Abstract
Background
Endogenous sodium pump inhibitors promote sodium excretion in normotensives and contribute to vasoconstriction in NaCl-sensitive hypertension. Marinobufagenin (MBG), an endogenous bufadienolide inhibitor of α-1 sodium pump, contributes to hypertension in Dahl salt-sensitive rats (DS). We hypothesized that in NaCl-loaded DS and normotensive Sprague-Dawley rats (S-D), MBG would elicit different patterns of sodium pump inhibition.
Methods
We compared systolic blood pressure (SBP), renal sodium excretion, activity of the sodium pump in aorta and renal medulla, and levels of MBG, atrial natriuretic peptide (ANP), and cyclic guanosine monophosphate (cGMP) in salt-loaded DS and S-D (20% NaCl, 2.5 ml/kg, intraperitoneally).
Results
NaCl loading produced sustained elevations in renal MBG excretion in both DS (2.41 ± 0.24 vs. 0.79 ± 0.08 pmol/h/kg, P < 0.01) and S-D (1.97 ± 0.37 vs. 0.60 ± 0.07 pmol/h/kg, P < 0.01) vs. that at baseline (n = 10 for each group). In NaCl-loaded DS, SBP rose by 18 mm Hg (P < 0.01) and aortic sodium pump was inhibited by 22% (P < 0.05 vs. control), while in S-D, SBP and activity of aortic sodium pump did not change. NaCl-loaded S-D excreted twice as much sodium as DS; in S-D, renal sodium pump was inhibited by 24% vs. 14% inhibition in DS (P < 0.05). NaCl loading elicited increases in plasma ANP and in renal cGMP excretion in S-D but not in DS.
Conclusions
Our present observations demonstrate that in NaCl-loaded S-D and DS, a comparable MBG response is associated with preferential inhibition of the sodium pump in the kidney and in vascular smooth muscle, respectively, resulting in an adaptive natriuresis in S-D but sodium retention and pressor response in DS.
Salt sensitivity contributes to elevated blood pressure (BP) in 30–40% of hypertensives worldwide.1,2 Endogenous digitalis-like cardiotonic steroids (CTS) are stimulated by renal sodium retention and by plasma volume expansion and are implicated in the homeostatic response to NaCl loading.3 In normo-tensives, CTS, acting via inhibition of renal sodium pump, promote sodium excretion.4,5 In salt-sensitive subjects, the impaired renal sodium pump is one of the factors underlying renal sodium retention.4,5 In these subjects, the excessive elaboration of CTS occurs with an adaptive effect, to override sodium retention. However, heightened CTS levels also exhibit a maladaptive effect, inhibit Na,K-ATPase (NKA) in vascular sarcolemma and raise BP.3–5 α-1 NKA in vitro and exhibits pressor and natriuretic effects in vivo, has been identified in human and rat tissues.7–11
Genetically determined hypertension that develops in Dahl salt-sensitive rats (DS) on a high NaCl diet results from renal sodium retention and, at least in part, is mediated by increased elaboration of MBG from adrenal cortex.12 In previous studies of mechanisms of salt sensitivity of BP, DS were usually compared to their salt-resistance counterparts, Dahl salt-resistant rats (DR).9,10,12 In the present study, we compared DS to Sprague-Dawley rats (S-D) because S-D, a genetic precursor of DS, at least with respect to CTS responses, may be more relevant to the normotensive human population than DR, in which salt-resistance has been genetically reinforced. 12 While in NaCl-loaded DS, markedly elevated MBG levels mediate NaCl-induced hypertension,13 in DR following acute and especially chronic NaCl loading, levels of MBG exhibit minimal changes.10,13 Moreover, renal NKA from DS, unlike that from other strains of rats, is remarkably insensitive to MBG.10 In NaCl-loaded S-D, however, a fourfold increase in renal MBG excretion does not increase BP but mediates a natriuretic response, as in these rats, the anti-MBG antibody administration in vivo markedly reduces renal sodium excretion and restores renal NKA activity.14 Likewise, in the normotensive NaCl-loaded human subjects, elevated levels of MBG correlate with renal sodium excretion in the presence of modestly elevated systolic BP (SBP).15
We hypothesize that in NaCl-loaded DS and in normotensive S-D, increased MBG levels would elicit different patterns of NKA inhibition and cause preferential inhibition of the sodium pump in vascular sarcolemma in DS and in kidney of S-D. Recently, we have demonstrated that atrial natriuretic peptide (ANP), via cyclic guanosine monophosphate (cGMP)-dependent NKA phosphorylation, potentiates the effect of MBG on renal sodium pump but exhibits an opposite effect in the vasculature.16 We, therefore, further hypothesized that impaired ANP response may be one of the factors underlying differential responses of the sodium pump to NaCl loading in S-D and DS. In the present experiment, we compared effects of NaCl loading on levels of endogenous ouabain (EO), MBG, ANP, and cGMP and the activity of sodium pump in thoracic aorta and renal outer medulla in DS and S-D.
