Abstract
Background
We tested whether single nucleotide polymorphisms (SNPs) in the PPARα gene (PPARA) are associated with variations in levels of plasma apolipoprotein CIII (apoCIII) levels, as well as other lipids and lipoproteins in African-Americans and Caucasians.
Methods and Results
We initially identified an intronic SNP (rs4253728) in PPARA that was associated with plasma apoCIII level (p<0.05) in a subset of 435 individuals from the total study population (n=944; 335 African Americans and 609 Caucasians). This SNP was then genotyped in a second subset of 476 individuals (total 911 subjects with available data), and a previously described PPARA coding SNP (L162V) which was shown to be in moderate linkage disequilibrium with the intronic SNP (r2=0.18) was genotyped in 928 subjects from the same study population. The minor allele frequencies for both SNPs were significantly lower in African-Americans compared with Caucasians (7.2% vs. 27.3% for rs4253728, 1.5% vs. 6.1% for L162V, both p<0.0001). African-Americans had significantly lower levels of TG and apoCIII compared with Caucasians after adjusting for age, sex, body mass index (BMI), waist circumference and other baseline characteristics. However, racial differences in TG levels were attenuated after adjusting for apoCIII levels. The minor alleles for both PPARA SNPs were associated with higher TG and apoCIII levels. Race modified the associations of L162V with TG (p for interaction=0.0056) and apoCIII (p for interaction=0.0011). Levels of both TG and apoCIII were lower in African-American but not Caucasian homozygotes for the major allele compared with carriers of the minor allele. Similar results were obtained for the intronic SNP, but the findings were no longer significant in a model that also contained L162V.
Conclusions
Two PPARA SNPs, L162V and rs4253728 (intronic), are less prevalent in African-Americans than in Caucasians and in African-Americans only are associated with higher apoCIII and TG levels.
Keywords: PPARα, gene polymorphism, race, apolipoprotein CIII, triglyceride
INTRODUCTION
Peroxisome proliferator activated receptor α (PPARα) regulates the transcription of multiple genes involved in lipid metabolism [1–2]. In vitro, activation of PPAR-α has been associated with increased lipoprotein lipase (LPL) gene transcription [3] and reduced apolipoprotein CIII (apoCIII) gene expression that can lead to increased LPL-mediated catabolism and plasma clearance of very low density lipoproteins (VLDLs) [4–6]. Fibrate drugs, which are agonists for PPARα, lower levels of plasma triglyceride (TG) and increase HDL-cholesterol concentrations in humans [7–10]. Fibrate-induced reductions in levels of apoCIII, mediated at the transcriptional level by a PPARα response element in the apoCIII gene, have been implicated in mediating the TG-lowering response to fibrates [11]. This effect is likely due to inhibition by apoCIII of both VLDL TG lipolysis by lipoprotein lipase and receptor-mediated clearance of VLDL lipolytic remnants [12].
There have been reports that PPARA single nucleotide polymorphisms (SNPs) are associated with blood lipid levels and with progression of coronary artery disease and type 2 diabetes mellitus, but the findings have been inconsistent [13–17]. Moreover, to date most of the studies have been conducted in Caucasian populations. There are no published reports of associations of PPARA SNPs with blood lipids and lipoproteins in black subjects.
In the present study, we sought to determine the existence of PPARA genetic polymorphisms associated with variations in plasma apoCIII levels, as well as other lipids and lipoproteins, in in a group of 944 Caucasian and African-American subjects.
RESEARCH DESIGN AND METHODS
Study population
The total study population consisted of 609 Caucasians and 335 African-Americans aged 30 years or older, with baseline total cholesterol 160 to 400 mg/dL and no use of drugs known to affect lipoprotein metabolism who participated in a trial of simvastatin therapy in the Cholesterol and Atherosclerosis Pharmacogenetics (CAP) substudy of the NIH-funded Pharmacogenomics and Risk of Cardiovascular Disease project [18]. The data for the present report were obtained after a two-week run-in period on placebo. Participants self-reported race/ethnicity. We required that three or more grandparents be reported as African-American or Caucasian for a participant to be classified as African American or Caucasian, respectively. Potential subjects from other racial/ethnic groups were not enrolled. Subjects were recruited from clinical centers in Los Angeles, CA and San Francisco, CA, and informed consent was obtained. The study was approved by local Institutional Review Boards as well as the University of California, San Francisco, University of California, Los Angeles, and Children’s Hospital and Research Center of Oakland. Baseline demographic data included self-reported age, sex, and ethnicity. Participants were asked about smoking status, alcohol consumption, level of physical activity, history of diabetes, hypertension, or coronary heart disease and use of oral estrogen preparations. They underwent a brief physical examination for measurement of seated blood pressure using an electronic manometer. Weight was measured using a standard balance beam scale or an electronic scale and height was measured using a height rod of a standard beam scale or a wall-mounted stadiometer. Body mass index (BMI) was calculated as weight in kg divided by height in meters squared. Waist circumference was measured twice to the nearest 0.1 cm with a flexible tape measure at the level of the minimum circumference, usually at the level of the navel. Blood was collected after overnight fast into tubes containing EDTA, and the plasma was separated and stored at −80°C.
