized that: 1) opioid receptors in the CeA would be involved in regulating the anxiolytic properties of both ethanol and diazepam; and 2) inactivation of the amygdala would attenuate the anxiolytic properties of diazepam.
Methods
Subjects
For all experiments, male Long Evans rats (Harlan, Indianapolis IN), weighing approximately 175 g upon arrival, were housed singly in an environmentally controlled animal facility on a 12:12 light/dark cycle with lights on at 07:00. Purina rat chow and water were available ad libitum. Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved animal facility, and all procedures were approved by the University of South Carolina Animal Care and Use Committee. Behavioral testing was initiated and completed during the light cycle between 08:00–12:00.
Surgery
One week before testing, bilateral cannula aimed at the CeA or BLA were implanted using stereotaxic procedures. Animals were anesthetized with sodium pentobarbital (75 mg/kg, i.p.), and given injections of local anesthetic (2% carbocaine, s.c.) at pressure points for the earbars and incision site. The rat was placed into a Kopf stereotaxic unit with the skull flat, the incision site was scrubbed with betadine wash, and a mid-saggital incision was used to expose the skull. The coordinates for CeA were A/P −2.3, M/L 4.1, D/V −6.2 from skull and for BLA were A/P −2.8, M/L +5.0, D/V −6.4 from skull (incisor bar −3.0), as determined from Bregma based on Paxinos & Watson (1997) (Paxinos and Watson 1997). The tips of the 26 gauge guide cannula (I.D. 0.433 inches, Plastics One, Roanoke, VA) were positioned 2 mm above the amygdalar target. Cannulas were anchored to three skull screws (0.80 × 3/32; Plastics One, Roanoke, VA) using Ortho-Jet cold-setting dental acrylic (Lang Dental, Wheeling, IL). Nalbuphine (1mg/kg, subcutaneous) was given post-operatively for pain management and the diet supplemented with bacon softies (Bio-serve, Frenchtown, NJ) in order to maintain postoperative weight.
Drugs and Microinjections
Rats were habituated to injection procedures on days four through six after surgery, and dummy cannula were checked and cleaned during these handling sessions. One week after surgery animals were lightly restrained in a towel in order to remove dummy cannula and insert the injector cannula. Bilateral intra-amygdalar injections were administered by two-2μl Hamilton microsyringes (Hamilton Co., Reno, NV) controlled by a Harvard Apparatus PHD 2000 microinfusion pump (Harvard, Holliston, MA). Microsyringes were connected to 33-gauge injector cannula (I.D. 0.004 inches; Plastics 1 Inc, Roanoke, VA) by polyethylene tubing (I.D. 0.023 inches). Displacement of an air bubble in the polyethylene tubing was used to confirm injection. Injections occurred over a two-minute period (0.3μl @ 0.15 μl/minute), with 60 seconds allowed after the injection to permit drug spread. Immediately following the intra-amygdalar injection, dummy cannula were replaced and animals were given systemic injections of either diazepam (2 mg/kg i.p.) or vehicle (see below) in experiments 1 and 3, or ethanol (1 g/kg, 20% v/v) or saline in experiment 2. In the first set of experiments, the nonselective opioid antagonist naltrexone hydrochloride (15 μg) was used to block opioid receptors in the CeA and BLA (Kang et al. 2000). This dose of naltrexone has been shown to affect anxiolytic drug responses when microinjected into the amygdala (Kang et al., 2000;Wilson et al., 2003), and similar doses modify behavioral responses when injected into the nucleus accumbens (Kelley et al., 1996). Lidocaine hydrochloride (10μg) (Vazdarjanova and McGaugh 1999) was used to reversibly inactivate the CeA and BLA immediately prior to systemic administration of diazepam (2 mg/kg) or vehicle administered intraperitoneally (i.p.). This dose of lidocaine was chosen because it (Vazdarjanova and McGaugh, 1999) and smaller doses (Helmstetter, 1992;Manning and Mayer, 1995) were effective in altering behavior when microinjected into amygdalar nuclei. Lidocaine hydrochloride (10μg) (Vazdarjanova and McGaugh 1999) was used to reversibly inactivate the CeA and BLA immediately prior to systemic administration of diazepam (2 mg/kg) or vehicle administered intraperitoneally (i.p.). The doses and timing of diazepam and ethanol administration regimens were selected based on our previous studies in order to produce effective and reliable anxiolytic responses in the elevated plus maze (Wilson et al. 2004).
Lidocaine hydrochloride was dissolved in 0.9% sterile saline (pH 7.4). Naltrexone hydrochloride (Research Triangle Park, NC) was dissolved in sterile double-deionized water and diluted to their final concentrations with 0.9% sterile saline (pH 7.4). Ethanol (20% v/v) was prepared in 0.9% sterile saline, and diazepam was prepared in vehicle (40% propylene glycol, 10% ethanol, and 50% sterile water).
Behavioral Testing
Animals were placed on the elevated plus maze thirty minutes after the systemic injection of diazepam or ten minutes after systemic injection of ethanol to allow for the peak anxiolytic effects of each drug (Wilson et al. 2004). This test was conducted as described in Kang et al (2000), as modified from Pellow et al. (1985) (Kang et al. 2000; Pellow et al. 1985). The black Plexiglas elevated plus maze consisted of two opposing open and two opposing closed arms in the shape of a cross, connected by a central square. The maze was elevated 50 cm above the ground and had a 0.5 cm edge on the open arms. Animals were placed in the center square facing an open arm and allowed to explore the maze for five minutes while their behavior was videotaped for later analysis. Behaviors known to be responsive to anxiolytic drugs were scored and included percent open arm time (open arm time/(open arm time + closed arm time)), and percent open arm entries (open arm entries/(open arm entries + closed arm entries)). Activity was determined by total distance moved by the rat (in centimeters). All behaviors were videotaped, and scoring was performed with the behavioral tracking system Ethovision (Noldus, Netherlands). A correlation of ≥95% between scoring by a trained observer and the Ethovision tracking system was determined prior to this set of experiments. The elevated plus maze has been successfully used as a test for anxiolytic agents (Lister 1987; Pellow et al. 1985). Both ethanol (Wilson et al. 2004; Lister 1987) and diazepam (Pellow et al. 1985; Wilson et al. 2004) increase open arm behavior in the elevated plus maze.