Methods
Experimental design
The experimental protocol was approved by the Animal Care and Use Committee of the National Institute on Aging. Twenty 10-week-old male DS (SS/JrHS-D) (351 ± 7 g) (Harlan Sprague Dawley, Indianapolis, IN) and twenty 10-week-old male S-D (440 ± 6 g) (Charles River Laboratories, Wilmington, MA) were studied after 1 week of adaptation to laboratory environment, metabolic chambers, and measurement of BP via tail-cuff plethysmography (IITC, Life Science, Woodland Hills, CA).
Following baseline urine collection and BP measurement, a single injection of 2.5 ml/kg, 0.9% NaCl (control groups; n = 10 for each strain) or hypertonic NaCl (experimental groups; 20% solution, 2.5 ml/kg; n = 10 for each strain) was administered intraperitoneally to DS or S-D under light anesthesia (25 mg/kg ketamine).10 Then, the animals were placed in metabolic chambers and urine was collected hourly for determination of renal excretion of sodium, MBG, EO, and cGMP. Within 2 h following acute NaCl loading, SBP was measured, and the animals were anesthetized with 60 mg/kg ketamine and exsanguinated from the abdominal aorta. Plasma was collected for determination of MBG, EO, and ANP 1–28. Kidneys and aortae were collected for measurement of sodium pump activity (below).
Concentration of sodium in the urine was measured using Roche-Hitachi 917 (Roche, Vienna, Austria) and natriuresis was expressed as mmol/h/kg.
Sodium pump in aortic rings
The sodium pump activity in the thoracic aorta was estimated by measurement of ouabain-sensitive 86Rb uptake in the presence or in the absence of 2 mmol/l ouabain, as reported previously.16 Aortic rings were equilibrated for 1 h in a medium containing NaCl 120 mmol/l, KCl 4 mmol/l, CaCl2 2.5 mmol/l, MgCl2 2.0 mmol/l, NaH2PO4 1.1 mmol/l, NaHCO3 24 mmol/l, and glucose 5.6 mmol/l and gassed with 95% O2 and 5% CO2 at 32 °C (pH 7.4). After addition of 86Rb (0.1 µCi/sample; NEN Life Science Products, Boston, MA), the aortic rings were incubated in the presence and absence of 2 mmol/l ouabain for 60 min at 37 °C. The aortic rings were then rinsed three times in ice-cold medium, blotted, weighed, placed in double distilled water, and counted in a gamma counter (Cherenkov radiation). The total 86Rb uptake was determined on a wet-weight basis and the activity of the sodium pump was estimated as the difference between the total uptake of 86Rb and the uptake in the presence of 2 mmol/l ouabain and expressed in nanomoles of 86Rb per gram of tissue per minute.