Measurements
Plasma total cholesterol and triglyceride (TG) levels were determined by enzymatic procedures on an Express 550 Plus analyzer (Ciba Corning, Oberlin, OH). These measurements were consistently in control as monitored by the CDC-NHLBI standardization program. HDL cholesterol was measured after dextran sulfate precipitation of plasma [19], and LDL-C was calculated from the formula of Friedewald et al. [20]. Apolipoproteins AI (apoAI) and B (apoB) were measured by immunoturbidometric assay (Express 550 Plus analyzer). Plasma apoCIII concentrations were determined in triplicate by sandwich assay using two polyclonal antibodies (International Immunology Corp., Murrieta, CA). Fasting plasma glucose and insulin were measured and the homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated as the product of [fasting plasma insulin (µU/L) × fasting plasma glucose (mmol/L)] divided by 22.5 [21].
SNP identification and genetic analysis
SNPs within PPARA were identified by genomic sequencing of the entire PPARA gene, with an additional 2.5 Kb upstream and 1.5 Kb downstream, in 24 African-American and 23 Caucasian (specifically European-American) individuals. The sequences from the 47 individuals were assembled with the Phred, Phrap and Consed, and PolyPhred was used to identify sequence variants. The University of California at Santa Cruz Golden Path human genome assembly was used as a reference sequence to identify gene architecture and map identified SNPs. One hundred ninety two polymorphic sites were identified in the PPARA gene and 24 tag SNPs were selected for genotyping using the LD-select algorithm. Genotypes for these SNPs were obtained in 435 of the first 458 subjects enrolled in CAP (179 African Americans and 256 Caucasians) by Illumina (San Diego, CA) using BeadArray™ technology. Among these SNPS, one intronic SNP (rs4253728) was selected to test for replication in the remaining 486 subjects in CAP because of its association (p<0.05) with apoCIII after adjustment for age, sex, BMI and race (See supplemental data). Another previously described non-synonymous SNP (L162V, rs1800206) was also genotyped in the entire CAP population. After exclusion of subjects with missing data for these two SNPs, there were a total of 911 individuals with results for rs 4253728, and 928 with results for L162V. The two genotypes were then tested for associations with apoCIII, as well as triglyceride, total and LDL cholesterol, HDL cholesterol, apoB and apoAI in the full study population.
Statistical analysis
We used JMP version 5.0 for Windows (SAS Ins., Chicago, IL, USA) for all statistical analyses. Each variable was examined for normality, and non-normally distributed variables (TG, apoCIII, and HOMA-IR) were log-transformed. Differences in blood lipids and apolipoproteins between racial groups and genotypes were compared using t-tests or Kruskall-Wallis test as appropriate. Chi-squared test was used to compare differences in allele frequency between African-Americans and Caucasians. General linear model and multiple linear regression analysis were used to examine the PPARα genotype effect and interaction effect between genotype and race on blood lipids and apolipoproteins. Tests for Hardy-Weinberg equilibrium and standardized linkage disequilibrium (D’) were determined by using Haploview 3.2 (http://www.broad.mit.edu/mpg/haploview). A p value of less than 0.05 was considered to be statistically significant.
RESULTS
Table 1 displays the baseline characteristics of the full population studied here. Age and sex distribution were similar between African-Americans and Caucasians. As described previously [18], African-Americans had significantly higher BMI and waist circumference, were more likely to be current smokers, and had higher prevalence of hypertension and type 2 diabetes mellitus compared with Caucasians.
Table 1.