Blood Alcohol Concentrations
Blood alcohol levels were determined in ethanol-treated rats, using a procedure adapted from Dudek & Abbot (Dudek and Abbott 1984) as described in Wilson et al. (2004) (Wilson et al. 2004). Immediately after testing 10 μl of blood was collected by tail stick. Absolute ethanol was diluted with double distilled H2O to produce working standards (10–400 mg%). The blood or standard was placed into tubes containing 190 μl of 0.53 N perchloric acid. Subsequently 200 μl of 0.3 M K2CO3 was added to the tube, vortexed, centrifuged (15 min, 25°C, 1500 × g), and stored at −20°C. Prior to assaying, blood samples and ethanol standards were thawed, centrifuged (15 min, 25°C, 1500 × g), and kept on ice. Borosilicate tubes (12 × 75 mm) were prepared with 400 μL NAD-Tris (1.875 mM NAD in 0.5 M Tris Base, pH 7.4) and 50 μl of alcohol dehydrogenase (89.25 units/mL). Fifty microliters of supernatant from each sample or standard was added, the mixture was incubated at room temperature for one hour, and absorbance was read on a Beckman Spectrophotometer at 340 nm. Concentration of ethanol in the blood samples was determined from linear regression analysis of the standard curve.
Verification of Cannula Placement
After behavioral testing was completed, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.), and bilateral injections of India ink (25% v/v) were administered with the same injection parameters used for drug injection (see above). Ten to twenty minutes following the injection, animals were perfused via intracardiac delivery of perfusion buffer (0.25 M EDTA, 0.1M phosphate buffered saline, and 9.1 mM NaNO2) followed by 10% formalin in 0.05M phosphate buffered saline. Brains were removed, blocked, and placed in a sucrose solution (3.5% sucrose in 0.1 M sodium phosphate buffer) for 48 hours. Brains were sectioned on a Micron HM560 Cryostat (Micron, Walldorf Germany) at 30μm with blade and chuck temperature set at −14°C. Sections were then thaw mounted on gelatin subbed glass microscope slides and stored at −80°C until further processing. Sections were stained using an acetylcholinesterase staining protocol modified from Hedreen et al (1985) (Hedreen et al. 1985). Briefly, sections were brought to room temperature and placed in a staining solution of 0.2M Tris maleate pH 7.5, 0.1M sodium citrate, 0.03M cupric sulfate, 5 mM potassium ferricyanide, and 25 mg acetylthiocholine iodide for 75 minutes. Following the incubation sections were dipped in deionized H2O, 70% ethanol, and cleared in Xylenes (Fisher Scientific, Fair Lawn, NJ). Sections were then coverslipped with Permount (Fisher Scientific, St. Louis, MO), and guide cannula and injector tip placement were determined and transcribed to corresponding Paxinos & Watson (1997) brain atlas plates (Paxinos and Watson 1997). Rats that did not have accurate bilateral cannula placement were excluded from analysis. Light microscope pictures of representative cannula tip placement are shown in Figure 1-d, e, & f.
Figure 1.

Photomicrograph of opioid receptor antagonist spread in the BLA (Panel a, b, and c), and placement into BLA or CeA (Panel d, e, and f). Panel ‘a’ is the acetylcholinesterase (AChE) stained horizontal section from which spread was determined corresponds with plate 90 in the Paxinos & Watson atlas (Paxinos and Watson 1997). The arrow in panel ‘a’ indicates a mark left by the injector cannula. Panel ‘b’ is a micrograph of the autoradiogram produced by exposing the coronal section in panel ‘a’ to Kodak BioMax MR film, the inset is a picture of the spread without color assigned to the density. Plate ‘c’ is a photomicrograph taken with the film of spread (Panel ‘b’) laid under the AChE stained section (panel ‘a’). Panel ‘d’ is a low magnification picture of India ink injection against AChE staining showing bilateral placement in the CeA. Panel ‘e’ and ‘f’ are higher magnification pictures of cannula placement into the CeA and BLA, respectively. The black bars in all micrographs represent 1mm.
Determination of Drug Spread
In order to determine the extent of opioid antagonist spread, the cannula were used to deliver a radiolabled version of the δ-receptor antagonist naltrindole in some subjects. Tritiated naltrindole (35 Ci/mmol, 1 mCi/ml; Perkin Elmer, Boston, MA) was diluted to a final concentration of 25 nCi/μl in 0.9% sterile saline. After behavioral testing was completed, rats were deeply anesthetized with sodium pentobarbitol (100 mg/kg, i.p.), and bilateral injections of 25 nCi/μl were administered with the same injection parameters used for drug injection (see above). Ten minutes following the injection, animals were perfused as described above. Brains were removed, blocked, and placed in a sucrose solution (3.5% sucrose in 0.1 M sodium phosphate buffer) for 48 hours. Coronal or Horizontal sections were collected (as described above) and stored at −80°C until further processing. Sections were warmed to room temperature and allowed to dry under a cool stream of air before being exposed to autoradiographic film (Kodak BioMax MR, Rochester, NY) for three weeks at −80°C. Drug spread occurred in an approximately spherical distribution with a diameter, in any plane, of approximately 0.7 mm (Figure 1-a, b, & c).
Statistical Analysis
All dependent variables (behavioral responses) in were analyzed by a two-way Analysis of Variance (ANOVA). The independent variable of systemic drug consisted of two levels (drug or vehicle). The independent variable of amygdalar microinjection consisted of two levels (drug or vehicle). Tukey’s honestly significant difference (HSD) was used for post-hoc analysis to determine differences among individual groups, but only when significant interactions were found. Blood alcohol levels were analyzed using a t-test. For all analyses statistical significance was set at p < 0.05. Graphs represent mean ± S.E.M. and Tables report mean ± S.D
Results
Experiment 1: Role of amygdalar opioid receptors in regulating the anxiolytic properties of diazepam in the elevated plus maze
This experiment evaluated the role of CeA versus BLA opioid receptors in regulating the anxiolytic properties of diazepam (See Figures 2 & 3). Elevated plus maze behavior was assessed after amygdalar microinjection of the non-selective opioid receptor antagonist naltrexone (15 μg) and systemic administration of diazepam (2 mg/kg, i.p.).
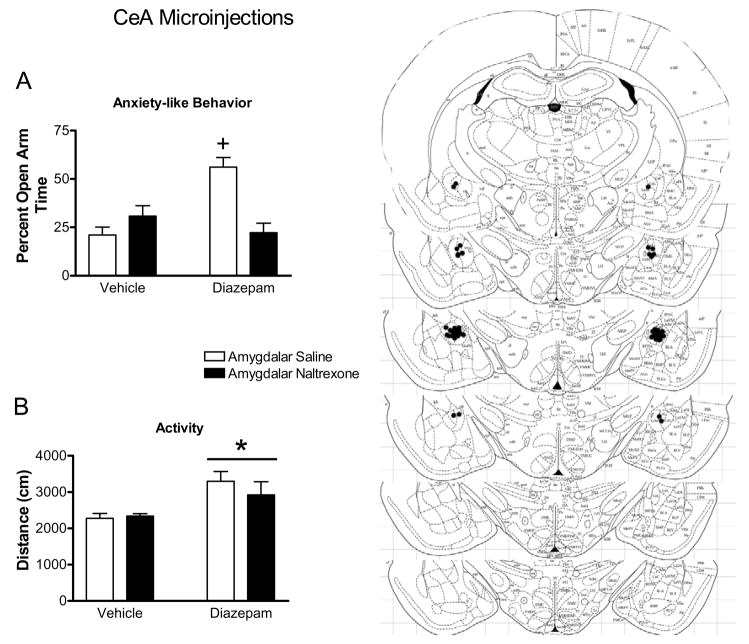
Figure 2.