Sodium pump in renal medulla
The activity of the sodium pump in the suspension of medullary tubules was estimated by measurement of ouabain-sensitive 86Rb uptake as reported recently in detail.13 Slices of outer medulla were rinsed in ice-cold modified Eagle’s minimal essential medium containing NaCl 137 mmol/l, KCl 4 mmol/l, MgSO4 1 mmol/l, CaCl2 1 mmol/l, Na2HPO4 0.33 mmol/l, NaH2PO4 0.44 mmol/l, NaHCO3 4 mmol/l, glucose 5 mmol/l, essential and nonessential amino acids 4 mmol/l, and HEPES 15 mmol/l (pH 7.4). Medullar fragments were incubated in the rinsing solution in the presence of 0.1% collagenase (wt/vol) (CLS II, 215 U/ mg; Worthington Biochemical, Freehold, NJ) and 0.1% bovine serum albumin for 1 h at 37 °C under aeration with 95% O2 and 5% CO2. The suspension of tubular fragments was obtained on ice by pouring tissue through graded filters (150 to 100 µm). After centrifugation at 100g for 3 min at 4 °C, tubular fragments were washed with ice-cold solution, resuspended in incubation medium to 2–4 mg protein/ml, and incubated for 1 h in oxygenated solution in the absence and presence of 5 mmol/l ouabain. 86Rb uptake was determined after addition of 10 µl incubation solution containing 86RbCl (0.1 µCi/sample; NEN Life Science Products, Boston, MA) for 10 min. Tubular fragments were then washed by ice-cold medium, and centrifuged and lysed in 1% sodium deoxycholate. The radioactivity was measured by liquid scintillation. Protein was measured by the Lowry method. 86Rb uptake was expressed as nanomoles of 86Rb per milligram of protein per minute.
Immunoassays
Plasma and urine samples were extracted on Sep-Pak C-18 cartridges (Waters, Milford, MA), and MBG or ouabain competitive fluoroimmunoassays, based on a polyclonal rabbit antibody, were performed as previously described.13 The crossreactivity of anti-MBG antibody (aMBG-P) is as follows: MBG: 100%, ouabain: 0.1%, digoxin: 1.0%, digitoxin: 3.0%, bufalin: 1.0%, cinobufagin: 1.0%, prednisone <0.1%, spironolactone <0.1%, proscillaridin <1.0%, and progesterone <0.1%. The crossreactivity of antiouabain antiserum ( anti-OU-M-2005) is as follows: ouabain: 100%, ouabagenin: 52%, digoxin: 1.8%, digitoxin: 0.47%, progesterone: 0.002%, prednisone: 0.001%, proscillaridin: 0.03%, bufalin: 0.10%, aldosterone: 0.04%, telocinobufagin: 0.02%, resibufagin: 0.15%, marinobufotoxin: 0.06%, cinobufagin: 0.02%, and MBG: 0.036%.
Plasma levels of rat ANP 1–28 were measured using enzyme immunoassay kit (Peninsula Laboratories, San Carlos, CA). Urinary cGMP was measured using enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI).
Statistics
Results are reported as the mean ± s.e.m. The significance of differences among the measured variables was assessed by one-way analysis of variance, followed by Newman–Keuls test and by two-way analysis of variance, where appropriate (GraphPad Prism software; GraphPad, San Diego, CA). A P value <0.05 was considered statistically significant.