Baseline characteristics of the study population
| Caucasians (n=609) | African-Americans (n=335) | p value | |
|---|---|---|---|
| Male/Female | 323/286 | 162/173 | NS |
| Age(years) | 54.6±0.5 | 54.3±0.7 | NS |
| BMI(kg/m2) | 27.7±0.2 | 30.1±0.4 | <0.001 |
| Waist (cm) | 94.5±0.6 | 98.7±0.8 | <0.001 |
| SBP (mmHg) | 123.2±0.7 | 128.5±0.9 | <0.001 |
| DBP (mmHg) | 70.6±0.4 | 73.9±0.5 | <0.001 |
| Hypertension (%) | 70 | 81 | <0.001 |
| Type 2 diabetes mellitus (%) | 2 | 8 | <0.001 |
| Current smoker (%) | 14 | 31 | <0.001 |
| Estrogen use (%)1 | 19 | 10 | 0.02 |
| Physical activity level | 0.04 | ||
| More active than peers | 43 | 42 | |
| Less active than peers | 15 | 21 | |
| About the same | 43 | 37 | |
| Alcohol intake | <0.001 | ||
| None | 27 | 45 | |
| <=2/wk | 45 | 42 | |
| >=3wk | 27 | 13 | |
Means ± S.E. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure
Based on the responses from 459 women
As shown in Table 2, the African-Americans had significantly lower levels of TG, total cholesterol, apoB and apoCIII than the Caucasians. After adjusting for age, sex, BMI, waist circumference and other baseline characteristics including current smoking, exercise level, alcohol consumption, estrogen use, and presence of hypertension and type 2 diabetes mellitus, the African-Americans had significantly higher levels of HDL-cholesterol and apoAI than the Caucasians. After adjusting for plasma apoCIII, the racial differences in apoB and TG levels were no longer significant. However, levels of HDL-cholesterol and apoAI remained significantly higher in the African-Americans compared with the Caucasians after adjusting for plasma apoCIII levels (Table 2).
Table 2.
Comparisons of blood lipids and apolipoproteins between Caucasians and African-Americans
| C (n=609) | AA (n=335) | p value | p value1 | p value2 | |
|---|---|---|---|---|---|
| Triglyceride (mg/dL) | 128.3 ± 2.8 | 109.6 ± 3.7 | <0.001 | <0.001 | NS |
| Total cholesterol (mg/dL) | 210.7 ± 1.5 | 205.9 ± 1.9 | 0.045 | NS | NS |
| LDL-cholesterol (mg/dL) | 131.9 ± 1.3 | 129.4 ± 1.9 | NS | NS | NS |
| HDL-cholesterol (mg/dL) | 53.2 ± 0.7 | 54.8 ± 0.9 | NS | <0.001 | <0.001 |
| ApoAI(mg/dL) | 126.8 ± 1.1 | 129.4 ± 1.5 | NS | <0.001 | <0.001 |
| ApoB (mg/dL) | 95.9 ± 0.9 | 91.7 ± 1.1 | 0.004 | <0.001 | NS |
| HOMA-IR | 3.2 ± 0.1 | 3.7 ± 0.2 | 0.027 | NS | NS |
| ApoCIII (µg/mL) | 13.5 ± 0.2 | 11.4 ± 0.2 | <0.001 | <0.001 | - |
After adjusting for age, sex, BMI, waist, current smoking, exercise, drink, estrogen use and presence of hypertension/type 2 diabetes mellitus
After adjusting age, sex, BMI, waist, current smoking, exercise, drink, estrogen use, presence of hypertension/type 2 diabetes mellitus and apoCIII
Mean ± S.E. C: Caucasians, AA: African-Americans
Table 3 presents the genotype distributions of the two PPARA SNPs. The minor allele frequencies for both SNPs were significantly lower in the African-Americans compared with the Caucasians (1.5% vs. 6.1% for L162V, 7.2% vs. 27.3% for rs4253728, p<0.001). The L162V SNP was not in Hardy-Weinberg equilibrium in the full study population (91.6 % CC, 7.7% GC and 0.6% GG) or in the Caucasians (88.7% CC, 10.3% GC and 1.0% GG). However, the genotype distributions were very similar to those reported in earlier studies [14–17]. In the African-Americans, the genotype distribution was in Hardy-Weinberg equilibrium (96.9% CC, 3.1% GC). The intronic SNP was in Hardy- Weinberg equilibrium in the whole study population (64.5% GG, 30.5% GA and 4.9% AA) and in Caucasians (52.4% GG, 40.7% GA and 6.9% AA). However, in the African- Americans, it was not in Hardy-Weinberg equilibrium (86.9% GG, 11.8% GA and 1.3% AA, p=0.03908). The intronic and coding SNPs were in linkage disequilibrium (D’= 0.965, r2= 0.18, p=0.003).
Table 3.