Effect of naltrexone microinjection into the CEA on the responses to diazepam in the elevated plus maze. Diazepam increased percent open arm time and entries in CeA injected rats (indicated by cross, panels ‘a’ and ‘b’, respectively). Microinjection of naltrexone into the CeA decreased percent open arm time and entries to control levels (panels ‘a’ and ‘b’, respectively). Diazepam increased the total distance traveled (panel ‘c’) in the elevated plus maze (indicated by bar with asterisk) in CeA injected rats. Panel ‘d’ represents plates −2.12 to −3.30 from Bregma (Paxinos and Watson 1997) showing placement of injection cannula tips in the CeA. All bars represent mean ± SEM of n=10–13 rats.
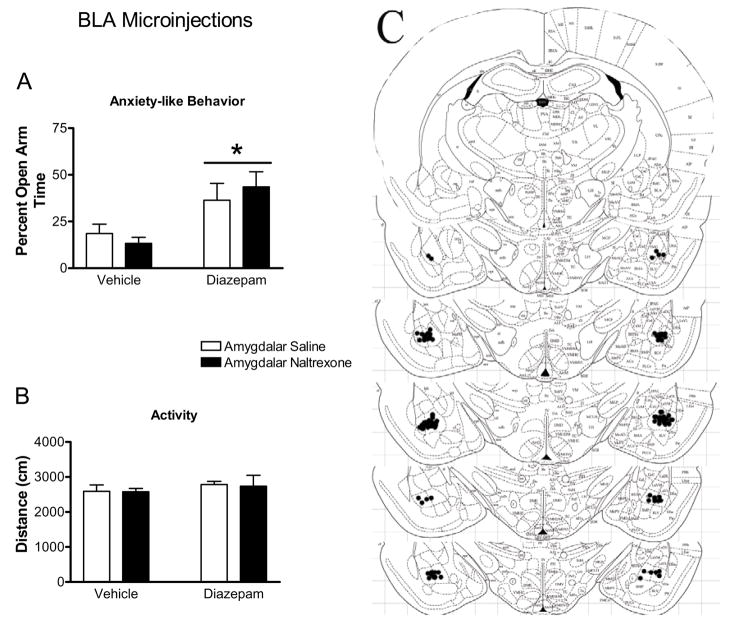
Figure 3.
Effect of naltrexone microinjection into the BLA on the responses to diazepam in the elevated plus maze. Diazepam increased percent open arm time and entries (indicated by bars with asterisks, panels ‘a’ and ‘b’, respectively) in BLA-injected rats. Microinjection of naltrexone into the BLA failed to affect percent open arm time and entries or the distance traveled (panel ‘c’) in the elevated plus maze. Panel ‘d’ represents plates adapted from Paxinos and Watson (1997) representing −2.12 to −3.30 from Bregma. These plates show placement of injection cannula tips in the BLA. All bars represent mean ± SEM of n=10–13 rats.
Microinjection of Naltrexone into the Central Nucleus of the Amygdala
As seen in Figure 2, diazepam increased open arm behavior in the elevated plus maze. Further, naltrexone microinjection into the CeA blocked the increase in open arm behavior seen with diazepam. Rats receiving microinjections of saline and systemic diazepam had a greater percent open arm time and entries compared to all other groups. Rats receiving amygdalar microinjection of naltrexone and systemic diazepam, however, did not differ from control groups, indicating that microinjection of naltrexone into the CeA blocks the anxiolytic properties of diazepam in the elevated plus maze. This result is supported by a statistically significant interaction between systemic diazepam injection and CeA microinjection for percent open arm time (F1,38=20.92, p<0.0001; Figure 2a) and percent open arm entries (F1,38=16.08, p=0.0003; Figure 2b). Post-hoc analysis indicated that groups receiving diazepam injection and CeA microinjection of saline showed increased levels of percent open arm time and entries compared to all other groups (p< 0.05 for all individual comparisons). Systemic delivery of diazepam also increased the distance traveled in the elevated plus maze (F1,38=14.33, p=0.0005; Figure 2c), but microinjection of naltrexone into the CeA had no effect on this index of activity (F1,38=0.15, p>0.7). Further, no interaction was found between systemic diazepam administration and microinjection of naltrexone on the total distance traveled in the plus maze (F1,38=1.77, p>0.1).
Microinjection of Naltrexone into the Basolateral Amygdala
As seen in Figure 3, microinjection of naltrexone into the BLA had no effect on diazepam-induced increases in open arm behavior. Systemic delivery of diazepam significantly increased percent open arm time (F1,41=11.65, p=0.0015; Figure 3a) and percent open arm entries (F1,41=11.89, p=0.0013, Figure 3b). Amygdalar microinjection of naltrexone into the BLA had no effect on percent open arm time (F1,41=0.05, p>0.8), percent open arm entries (F1,41=0.30, p>0.5), or the distance traveled in the elevated plus maze (Figure 3c), and no significant interactions were found between systemic diazepam administration and microinjection of naltrexone into the BLA in any measure (P>0.1), Neither diazepam administration (F1,41=0.88, p>0.3) nor naltrexone microinjection in the BLA (F1,41=0.04, p>0.8) altered the total distance traveled in the elevated plus maze.
Experiment 2: Role of amygdalar opioid receptors in regulating the anxiolytic properties of ethanol in the elevated plus maze
In this experiment elevated plus maze behavior was assessed after bilateral amygdalar microinjection of the non-selective opioid receptor antagonist naltrexone (15 μg) and systemic administration of ethanol (1 g/kg, i.p.) or saline (See Figures 4 & 5). No difference in blood alcohol level was found between groups of rats (t(38)=0.5, p=0.6) receiving microinjection of naltrexone (106 ± 24 mg%) or saline(100 ± 20 mg%).