Results
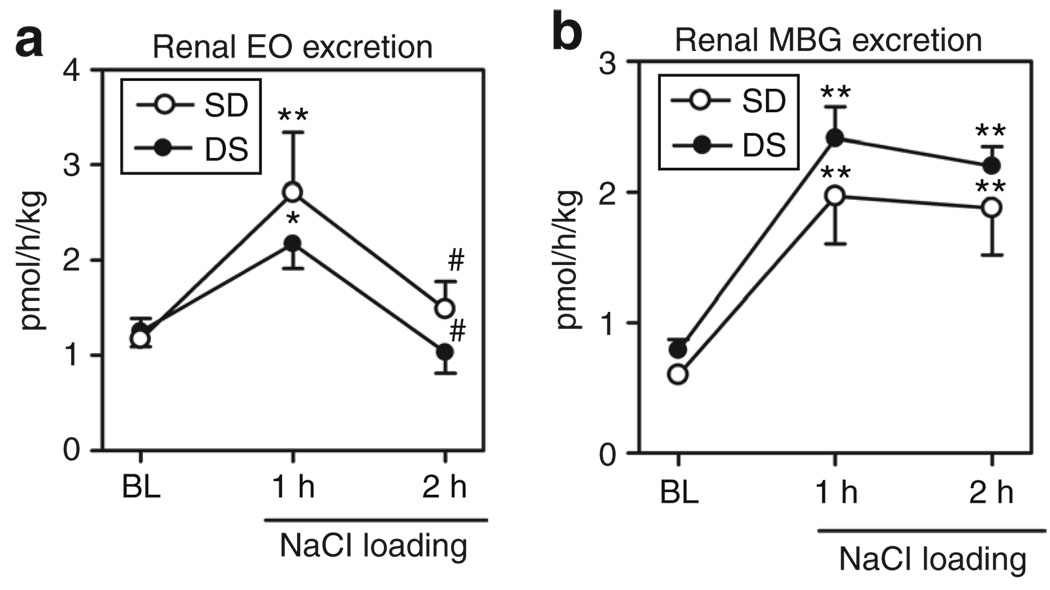
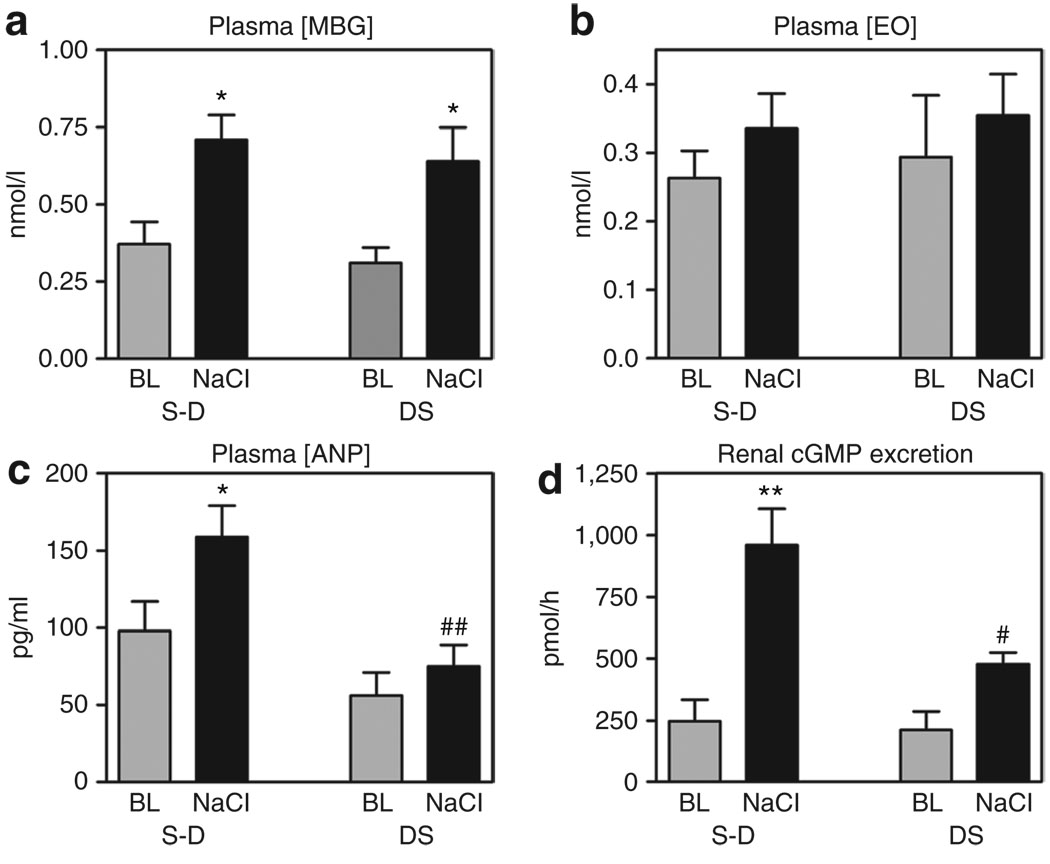
As presented in Figure 1a, in both S-D and DS, renal EO excretion exhibited a comparable transient increase at 1 h following NaCl loading and then decreased to baseline levels. Unlike that of EO, acute NaCl administration elicited sustained elevations in renal MBG excretion, which were similar in DS and S-D (Figure 1b). Within 2 h of NaCl loading, plasma levels of MBG, but not that of EO, were elevated in rats from both strains, as compared to that at baseline (Figure 2a,b).