PPARα genotype distribution of study population
| Whole population (n=928) |
Caucasians (n=602) |
African-Americans (n=326) |
p-value1 | ||||
|---|---|---|---|---|---|---|---|
| L162V (rs1800206) |
CC | 850 | CC | 534 | CC | 316 | <0.001 |
| GC | 72 | GC | 62 | GC | 10 | ||
| GG | 6 | GG | 6 | GG | 0 | ||
|
Whole population (n=911) |
Caucasians (n=590) |
African-Americans (n=321) |
|||||
| Intronic SNP (rs4253728) |
GG | 588 | GG | 309 | GG | 279 | <0.001 |
| GA | 278 | GA | 240 | GA | 38 | ||
| AA | 45 | AA | 41 | AA | 4 | ||
comparison of minor allele frequency between Caucasians and African-Americans using chi-squared test
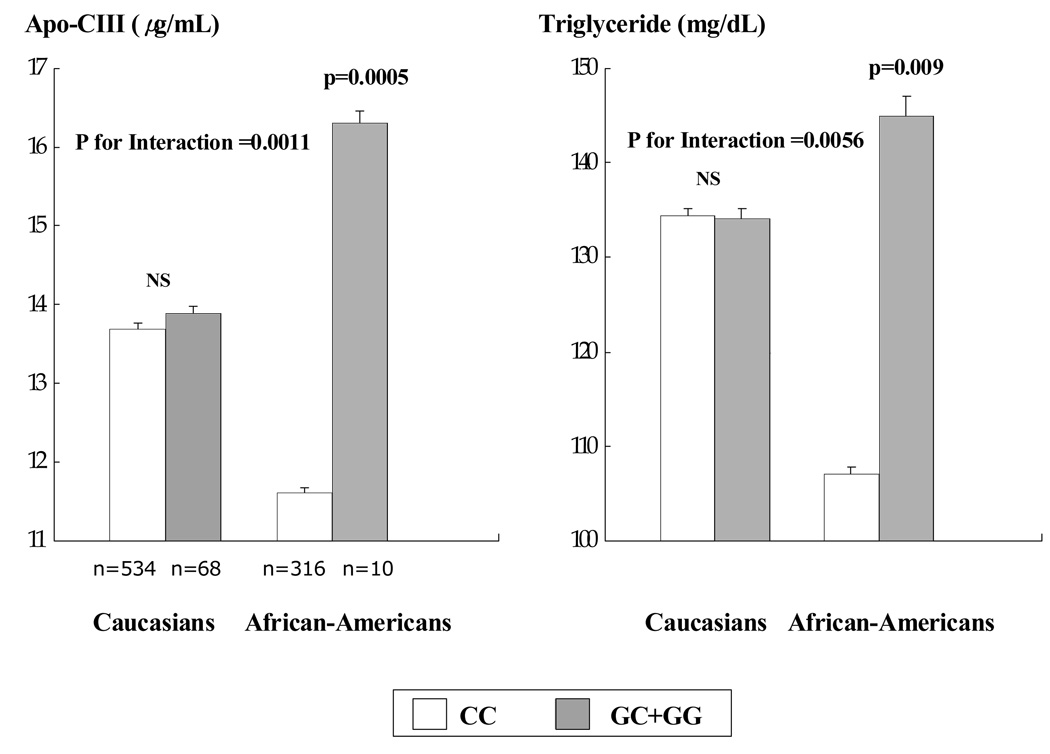
Table 4 displays the associations between the L162V SNP/intronic SNP and blood lipids, lipoproteins, and apoCIII in the entire study population. Plasma levels of apoCIII and TG were significantly lower in L162 homozygotes than in carriers of the V162 allele after adjusting for age, BMI, sex, race, interaction of race with genotype and other baseline characteristics. Levels of apoAI and HDL-cholesterol tended to be higher in the carriers of the V162 allele compared with L162 homozygotes, although the differences did not reach statistical significance. Race modified the genotype effects with TG (p for interaction=0.0056) and apoCIII (p for interaction=0.0011). In African-Americans, the carriers of the V162 allele had higher levels of plasma apoCIII (p=0.0005) and TG (p=0.009) than L162 homozygotes, whereas there were no differences in plasma apoCIII and TG between the genotypes in Caucasians (Fig. 1).
Table 4.