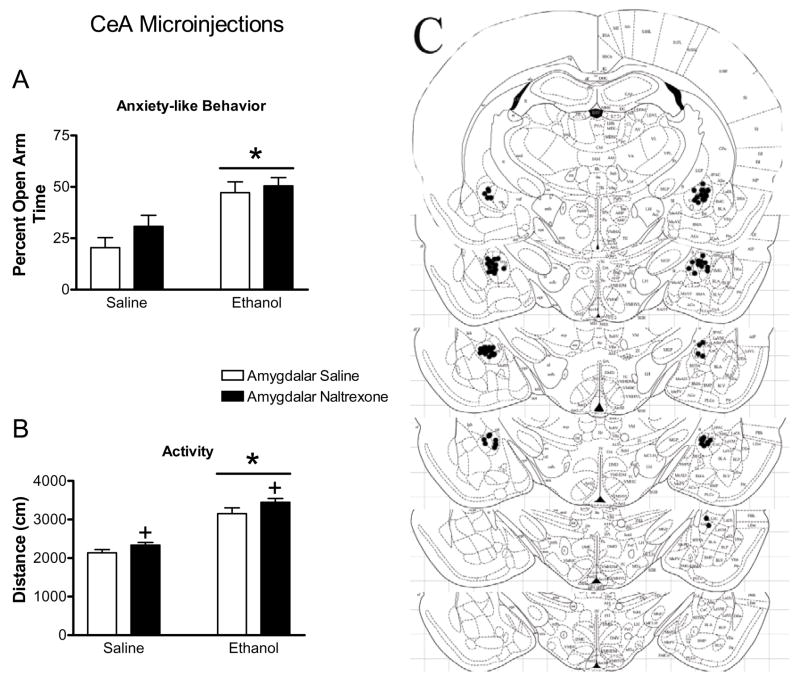
Figure 4.
Effect of naltrexone microinjection into the CEA on the responses to ethanol in the elevated plus maze. Ethanol increased percent open arm time and entries (indicated by bars with asterisks, panel ‘a’ and ‘b’, respectively) in CeA injected rats. Ethanol also increased the total distance traveled in the elevated plus maze (indicated by bars with asterisks, panel ‘c’) in BLA-injected rats. Microinjection of naltrexone into the CeA increased distance traveled regardless of systemic drug treatment (indicated by cross). Panel ‘d’ represents plates adapted from Paxinos and Watson (1997) for representing −2.12 to −3.30 from Bregma. These plates show placement of injection cannula tips in the CeA. All bars represent mean ± SEM of n=9–10 rats.
Figure 5.
Effects of naltrexone microinjection into the BLA on the responses to ethanol in the elevated plus maze. Ethanol increased percent open arm time and entries (indicated by bars with asterisks in panels ‘a’ and ‘b’, respectively) in BLA-injected rats. Microinjection of naltrexone into the BLA decreased percent open arm (panel ‘a’) time regardless of systemic drug treatment (indicated by cross). Ethanol increased the total distance traveled in the elevated plus maze (panel ‘c’, indicated by bars with asterisks) in BLA-injected rats. Panel ‘d’ represents plates adapted from Paxinos and Watson (1997) for representing −2.12 to −3.30 from Bregma. These plates show placement of injection cannula tips in the BLA. All bars represent mean ± SEM of n=9–10 rats.
Microinjection of Naltrexone into the Central Nucleus of the Amygdala
As seen in Figure 4, ethanol increased open arm behavior and activity in the elevated plus maze, but microinjection of naltrexone into the CeA failed to attenuate the anxiolytic actions of ethanol. Systemic ethanol injections significantly increased percent open arm time (F1,36=22.28, p<0.0001; Figure 4a) and percent open arm entries (F1,36=17.83, p=0.0002; Figure 4b). There was no effect of naltrexone delivered to the CeA on percent open arm time (F1,36=1.93, p>0.1) or percent open arm entries (F1,36=3.62, p=0.07), and no interactions between ethanol administration and naltrexone microinjection into the CeA were observed in any plus maze measure (p>0.1). Both ethanol (F1,36=99.58, p<0.0001, Figure 4c) and microinjection of naltrexone into the CeA (F1,36=5.47, p=0.025) increased the distance traveled in the elevated plus maze.
Microinjection of Naltrexone into the Basolateral Amygdala
As seen in Figure 5, microinjection of naltrexone into the BLA decreased open decreased percent open arm time (F1,34=4.78, p=0.0343; Figure 5a) regardless of systemic ethanol administration. Post-hoc analysis, however, showed no statistically significant difference between rats receiving saline or naltrexone microinjections and systemic ethanol for percent open arm time. Microinjection of naltrexone into the BLA had no effect on percent open arm entries (F1,34=2.96, p>0.09; Figure 5b). Although systemic ethanol administration significantly increased percent open arm time (F1,34=17.6, p=0.0002) and percent open arm entries (F1,34=11.33, p=0.0019), no interactions between systemic ethanol administration and microinjection of naltrexone into the BLA was seen for percent open arm time (F1,34=0.48, p>0.4). This finding supports the suggestion that BLA naltrexone injections decreased open arm time, but did not attenuate the anxiolytic actions of ethanol. Ethanol also slightly increased the total distance traveled in the elevated plus maze (F1,34=22.64, p<0.0001; Figure 5c), but naltrexone had no effect on this measure of activity in the plus maze (F1,34=0.76, p>0.3).
Experiment 3: Effect of amygdalar inactivation with lidocaine on the anxiolytic properties of diazepam
This experiment determined the role of the BLA and CeA in regulating the anxiolytic properties of diazepam and addressed the specificity of the naltrexone microinjection into the CeA to block the anxiolytic properties of diazepam. Elevated plus maze behavior was assessed after CeA or BLA inactivation by lidocaine microinjection (10 μg) and systemic administration of diazepam (2 mg/kg, i.p.) or vehicle (See Figures 6 & 7).
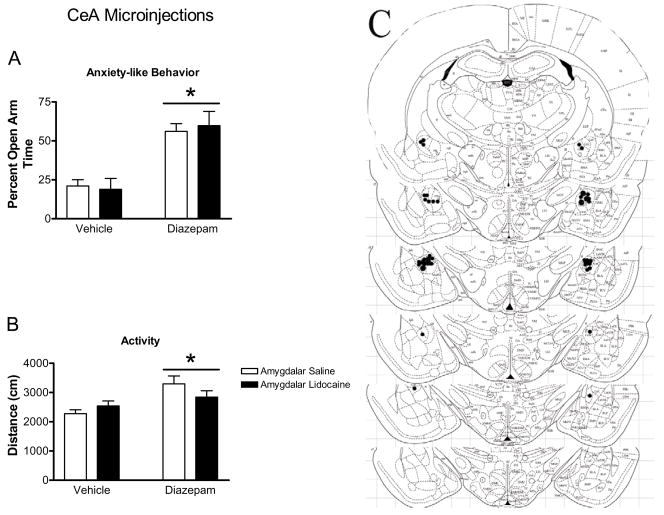
Figure 6.
Effect of CEA inactivation with lidocaine on the properties of diazepam in the elevated plus maze. Diazepam increased percent open arm time and entries (indicated by bars with asterisks panels ‘a’ and ‘b’, respectively) and the distance traveled (panel ‘c’) in the elevated plus maze by CeA injected rats. Microinjection of lidocaine into the CeA failed to affect the anxiolytic properties of diazepam. Panel ‘d’ represents plates adapted from Paxinos and Watson (1997) for representing −2.12 to −3.30 from Bregma. These plates show placement of injection cannula tips in the CeA. All bars represent mean ± SEM of n=4–13 rats.
Figure 7.