Figure 1.
Effect of NaCl loading on renal excretion of cardiotonic steroids. (a) Renal excretion of endogenous ouabain (EO) in Sprague-Dawley (S-D) and in Dahl salt-sensitive rats (DS). (b) Renal excretion of marinobufagenin (MBG) in S-D and in DS. Each point represents means ± s.e.m. from 10 observations. *P < 0.05 and **P < 0.01 vs. baselines (BL) for both S-D and DS; #P < 0.05 vs. 1 h of an acute NaCl loading (one-way analysis of variance followed by the Newman–Keuls test).
Figure 2.
Effect of NaCl loading on plasma levels of marinobufagenin (MBG), endogenous ouabin (EO), atrial natriuretic peptide (ANP), and on renal excretion of cyclic guanosine monophosphate (cGMP). Plasma levels of (a) MBG, (b) EO, (c) ANP, and (d) renal excretion of cGMP in Sprague-Dawley (S-D) and Dahl salt-sensitive rats (DS) following intraperitoneal administration of hypertonic solution of NaCl, and at baseline (BL). Each bar represents means ± s.e.m. from 10 observations. By one-way analysis of variance (ANOVA) followed by the Newman–Keuls test: *P < 0.05, **P < 0.001 vs. BL; #P < 0.05, ##P < 0.01 vs. NaCl-loaded S-D. Source of variations by two-way ANOVA: plasma MBG (NaCl effect: P = 0.002; strain effect: P = 0.43), plasma EO (NaCl effect: P = 0.29; strain effect: P = 0.69), plasma ANP (NaCl effect: P = 0.038; strain effect: P = 0.002), renal cGMP excretion (NaCl effect: P < 0.0001; strain effect: P = 0.026).
In NaCl-loaded S-D, plasma concentration of ANP increased vs. baseline levels, while no such response has occurred in DS (Figure 2c). Renal excretion of cGMP exhibited a threefold increase in NaCl-loaded S-D, while in DS, cGMP response was of marginal statistical significance (Figure 2d).