Comparisons of blood lipids and apolipoproteins among PPARA genotypes
| SNP L162V | SNP rs4253728 | |||||
|---|---|---|---|---|---|---|
| CC (n=850) | GC+GG (n=78) | p value | GG (n=588) | GA+AA (n=323) | p value | |
| Triglyceride (mg/dL) | 120.9 ± 6.7 | 139.7 ± 13.2 | 0.024 | 119.9 ± 6.8 | 134.8 ± 8.5 | 0.016 |
| Total cholesterol (mg/dL) | 203.6 ± 3.9 | 210.0 ± 7.9 | NS | 204.3 ± 4.0 | 208.1 ± 4.9 | NS |
| LDL-cholesterol (mg/dL) | 124.5 ± 3.8 | 122.5 ± 7.4 | NS | 125.1 ± 3.8 | 125.8 ± 4.7 | NS |
| HDL-cholesterol (mg/dL) | 55.0 ± 1.5 | 59.6 ± 2.9 | NS | 55.3 ± 1.5 | 55.2 ± 1.9 | NS |
| ApoAI(mg/dL) | 135.2 ± 2.4 | 143.1 ± 4.7 | NS | 135.2 ± 2.5 | 136.7 ± 3.0 | NS |
| ApoB (mg/dL) | 92.1 ± 2.3 | 94.1 ± 4.5 | NS | 92.5 ± 2.3 | 94.9 ± 2.9 | NS |
| ApoCIII (µ/mL) | 12.7 ± 0.5 | 15.1 ± 0.9 | 0.0008 | 12.5 ± 0.5 | 13.7 ± 0.6 | 0.0077 |
Mean ± S.E.
Means were adjusted for age, sex, BMI, waist, race, HOMA-IR, current smoking, exercise, drink, estrogen use, presence of hypertension/type 2 diabetes mellitus and interaction between race and the PPARα SNP
Figure 1. Plasma apo-CIII and TG levels in Caucasians and African-Americans according to the PPARα L162V SNP.
Means ± S.E. Means were adjusted for age, sex, BMI, waist, current smoking, alcohol consumption, exercise levels, estrogen use and presence of hypertension and type 2 diabetes mellitus
For the intronic SNP (rs4253728), plasma levels of apoCIII and TG were significantly lower in GG homozygotes than in the carriers of the A allele after adjusting for age, BMI, sex, race, interaction of race with genotype and other baseline characteristics (Table 4). Again, the association between genotype with TG and apoCIII varied by race (p for interaction=0.01 for TG and 0.03 for apoCIII). In the African-Americans, plasma levels of apoCIII and TG were significantly higher in carriers of the A allele than GG homozygotes (apoCIII: 13.5±0.5 vs. 11.5±0.5 µg/mL, p=0.03; TG: 135.0±7.5 vs. 104.8±7.3 mg/dL, p=0.02), whereas there were no differences in plasma apoCIII and TG among the genotypes in the Caucasians. The associations with the intronic SNP, however, were no longer significant in a model including the L162V SNP. After further adjusting for apoCIII, neither the L162V SNP nor the intronic SNP were associated with plasma levels of TG in the African-Americans.
DISCUSSION
We have found significant associations of plasma levels of apoCIII and TG with two SNPs in the gene for PPARα, a functional leucine 162 to valine (L162V) variant and a novel intronic SNP. These associations however varied by race, suggesting differential regulation by PPARα of apoCIII and TG levels between African-Americans and Caucasians. Specifically, in African-Americans, both L162 homozygotes and GG homozygotes for the intronic SNP had lower levels of plasma apoCIII and TG than carriers of the 162V allele and the A allele of the intronic SNP. On the other hand, there were no differences in plasma apoCIII and TG levels between carriers of these alleles in Caucasians.
The PPARA L162V polymorphism has been reported to alter transcriptional activation of PPARA associated with fibrate treatment in vitro [15, 22]. Flavell et al. [15] reported that V162 PPARα showed greater transactivation of a reporter gene construct compared with L162 PPARα. In that study, however, protein concentrations of both variants were not different after transient transfection as assessed by western blot analysis. There is also evidence that the effect of the L162V polymorphism on the transcriptional activation of PPARα depends on the concentration of the ligand to which it is exposed [22]. It has been reported that PPARα genetic variations contribute to inter-individual variability in total cholesterol [15, 23], LDL-cholesterol [13, 23], HDL-cholesterol [15], apoB [13, 23] and apoAI (15). Despite the prominent role of PPARα in the regulation of proteins involved in TG-rich lipoprotein metabolism, reports of associations between genetic variations in the PPARA and plasma TG and apoCIII concentrations have been inconsistent. In particular, some have found no associations between the L162V polymorphism and plasma levels of TG [13, 15–16]. However, a study by Nielsen et al. [24] found that the V162 allele of PPARα was associated with a decreased level of TG in glucose tolerant white subjects. Tai et al. [25] reported that apoCIII levels were higher in Caucasian carriers of the V162 allele, with a significant interaction between the PPARA L162V polymorphism and polyunsaturated fat (PUFA) intake with respect to plasma levels of apoCIII and TG. As a result, the L162 allele was associated with lower TG and apoCIII levels only in subjects with PUFA fat intake less than 6% of energy. The possibility might therefore be considered that the results of the current study could reflect lower PUFA intake in African-Americans than Caucasians. There is no evidence, however, from the National Health and Nutrition Evaluation Survey (NHANES) that this is the case (Mangravite LM and Krauss RM, unpublished), and detailed dietary information is not available for participants in the present study.