Effect of BLA inactivation with lidocaine on the properties of diazepam in the elevated plus maze. Diazepam increased percent open arm time and entries (indicated by bars with asterisks, panel ‘a’ and ‘b’, respectively) in BLA-injected rats. Microinjection of lidocaine into the BLA failed to affect the anxiolytic properties of diazepam. Panel ‘d’ represents plates adapted from Paxinos and Watson (1997) for representing −2.12 to −3.30 from Bregma. These plates show placement of injection cannula tips in the BLA. All bars represent mean ± SEM of n=4–13 rats.
Microinjection of Lidocaine into the Central Nucleus of the Amygdala
As seen in Figure 6, CeA inactivation using lidocaine microinjection did not alter the increase in open arm behavior caused by systemic diazepam administration. Systemic delivery of diazepam significantly increased percent open arm time (F1,30=38.39, p<0.0001; Figure 6a), percent open arm entries (F1,30=32.23, p=0.0001; Figure 6b), and total distance traveled in the elevated plus maze (F1,30=6.36, p=0.0172, Figure 6c). Microinjection of lidocaine into the CeA had no effect on percent open arm time (F1,30=0.02, p>0.8), percent open arm entries (F1,30=0.06, p>0.6), or total distance traveled (F1,30=0.13, p>0.7) in the elevated plus maze, and no significant interactions were found between systemic diazepam administration and lidocaine microinjection into the CeA for any measure (Figure 6a–c).
Microinjection of Lidocaine into the Basolateral Amygdala
As seen in Figure 7, inactivation of the BLA by lidocaine microinjection did not alter the increase in open arm behavior caused by systemic diazepam administration. Systemic diazepam administration significantly increased percent open arm time (F1,26=11.10, p=0.0026; Figure 7a), and percent open arm entries (F1,26=9.29, p=0.0052; Figure 7b), but had no effect on the total distance traveled in the elevated plus maze (F1,26=1.12, p>0.2; Figure 7c). Microinjection of lidocaine into the BLA had no effect on percent open arm time (F1,26=0.04, p>0.8), percent open arm entries (F1,26=0.12, p>0.7), or distance traveled (F1,26=0.00, p>0.9), and no significant interactions between diazepam and lidocaine injections were seen for these measures. (p>0.05).
Discussion
Both diazepam and ethanol show anxiolytic properties in the elevated plus maze. The present study indicates that a non-selective opioid receptor antagonist delivered to the CeA, but not BLA, blocks the anxiolytic properties of diazepam in the elevated plus maze. Interestingly, these results suggest that opioid receptors in the BLA and CeA are not involved in regulating the anxiolytic effects of ethanol in the plus maze. Further, the reversible inactivation of the CeA or BLA does not attenuate the anxiolytic properties of diazepam.
Microinjection of naltrexone into the CeA blocked the anxiolytic properties of diazepam in the elevated plus maze. This supports the concept that the effects of anxiolytic drugs are localized to, or at least regulated by, the amygdala (Pesold and Treit 1995; Killcross et al. 1997; Kang et al. 2000; Wilson et al. 2003). It also supports the finding that systemic administration of opioid antagonists inhibits the anxiolytic properties of benzodiazepines in an anticonflict paradigm (Agmo et al. 1995; Yadin et al. 1991) and in the elevated plus maze (Agmo and Belzung 1998). Although opioid receptor knockout animals commonly show changes in baseline behavior (Filliol et al. 2000; Sasaki et al. 2002), microinjection of naltrexone into the CeA in this set of experiments had no effect on baseline anxiety-related behaviors. These results indicate that the effects of naltrexone delivered into the CeA are selectively regulating the anxiolytic effects of diazepam.
In these experiments lidocaine microinjection (10 μg) failed to affect behavior in the elevated plus maze. This lack of effect was surprising and at odds with the second hypothesis of this study. This dose of lidocaine was chosen because it (Vazdarjanova et al. 1999) and smaller doses (Helmstetter et al., 1992; Manning et al. 1995) were effective in altering behavior when microinjected into amygdalar nuclei. Additional studies (Burghardt et al., unpublished) using this dose and timing of lidocaine injections suggest that this regimen effectively decreases neuronal activity. First, a separate set of rats with cannula aimed at the CeA were trained in a context conditioned freezing paradigm as described by (Fanselow 1980) one week after plus maze testing. Twenty-four hours after receiving the context-footshock pairing, rats received microinjection of lidocaine into the CeA; no systemic drugs were used in these studies. Ten minutes after lidocaine microinjection the rats were returned to the context and freezing behavior was assessed. Rats that received lidocaine microinjection froze significantly less (34.7 ± 7.8%) than rats that received microinjection of saline (58.4 ± 2.9%) into the CeA (t6=3.43). In addition, microinjection of lidocaine (10 μg) into the BLA significantly altered open field behavior in diazepam-treated rats (F(1, 26)=4.73, p<0.04; unpublished). The ability of lidocaine microinjection to alter open field behavior and contextually conditioned freezing indicate that this was an effective dose and timecourse for inactivation of the BLA and CeA.
In previous studies, lesions of the amygdala have failed to alter behavior in the elevated plus maze or other tests of novelty (McHugh et al. 2004; Treit et al. 1993b; Treit et al. 1993a). Similarly in this set of experiments, lidocaine inactivation of the CeA or BLA did not attenuate the anxiolytic properties of diazepam or baseline anxiety-like behaviors in the plus maze. Although this is in agreement with other literature, it is at odds with the idea that the anxiolytic actions of these drugs are localized to amygdalar nuclei. Lesion techniques, however, induce permanent destruction of a large areas encompassing much of the amygdaloid complex (Treit et al. 1993b; Treit et al. 1993a; McHugh et al. 2004). They also require animals to recover from surgery for several days after the lesion is introduced, potentially allowing for neural adaptations. It is possible that the lack of effect of lidocaine microinjection on diazepam’s anxiolytic properties in the current set of experiments is due to the inactivation of all neuronal activity in the region. In contrast, the use of a receptor antagonist that selectively affects the opioid receptor system within the CeA was effective in blocking the anxiolytic properties of diazepam. Since the CeA is a point of integration for efferent information, complete inactivation may mask any regulatory role of the CeA when an anxiolytic compound has been administered. It also suggests diazepam’s effects are not localized solely to the CeA, and that other constituents of the brain’s emotional system, such as the bed nucleus of the stria terminalis (Walker et al. 2003), lateral septum (Menard and Treit 2000), hippocampus (Treit and Menard 1997), or even other amygdalar subnuclei, work in parallel with the CeA and are responsible for the anxiolytic effects of diazepam when the CeA is inactivated (Pitkanen et al. 1997; Menard and Treit 1999). Others have similarly shown that amygdala lesions do not eliminate the anxiolytic effects of the benzodiazepines (Yadin et al. 1991). Since local inactivation of the CeA had no effect on the anxiolytic properties of diazepam, the results suggest that a complex circuitry may be involved in the regulation of benzodiazepine effects by opioid receptor systems in the CeA. The results also crucially indicate that the effects of naltrexone were not related to a non-selective inactivation of the CeA. Recent studies have also demonstrated divergent roles of the CeA and BLA in conditioned responding using electrolytic versus axon-sparing lesion techniques (Koo et al. 2004), indicating the technical approach used in these lesion studies is critical to understanding the role of amygdalar nuclei in anxiety-related responses.