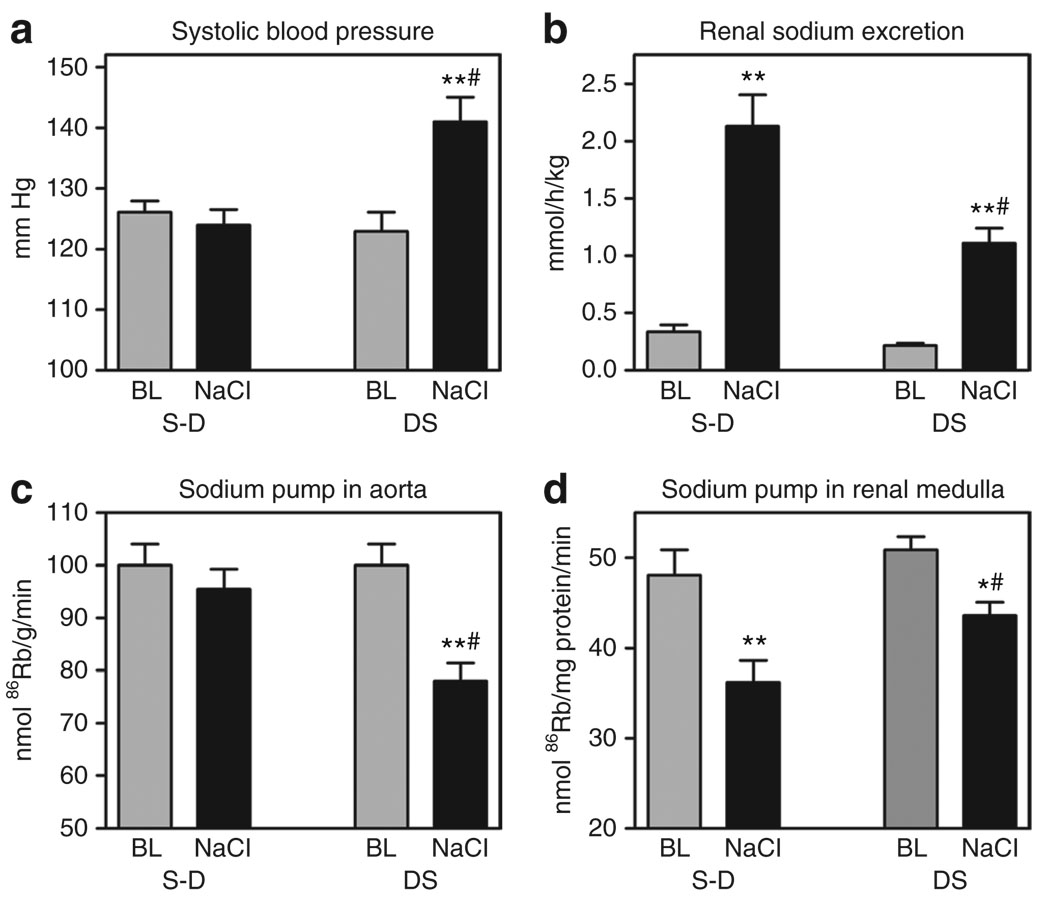
NaCl-loading produced pressor response in DS; SBP in DS increased by 18 mm Hg, while no SBP changes has been observed in NaCl-loaded S-D (Figure 3a). In NaCl-loaded S-D, the activity of aortic sodium pump was not altered, while in NaCl-loaded DS, the aortic sodium pump was inhibited by 22%, as compared to that at baseline (Figure 3c).
Figure 3.
Effect of NaCl loading on systolic blood pressure (SBP), renal sodium excretion and sodium pump activity in aorta and renal medulla. SBP (a), renal sodium excretion (b), and activity of sodium pump in aorta (c) and in renal medulla (d) in Sprague-Dawley (S-D) and Dahl salt-sensitive rats (DS) following intraperitoneal administration of hypertonic solution of NaCl, and at baseline (BL). Each bar represents means ± s.e.m. from 10 observations. By one-way analysis of variance (ANOVA) followed by the Newman–Keuls test: *P < 0.05, **P < 0.001 vs. BL; #P < 0.05 vs. NaCl-loaded S-D. Source of variations by two-way ANOVA: SBP (NaCl effect: P = 0.01; strain effect: P = 0.02), renal sodium excretion (NaCl effect: P < 0.0001; strain effect: P = 0.002), activity of sodium pump in aorta (NaCl effect: P = 0.045; strain effect: P = 0.013), activity of sodium pump in renal medulla (NaCl effect: P = 0.0002; strain effect: P = 0.025).