Another possible explanation for our findings is that PPARA effects on apoCIII in the African-American population can be influenced by variations in other genes whose prevalence may differ from those in Caucasians. For example, there is evidence that the PPARA L162V SNP interacts with the G3238G in the apoCIII gene and with the APOE locus in modulating plasma apoCIII concentrations [23]. Also, PPARα has been reported to be regulated by hepatocyte nuclear factor 4α [26] and retinoid X receptor α [27]. It is possible that differing combinations of SNPs in these or other genes in African-Americans vs. Caucasians may be responsible for the differences in the PPARA SNP associations that have been shown here for these two populations. It is also possible that there are differences between African-American and Caucasians in linkage disequilibrium of these SNPs with unknown SNPs that affect PPARα function.
With regard to the intronic SNP, there is no evidence that this SNP is itself functional. This SNP was in linkage disequilibrium with the functional L162V variant, and thus it is possible that the intronic SNP might be simply tagging the functional SNP. However, the r2 for the linkage disequilibrium was relatively small, and the two SNPs were not within the same LD block (Supplementary Figure). Thus, the possibility cannot be excluded that the intronic SNP may affect the expression level of the gene via an unknown mechanism, similar, for example, to the actions of intronic SNPs in Calpain10 [28] and COLIA1 [29].
ApoCIII can modulate plasma TG levels by inhibiting LPL and reducing apoE mediated hepatic uptake of TG rich lipoprotein remnants [12]. Recently, Florez et al. reported that African-Americans had significantly lower levels of apoCIII and TG compared with Hispanics and White non-Hispanics independently of gender and type 2 diabetes mellitus. However, in that study, multiple linear regression revealed that apoCIII was the most important contributor to ethnicity-adjusted TG levels, suggesting that ethnic differences in apoCIII levels partially contribute to differences in TG levels [30]. In the present study, our results showed that the effects of both the L162V and intronic PPARA SNPs on plasma TG were no longer significant when adjusting for apoCIII. These results raise the possibility that plasma apoCIII levels modulated by PPARA SNPs may be involved in regulation of plasma TG levels. On the other hand, plasma apoCIII and TG levels are strongly correlated (r2=0.48 in the present study population), and it is possible that the primary associations of the SNPs with apoCIII vs. TG reflect greater analytic variability in the TG measurement rather than a causal relationship. Testing of the present findings in larger population groups, as well as studies designed to test the functional effects of these SNPs on TG metabolism, would be required to address this issue.
Limitations of the study include that participants were volunteers in a clinical trial, and hence may not be representative of the general population, and their ethnicity was defined using self-reported information rather than genetic markers.
In conclusion, a functional variant (L162V) and an intronic SNP in the PPARA gene were associated with the plasma levels of apoCIII and TG in African- Americans, while there was no association between these two SNPs and plasma levels of apoCIII and TG in Caucasians. These results indicate that ethnicity should be considered in evaluating the associations of PPARA SNPs with plasma lipoprotein metabolism.
Supplementary Material
Acknowledgements
We thank Debbie Nickerson, Mark Rieder, and Joshua Smith for performing genotyping assays, David Waters and Mohammed Saad for recruitment and sampling of subjects, and Patricia Blanche and the staff of the Lipoprotein Core Laboratory at Children’s Hospital Oakland Research Institute for performing lipid, lipoprotein, and apoprotein analyses.