Our results indicate an essential opioidergic mechanism within the CeA, and perhaps a complex amygdalar circuitry, underlies the anxiolytic effects of benzodiazepines. Previous studies looked solely at the direct effects of benzodiazepines in each region. Although opioid receptor mRNA (Mansour et al. 1995a) and benzodiazepine binding (Niehoff and Kuhar 1983) have relatively higher levels in the BLA compared to CeA, μ-opioid and δ-opioid receptor immunoreactivity is found in the CeA (Wilson et al. 2002). The colocalization of opioid receptors and benzodiazepine receptors has not been determined and will be integral to explaining the ability of opioid receptors in the CeA to regulate the anxiolytic properties of diazepam. Further, the intercalated cell masses lie between the medial border of the BLA and the lateral border of the capsular region of the CeA (McDonald 1998). The potential for drug spread into these nuclei, that heavily express μ-opioid receptors (Wilson et al. 2002), cannot be ruled out. This is of particular importance since the intercalated nuclei contain GABAergic cell bodies that are believed to gate the flow of information from the BLA to the CeA (Royer et al. 1999). The specific role of opioid receptors subtypes within the CeA in modulating the anxiolytic properties of diazepam remains uncertain, but may help clarify the mechanistic basis for this effect of naltrexone.
Since benzodiazepines are known to enhance GABAA receptor function (as reviewed by (Wilson 1996)) and have specific binding sites on GABA receptors the interaction between opioid systems and benzodiazepines could involve opioid-induced alterations in GABAergic function within the CeA. One possibility is that opioid peptides serve to modulate inhibitory interneurons in the CeA, since GABA release from presynaptic terminals is regulated by opioid systems in the hippocampus (Cohen et al. 1992) and the lateral amygdala (Sugita and North 1993). Therefore, enhanced GABAergic receptor function in the CeA caused by systemic administration of diazepam could be regulated by opioid receptors in several complex ways. One possibility is that opioid receptors found on GABAergic terminals in CeA (originating in the intercalated cell masses) could regulate the inhibition of interneurons within the CeA. Since opioid receptors are linked to G-proteins that generally exert inhibitory control on the cell (for review see (Minami and Satoh, 1995)), microinjection of naltrexone into the CeA could enhance GABA release from intercalated terminals by relieving opioid receptor-mediated inhibition on these terminals. This enhanced GABA release would inhibit the interneurons of the CeA allowing for increased activity of projection neurons leaving the CeA. In contrast, lidocaine microinjection would not only inhibit the interneurons of the CeA, but also the neurons with cell bodies residing in the CeA and fibers of passage through the CeA. Thus, lidocaine injections might produce different changes in neuronal activity in CeA output compared with those induced by selective receptor antagonists. It should be noted, however, that several variations on this hypothetical circuit are possible since opioid receptors can exert their function pre-synaptically by μ- and δ-opioid receptors (Mansour et al., 1995b;Wilson et al., 2002), or post-synaptically by μ-opioid receptors (Mansour et al., 1995a). Opioid receptors could also regulate the phosphorylation of NMDA and GABAA receptors (Xie and Lewis, 1997;Brandon et al., 2000). Whether opioid-mediated phosphorylation results in decreased or increased GABA-activated currents, is contingent upon the GABAA receptor subunit composition (McDonald et al., 1998). It may also be that opioid receptors modulate the balance of excitatory/inhibitory inputs that converge on a given cell, since it was recently shown that opioids modulate both inhibitory and excitatory neurotransmission in the CeA (Zhu and Pan, 2004).
Taken together the results of these experiments suggest that there may be an essential opioidergic-GABAergic interaction in the CeA involved in regulating the behavioral responses to diazepam in the elevated plus maze. The exact nature of this regulation, however, will likely involve a complex circuitry that will require both anatomical and electrophysiological studies to thoroughly describe.
Microinjection of naltrexone and lidocaine into the BLA failed to affect the anxiolytic properties of diazepam in the elevated plus maze, although naltrexone administration into this area increased open arm avoidance. Thus, diazepam effects in the plus maze were not affected by amygdalar inactivation, but there appears to be a role for opioid receptor systems within the BLA in baseline anxiety-related behavioral responses in the elevated plus maze. This finding also explains our earlier results using larger amygdalar injection volumes of naltrexone, which demonstrated attenuation of the anxiolytic effects of ethanol in the plus maze (Wilson et al. 2003). This inhibition was likely the result of a reduction in overall open arm behaviors, rather than a selective inhibition of ethanol’s ability to enhance open arm time. These results using the plus maze are interesting, since benzodiazepine and GABAA receptor antagonists microinjected into the BLA block the anxiolytic effects of chlordiazepoxide in the social interaction test (Sanders and Shekhar 1995). Further, pharmacological interventions in the CeA produce divergent blockade of stress-induced changes in anxiety-related behaviors using the elevated plus maze and social interaction tests (Cecchi et al. 2002). This reinforces the notion that amygdalar nuclei appear to play distinct roles in the anxiolytic actions of drugs depending upon the targeted region and the paradigm utilized to assess anxiety-related behaviors.
Although the anxiolytic effects of diazepam appear to involve amygdalar processes (current study, and (Kang et al. 2000; Sanders and Shekhar 1995)), microinjection of naltrexone into the BLA or CeA did not affect the anxiolytic properties of ethanol in the elevated plus maze. These results suggest that the anxiolytic properties of ethanol in the elevated plus maze are not regulated by opioid receptor systems within central or basolateral nuclei of the amygdala. This is surprising since ethanol increases GABA release, GABAA receptor function (Roberto et al. 2003), GABAergic neurotransmission (Nie et al. 2004), and c-fos expression in GABAergic neurons (Morales et al. 1998) in the CeA. These results are also somewhat discrepant with previous reports from this lab (Wilson et al 2003), however some important technical differences between the previous report and the current study warrant discussion. In the current experiment the total dose and injection volume of naltrexone delivered was smaller (0.3 μl) compared to previous work (1.0 μl used in Wilson et al, 2002). The larger dose in combination with a significantly larger injection volume could explain the discrepancy between the previous and current results, since it is likely that this previous injection paradigm affected both CeA and BLA. Further, the previous report with ethanol did not have the full range of control groups. Specifically a control group that received systemic saline and the large volume (1 μl) microinjection of naltrexone would have indicated that the effects of naltrexone on ethanol’s anxiolytic properties were actually a general effect of the naltrexone microinjection into the amygdala, rather than a specific attenuation of ethanol effects.