Changes of sodium pump activity in renal medulla in NaCl-loaded rats from both strains exhibited a pattern different from that observed in the aortae (Figure 3d). In NaCl-loaded S-D, renal sodium pump was inhibited by 24%, while in DS, only a 14% inhibition of sodium pump from renal medulla has occurred (Figure 3d). As illustrated in Figure 3b, within 2 h of NaCl loading, rats from both strains developed natriuresis, but DS exhibited a lesser natriuretic response as compared to that in S-D. Thus, following administration of an equal amount of NaCl (8.5 mmol Na/kg), sodium excretion in S-D exceeded that in DS by two times. Importantly, animals from both strains exhibited comparable diuresis at baseline (2.2 ± 0.3 ml/h/kg in S-D and 2.0 ± 0.3 ml/h/kg in DS) as well as within 2 h of NaCl administration (7.4 ± 0.8 ml/h/kg in S-D and 6.5 ± 1.3 ml/h/kg in DS).
Discussion
The main observation of our study is that acute NaCl loading of normotensive (S-D) and salt-sensitive (DS) rats generated comparable elevations in plasma levels and renal excretion of MBG, an endogenous sodium pump inhibitor, as well as different patterns of sodium pump inhibition in the vascular sarcolemma and the renal medulla. While the aortic sodium pump was inhibited in DS only, S-D, by contrast, exhibited greater inhibition of the renal sodium pump than did DS. Therefore, the pattern of sodium pump inhibition is associated with the differences in pressor and natriuretic responses of the two strains of rats to acute NaCl loading. The hypertension- prone NaCl-loaded DS showed increased BP, while no pressor response occurred in the S-D. Conversely, the normotensive S-D exhibited a greater natriuretic response than DS. In rats from both strains, renal excretion of another CTS, EO, exhibited a transient increase within 1 h of NaCl loading.
Previously, in NaCl-loaded DS, we demonstrated that a transient response of brain EO is followed by a transient increase in renal EO excretion, and that EO, via activation of central and adrenocortical renin–angiotensin system, stimulates the release of MBG from the adrenal cortex.17,18 In the present study, both NaCl-loaded DS and S-D exhibited a similar pattern of CTS responses, that is, transient increase in EO excretion and a more sustained increase in the excretion of MBG. This similarity suggests that a causative link between EO and MBG may not be limited to pathogenesis of hypertension in DS but may also underlie the physiological response to NaCl loading in the normotensives. Our recent data demonstrating that in NaCl-supplemented normotensive human subjects renal excretion of EO also exhibit a transient increase while MBG excretion is sustained15 agree with our previous13,17,18 and present experimental observations.
As demonstrated in previous studies in normotensive rats, NaCl loading stimulates CTS to induce natriuresis.14,19 In salt-sensitive rats, such as DS, production of CTS occurs to override renal sodium retention, but CTS inhibit the NKA in the vasculature and thereby contribute to hypertension.13,17 In our previous studies, the magnitude of the NaCl-induced response of plasma and urinary MBG in DS substantially exceeded that in DR.10,13 In the present study, the magnitude of the NaCl-induced plasma and urinary MBG response in DS and S-D was comparable, but the patterns of sodium pump inhibition differed dramatically between the two strains of rats.
CTS promote natriuresis via inhibition of the renal tubular sodium pump and reduction of tubular sodium reabsorption.5 In the present experiment, elevated plasma and urinary levels of MBG in NaCl-loaded normotensive S-D were accompanied by a 24% inhibition of the renal sodium pump. However, in NaCl-loaded DS, although MBG levels increased to the same extent as in S-D, sodium pump from renal medulla was inhibited by only 14%. Furthermore, NaCl-loaded DS excreted much less sodium compared to that in S-D. These data are in accordance with our previous observation that as compared to renal NKA from normotensive Wistar rats, renal NKA in DS is less sensitive to MBG.10
In the present experiment, the two strains of rats exhibited different patterns of sodium pump inhibition in the presence of comparable MBG response. Thus, NaCl-loaded S-D exhibited adequate natriuresis in the absence of BP elevation as well as inhibition of the sodium pump in the kidney but not in aortae. As compared to S-D, NaCl-loading of DS was associated with a pressor response and inhibition of the aortic sodium pump as well as lower natriuresis and renal sodium pump inhibition. Thus, NaCl-sensitivity of BP is associated with a shift in the responses of renal and vascular sodium pump to NaCl loading. This shift in the balance between inhibition of renal and vascular NKA could be one of the factors underlying the development of NaCl-sensitive hypertension. Whether the two patterns of sodium pump inhibition observed in the present experiment in DS and in S-D are relevant to the specific salt-sensitive and salt-insensitive subgroups of human population remains to be established.