Funding Resource This work was supported by NIH U01 HL69757, a grant from the National Dairy Council and the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF- 2005-214-C00250)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure There are no disclosures
REFERENCES
- 1.Fruchart JC, Duriez P, Staels B. Peroxisome proliferator-activated receptor-alpha activators regulate genes governing lipoprotein metabolism, vascular inflammation and atherosclerosis. Curr Opin Lipidol. 1999;10:245–257. doi: 10.1097/00041433-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Schonfeld G. The effects of fibrates in lipoprotein and hemostatic coronary risk factors. Atherosclerosis. 1994;111:161–174. doi: 10.1016/0021-9150(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 3.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S. PPAR alpha and PPAR gamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 4.Hertz R, Bishara-Shieban J. Mode of action of peroxisome proliferators as hypolipidemic drugs, suppression of apolipoprotein C-III. J Biol Chem. 1995;270:13470–13475. doi: 10.1074/jbc.270.22.13470. [DOI] [PubMed] [Google Scholar]
- 5.Haubenwallner S, Essenburg AD, Barnet BC. Hypolipidemic activity of select fibrates correlates to changes in hepatic apolipoprotein C-III expression: a potential physiologic basis for their mode of action. J Lipid Res. 1995;36:2541–2551. [PubMed] [Google Scholar]
- 6.Staels B, Vu-Dac N, Kosykh VA, Saladin R, Fruchart JC, Dallongeville J, Auwerx J. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J Clin Invest. 1995;95:705–712. doi: 10.1172/JCI117717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB. for the Veterans Affairs High-density Lipoprotein Cholesterol Intervention Trial Study Group: Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of highdensity lipoprotein cholesterol. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 8.Bard JM, Parra HJ, Camare R, Luc G, Ziegler O, Dachet C, Bruckert E, Douste-Blazy P, Drouin P, Jacotot B. A multicenter comparison of the effects of simvastatin and fenofibrate therapy in severe primary hypercholesterolemia, with particular emphasis on lipoproteins defined by their apolipoprotein composition. Metabolism. 1995;41:498–503. doi: 10.1016/0026-0495(92)90208-r. [DOI] [PubMed] [Google Scholar]
- 9.Lussier-Cacan S, Bard JM, Boulet L, Nestruck AC, Grothe AM, Fruchart JC, Davignon J. Lipoprotein composition changes induced by fenofibrate in dysbetalipoproteinemia type III. Atherosclerosis. 1989;78:167–182. doi: 10.1016/0021-9150(89)90221-9. [DOI] [PubMed] [Google Scholar]
- 10.Genest J, Nguyen NH, Theroux P, Davignon J, Cohn JS. Effect of Micronized Fenofibrate on Plasma Lipoprotein Levels and Hemostatic Parameters of Hypertriglyceridemic Patients with Low Levels of High-Density Lipoprotein Cholesterol in the Fed and Fasted State. J Cadiovasc Pharmacol. 2000;35:164–172. doi: 10.1097/00005344-200001000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Auwerx J, Schoonjans K, Fruchart JC, Staels B. Transcriptional control of triglyceride metabolism: fibrates and fatty acids change the expression of the LPL and apo C-III genes by activating the nuclear receptor PPAR. Atherosclerosis. 1996;124:S29–S37. doi: 10.1016/0021-9150(96)05854-6. [DOI] [PubMed] [Google Scholar]
- 12.Clavey V, Lestavel-Delattre S, Copin C, Bard JM, Fruchart JC. Modulation of lipoprotein B binding to the LDL receptor by exogenous lipids and apolipoproteins CI, CII, CIII, and E. Arterioscler Thromb Vasc Biol. 1995;15:963–971. doi: 10.1161/01.atv.15.7.963. [DOI] [PubMed] [Google Scholar]
- 13.Vohl MC, Lepage P, Gaudet D, Brewer CG, Betard C, Perron P, Houde G, Cellier C, Faith JM, Despres JP, Morgan K, Hudson TJ. Molecular scanning of the human PPARα gene: association of the L162v mutation with hyperapobetalipoproteinemia. J Lipid Res. 2000;41:945–952. [PubMed] [Google Scholar]
- 14.Tai ES, Collins D, Robins SJ, O’Connor JJ, Bloomfield HE, Ordovas JM, Schaefer EJ, Brousseau ME. The L162V polymorphism at the peroxisome proliferator activated receptor alpha locus modulates the risk of cardiovascular events associated with insulin resistance and diabetes mellitus: The Veterans Affairs HDL Intervention Trial (VA-HIT) Atherosclerosis. 2006;187:153–160. doi: 10.1016/j.atherosclerosis.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 15.Flavell DM, Pineda Torra I, Jamshidi Y, Evans D, Diamond JR, Elkeles RS, Bujac SR, Miller G, Talmud PJ, Staels B, Humphries SE. Variation in the PPARα gene is associated with altered function in vitro and plasma lipid concentrations in type II diabetic subjects. Diabetologia. 2000;43:673–680. doi: 10.1007/s001250051357. [DOI] [PubMed] [Google Scholar]