Another potential problem is that ethanol is known to affect several neurotransmitter systems including GABA (Roberto et al., 2003), glutamate (Lovinger et al., 1989), neuroactive steroids (Sanna et al., 2004), and a variety of neuropeptides including opioids (de Gortari et al., 2000) and CRF (Nie et al., 2004). Therefore even if opioid receptors modulate GABAA receptor function in the amygdala, the ability of ethanol to affect other neurotransmitter and neuromodulatory systems may be sufficient to circumvent any opioid receptor effects on GABAA receptor function localized selectively within the CeA or BLA.
At the doses used in this set of experiments both ethanol (1 g/kg) and diazepam (2 mg/kg) slightly increased or had no effect on activity levels in the elevated plus maze. This indicates that these doses were not sedative, and is similar to results seen in other studies (Boerngen-Lacerda and Souza-Formigoni 2000; Wilson et al. 2004). Interestingly, the increases in activity were somewhat variable, and it appeared that systemic ethanol caused a larger increase in activity when administered to rats with cannula aimed at the BLA compared to rats with cannula aimed at the CeA. This does not appear to be a generalized effect of BLA cannulation, since diazepam increased activity in CeA-cannulated rats, but not BLA-cannulated rats. Although the reason for the discrepant results on activity measures in this test are unclear, most critically these results indicate that these drug doses were anxiolytic without sedation, and that naltrexone microinjection into the CeA and BLA had no effect on activity-related measures in the plus maze.
Conclusions
These experiments demonstrate a critical role for opioid receptor systems of the central nucleus of the amygdala in regulating the anxiolytic properties of diazepam, but not ethanol. Further, it seems that opioid receptor function in the BLA plays a subtle role in behavioral responses in the elevated plus maze, but not the anxiolytic effects of ethanol or diazepam. These effects are specific to anxiety-like behavior (BLA) and the anxiolytic effects of diazepam (CEA), since microinjection of naltrexone did not affect activity levels. It appears that the anxiolytic effects of ethanol and diazepam are regulated by distinct mechanisms within the amygdala, despite their similar influences on the GABAA receptor system.
Acknowledgments
This work was supported by RO1 MH63344 from the National Institutes of Health to M.A. Wilson
Reference List
- 1.Agmo A, Belzung C. The role of subtypes of the opioid receptor in the anxiolytic action of chlordiazepoxide. Neuropharmacology. 1998;37:223–232. doi: 10.1016/s0028-3908(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 2.Agmo A, Galvan A, Heredia A, Morales M. Naloxone blocks the antianxiety but not the motor effects of benzodiazepines and pentobarbital: experimental studies and literature review. Psychopharmacology (Berl) 1995;120:186–194. doi: 10.1007/BF02246192. [DOI] [PubMed] [Google Scholar]
- 3.Belzung C, Agmo A. Naloxone potentiates the effects of subeffective doses of anxiolytic agents in mice. Eur J Pharmacol. 1997;323:133–136. doi: 10.1016/s0014-2999(97)00142-8. [DOI] [PubMed] [Google Scholar]
- 4.Boerngen-Lacerda R, Souza-Formigoni ML. Does the increase in locomotion induced by ethanol indicate its stimulant or anxiolytic properties? Pharmacol Biochem Behav. 2000;67:225–232. doi: 10.1016/s0091-3057(00)00360-9. [DOI] [PubMed] [Google Scholar]
- 5.Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, et al. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275:38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- 6.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 7.Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 8.Cohen GA, Doze VA, Madison DV. Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 1992;9:325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- 9.Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 10.de Gortari P, Mendez M, Rodriguez-Keller I, Perez-Martinez L, Joseph-Bravob P. Acute ethanol administration induces changes in TRH and proenkephalin expression in hypothalamic and limbic regions of rat brain. Neurochem Int. 2000;37:483–496. doi: 10.1016/s0197-0186(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 11.Dudek BC, Abbott ME. A biometrical genetic analysis of ethanol response in selectively bred long-sleep and short-sleep mice. Behav Genet. 1984;14:1–19. doi: 10.1007/BF01066065. [DOI] [PubMed] [Google Scholar]
- 12.Duka T, Millan MJ, Ulsamer B, Doenicke A. Naloxone attenuates the anxiolytic action of diazepam in man. Life Sci. 1982;31:1833–1836. doi: 10.1016/0024-3205(82)90222-3. [DOI] [PubMed] [Google Scholar]
- 13.Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- 14.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 15.File SE. The amygdala: anxiety and benzodiazepines. In: Aggleton J, editor. The Amygdala: A functional analysis. Oxford: Oxford University Press; 2000. pp. 195–212. [Google Scholar]
- 16.File SE, Rodgers RJ. Partial anxiolytic action of morphine sulphate following microinjection into the central nucleus of the amygdala in rats. Pharmacol Biochem Behav. 1979;11:313–318. doi: 10.1016/0091-3057(79)90141-2. [DOI] [PubMed] [Google Scholar]
- 17.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 18.Froehlich JC, Harts J, Lumeng L, Li TK. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol Biochem Behav. 1990;35:385–390. doi: 10.1016/0091-3057(90)90174-g. [DOI] [PubMed] [Google Scholar]
- 19.Hedreen JC, Bacon SJ, Price DL. A modified histochemical technique to visualize acetylcholinesterase-containing axons. J Histochem Cytochem. 1985;33:134–140. doi: 10.1177/33.2.2578498. [DOI] [PubMed] [Google Scholar]
- 20.Helmstetter FJ. Contribution of the amygdala to learning and performance of conditional fear. Physiol Behav. 1992;51:1271–1276. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- 21.Ho AK, Rossi N. Suppression of ethanol consumption by MET-enkephalin in rats. J Pharm Pharmacol. 1982;34:118–119. doi: 10.1111/j.2042-7158.1982.tb04199.x. [DOI] [PubMed] [Google Scholar]
- 22.Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 23.Hyytia P, Sinclair JD. Responding for oral ethanol after naloxone treatment by alcohol- preferring AA rats. Alcohol Clin Exp Res. 1993;17:631–636. doi: 10.1111/j.1530-0277.1993.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 24.Kang W, Wilson SP, Wilson MA. Overexpression of proenkephalin in the amygdala potentiates the anxiolytic effects of benzodiazepines. Neuropsychopharmacology. 2000;22:77–88. doi: 10.1016/S0893-133X(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 25.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 26.Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan-Sarin S, Jing SL, Kurtz DL, Zweifel M, Portoghese PS, Li TK, et al. The delta opioid receptor antagonist naltrindole attenuates both alcohol and saccharin intake in rats selectively bred for alcohol preference. Psychopharmacology (Berl) 1995;120:177–185. doi: 10.1007/BF02246191. [DOI] [PubMed] [Google Scholar]