- 16.Flavell DM, Jamshidi Y, Hawe E. Peroxisome proliferator-activated receptor alpha gene variants influence progression of coronary atherosclerosis and risk of coronary artery disease. Circulation. 2002;105:1440–1445. doi: 10.1161/01.cir.0000012145.80593.25. [DOI] [PubMed] [Google Scholar]
- 17.Flavell DM, Ireland H, Stephens JW, Hawe E, Acharya J, Mather H, Hurel SJ, Humphries SE. Peroxisome proliferator-activated receptor alpha gene variation influences age of onset and progression of type 2 diabetes. Diabetes. 2005;54:582–586. doi: 10.2337/diabetes.54.2.582. [DOI] [PubMed] [Google Scholar]
- 18.Simon JA, Lin F, Hulley SB, Blanche PJ, Waters D, Shiboski S, Rotter JI, Nickerson DA, Yang H, Saad M, Krauss RM. Phenotypic predictors of response to simvastatin therapy among African-Americans and Caucasians: the Cholesterol and Pharmacogenetics (CAP) Study. Am J Cardiol. 2006;97:843–850. doi: 10.1016/j.amjcard.2005.09.134. [DOI] [PubMed] [Google Scholar]
- 19.Warnick GR, Nguyen T, Albers AA. Comparison of improved precipitation methods or quantification of high-density lipoprotein cholesterol. Clin Chem. 1985;31:217–222. [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI. Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Mathews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Sapone A, Peters JM, Sakai S, Tomita S, Papiha SS, Dai R, Friedman FK, Gonzalez FJ. The human peroxisome proliferator-activated receptor [alpha] gene: identification and functional characterization of two natural allelic variants. Pharmacogenetics. 2000;10:321–333. doi: 10.1097/00008571-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Tai ES, Demissie S, Cupples LA, Corella D, Wilson PW, Schaefer EJ, Ordovas JM. Association Between the PPARA L162V Polymorphism and Plasma Lipid Levels: The Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2002;22:805–810. doi: 10.1161/01.atv.0000012302.11991.42. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen EMD, Hansen L, Echwald SM, Drivsholm T, Borch-Johnsen K, Ekstrøm CT, Hansen T, Pedersen O. Evidence for an association between the Leu162Val polymorphism of the PPAR[alpha] gene and decreased fasting serum triglyceride levels in glucose tolerant subjects. Pharmacogenetics. 2003;13:417–423. doi: 10.1097/01.fpc.0000054105.48725.5c. [DOI] [PubMed] [Google Scholar]
- 25.Tai ES, Corella D, Demissie S, Cupples LA, Coltell O, Schaefer EJ, Tucker KL, Ordovas JM. Polyunsaturated fatty acids interact with the PPAR-L162V polymorphism to affect plasma triglyceride and apolipoprotein CIII concentrations in the Framingham Heart Study. J Nutr. 2005;135:397–403. doi: 10.1093/jn/135.3.397. [DOI] [PubMed] [Google Scholar]
- 26.Pineda Torra I, Jamshidi Y, Flavell DM, Fruchart JC, Staels B. Characterization of the human PPARalpha promoter: identification of a functional nuclear receptor response element. Mol Endocrinol. 2002;16:1013–1028. doi: 10.1210/mend.16.5.0833. [DOI] [PubMed] [Google Scholar]
- 27.Faul MM, Grese TA. Selective RXR modulators for the treatment of type II diabetes. Curr Opin Drug Discov Devel. 2002;5:974–985. [PubMed] [Google Scholar]
- 28.Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M. Genetic variation in the gene encoding calpain –10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163–175. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 29.Uitterlinden AG, Burger H, Huang Q, Yue F, McGuigan FE, Grant SF, et al. Relation of alleles of the collagen type Ia1 gene to bone density and the risk of osteoporotic fractures in postmenopausal women. N Engl J Med. 1998;338:1016–1021. doi: 10.1056/NEJM199804093381502. [DOI] [PubMed] [Google Scholar]
- 30.Florez H, Mendez A, Casanova-Romero P, Larreal-Urdaneta C, Castillo-Florez S, Lee D, Goldberg R. Increased apolipoprotein C-III levels associated with insulin resistance contribute to dyslipidemia in normoglycemic and diabetic subjects from a triethnic population. Atherosclerosis. 2006;188:134–141. doi: 10.1016/j.atherosclerosis.2005.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.