- 28.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 29.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 30.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 31.Macdonald RL. Ethanol, gamma-aminobutyrate type A receptors, and protein kinase C phosphorylation. Proc Natl Acad Sci U S A. 1995;92:3633–3635. doi: 10.1073/pnas.92.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning BH, Mayer DJ. The central nucleus of the amygdala contributes to the production of morphine antinociception in the rat tail-flick test. J Neurosci. 1995;15:8199–8213. doi: 10.1523/JNEUROSCI.15-12-08199.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995a;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 34.Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995b;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- 35.Maren S. What the amygdala does and doesn’t do in aversive learning. Learn Mem. 2003;10:306–308. doi: 10.1101/lm.68403. [DOI] [PubMed] [Google Scholar]
- 36.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 37.McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- 38.McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- 39.Menard J, Treit D. Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci Biobehav Rev. 1999;23:591–613. doi: 10.1016/s0149-7634(98)00056-6. [DOI] [PubMed] [Google Scholar]
- 40.Menard J, Treit D. Intra-septal infusions of excitatory amino acid receptor antagonists have differential effects in two animal models of anxiety. Behav Pharmacol. 2000;11:99–108. doi: 10.1097/00008877-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Minami M, Satoh M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci Res. 1995;23:121–145. doi: 10.1016/0168-0102(95)00933-k. [DOI] [PubMed] [Google Scholar]
- 42.Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- 43.Morales M, Criado JR, Sanna PP, Henriksen SJ, Bloom FE. Acute ethanol induces c-fos immunoreactivity in GABAergic neurons of the central nucleus of the amygdala. Brain Res. 1998;798:333–336. doi: 10.1016/s0006-8993(98)00457-0. [DOI] [PubMed] [Google Scholar]
- 44.Nestoros JN. Ethanol specifically potentiates GABA-mediated neurotransmission in feline cerebral cortex. Science. 1980;209:708–710. doi: 10.1126/science.7394531. [DOI] [PubMed] [Google Scholar]
- 45.Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- 46.Niehoff DL, Kuhar MJ. Benzodiazepine receptors: localization in rat amygdala. J Neurosci. 1983;3:2091–2097. doi: 10.1523/JNEUROSCI.03-10-02091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Malley SS, Krishnan-Sarin S, Farren C, O’Connor PG. Naltrexone-induced nausea in patients treated for alcohol dependence: clinical predictors and evidence for opioid-mediated effects. J Clin Psychopharmacol. 2000;20:69–76. doi: 10.1097/00004714-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The rat brain in steriotaxic coordinates. San Diego: Academic Press; 1997 . [Google Scholar]
- 49.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 50.Pesold C, Treit D. The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res. 1995;671:213–221. doi: 10.1016/0006-8993(94)01318-c. [DOI] [PubMed] [Google Scholar]
- 51.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 52.Pohorecky LA. The interaction of alcohol and stress. A review. Neurosci Biobehav Rev. 1981;5:209–229. doi: 10.1016/0149-7634(81)90003-8. [DOI] [PubMed] [Google Scholar]
- 53.Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanders SK, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry. 1995;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- 56.Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, et al. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasaki K, Fan LW, Tien LT, Ma T, Loh HH, Ho IK. The interaction of morphine and gamma-aminobutyric acid (GABA)ergic systems in anxiolytic behavior: using mu-opioid receptor knockout mice. Brain Res Bull. 2002;57:689–694. doi: 10.1016/s0361-9230(01)00785-7. [DOI] [PubMed] [Google Scholar]
- 58.Sugita S, North RA. Opioid actions on neurons of rat lateral amygdala in vitro. Brain Res. 1993;612:151–155. doi: 10.1016/0006-8993(93)91655-c. [DOI] [PubMed] [Google Scholar]
- 59.Treit D, Menard J. Dissociations among the anxiolytic effects of septal, hippocampal, and amygdaloid lesions. Behav Neurosci. 1997;111:653–658. doi: 10.1037//0735-7044.111.3.653. [DOI] [PubMed] [Google Scholar]
- 60.Treit D, Pesold C, Rotzinger S. Dissociating the anti-fear effects of septal and amygdaloid lesions using two pharmacologically validated models of rat anxiety. Behav Neurosci. 1993a;107:770–785. doi: 10.1037//0735-7044.107.5.770. [DOI] [PubMed] [Google Scholar]
- 61.Treit D, Pesold C, Rotzinger S. Noninteractive effects of diazepam and amygdaloid lesions in two animal models of anxiety. Behav Neurosci. 1993b;107:1099–1105. doi: 10.1037//0735-7044.107.6.1099. [DOI] [PubMed] [Google Scholar]
- 62.Vaccarino AL, Kastin AJ. Endogenous opiates: 2000. Peptides. 2001;22:2257–2328. doi: 10.1016/s0196-9781(01)00566-6. [DOI] [PubMed] [Google Scholar]
- 63.Vazdarjanova A, McGaugh JL. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J Neurosci. 1999;19:6615–6622. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volpicelli JR, Ulm RR, Hopson N. Alcohol drinking in rats during and following morphine injections. Alcohol. 1991;8:289–292. doi: 10.1016/0741-8329(91)90401-h. [DOI] [PubMed] [Google Scholar]
- 65.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 66.Wilson MA. GABA physiology: modulation by benzodiazepines and hormones. Crit Rev Neurobiol. 1996;10:1–37. doi: 10.1615/critrevneurobiol.v10.i1.10. [DOI] [PubMed] [Google Scholar]
- 67.Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD. Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacol Biochem Behav. 2004;78:445–458. doi: 10.1016/j.pbb.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 68.Wilson MA, Burghardt PR, Lugo JN, Jr , Primeaux SD, Wilson SP. Effect of amygdalar opioids on the anxiolytic properties of ethanol. Ann N Y Acad Sci. 2003;985:472–5. doi: 10.1111/j.1749-6632.2003.tb07102.x. [DOI] [PubMed] [Google Scholar]
- 69.Wilson MA, Mascagni F, McDonald AJ. Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci Lett. 2002;328:160–164. doi: 10.1016/s0304-3940(02)00481-0. [DOI] [PubMed] [Google Scholar]
- 70.Xie CW, Lewis DV. Involvement of cAMP-dependent protein kinase in mu-opioid modulation of NMDA-mediated synaptic currents. J Neurophysiol. 1997;78:759–766. doi: 10.1152/jn.1997.78.2.759. [DOI] [PubMed] [Google Scholar]
- 71.Yadin E, Thomas E, Strickland CE, Grishkat HL. Anxiolytic effects of benzodiazepines in amygdala-lesioned rats. Psychopharmacology (Berl) 1991;103:473–479. doi: 10.1007/BF02244247. [DOI] [PubMed] [Google Scholar]
- 72.Zhu W, Pan ZZ. Synaptic properties and postsynaptic opioid effects in rat central amygdala neurons. Neuroscience. 2004;127:871–879. doi: 10.1016/j.neuroscience.2004.05.043. [DOI] [PubMed] [Google Scholar]