Interactions of CTS with NKA are modulated by many factors, including cGMP-induced NKA phosphorylation.20 Activation of the particulate guanylyl cyclase–cGMP-protein kinase G pathway underlies both renal and vascular effects of ANP peptides.16,21 Recently, in rats, we demonstrated that ANP, via a cGMP-dependent mechanism, induced phosphorylation of α-1 NKA in the renal medulla and exhibited an opposite effect on NKA from the aortic sarcolemma.16 In the same study, ANP sensitized renal NKA to the inhibitory effect of MBG. In the aortic sarcolemma, ANP, by contrast, markedly decreased sensitivity of NKA to MBG. Thus, ANP can potentiate the physiological effect of MBG, e.g., natriuresis, and offset MBG-induced vasoconstriction.
In the present experiment, NaCl loading of S-D was associated with heightened plasma levels of ANP and a threefold increase in cGMP excretion, while in NaCl-loaded DS, ANP was not stimulated and cGMP excretion exhibited a lesser increase than that in S-D. Our present data suggest, but do not prove, that in NaCl-loaded DS, impaired responsiveness of renal sodium pump, along with heightened responsiveness of vascular sodium pump to MBG, may be, at least in part, explained by the absence of ANP response. This scenario is in agreement with the notion that down-regulation of reno- and vasoprotective cGMP-dependent mechanisms is an important feature of salt-sensitive hypertension.22,23
Previously, Simchon et al. compared responses of ANP to chronic administration of 8% NaCl diet to DS and to DR and demonstrated that while in NaCl-loaded DR, plasma levels of ANP increased more than twofold, in DS plasma, ANP concentration increased by only 50%.24 Later, the same group demonstrated that chronic NaCl loading of DS produces a lesser cGMP response as compared to that in DR.25 Our present observations are in accordance with these previous findings.
In the present experiment, response of plasma ANP to NaCl loading was abolished in DS unlike that in S-D. Natriuretic peptides are implicated in physiological response to NaCl loading, and blockade of ANP with a monoclonal antibody has been reported to aggravate NaCl-sensitive hypertension in rats.26 Accordingly, ANP knockout mice on a high salt diet increased BP, unlike their wild-type counterparts.27 Absence of ANP response to NaCl loading in DS, observed in the present experiment, may be explained by a variety of factors, one of which may be increased activity of neutral endopeptidase (NEP), which catalyzes ANP degradation.28 Indeed, the experimental evidence suggests that NaCl loading is associated with enhanced activity of NEP. Thus, in Wistar rats, NaCl loading was reported to increase levels of NEP in the urine and activity of this enzyme in the proximal tubules.29 Furthermore, activity and expression of NEP in the vascular smooth muscle was increased in rats with deoxycorticosterone-salt hypertension but not with one-kidney one-clip and NG-nitro-l-arginine methyl ester–induced hypertension.30 In DS, chronic NEP inhibition has been reported to increase levels of circulating ANP and to facilitate natriuresis.28 Mechanistic experiments are needed to prove that diminished ANP response underlies differential patterns of MBG-induced sodium pump inhibition, observed in NaCl-loaded S-D and DS, and contributes to the development of salt-sensitive hypertension.
In conclusion, NaCl loading of DS and of salt-insensitive S-D, in the presence of comparable response of plasma and urinary MBG, produces preferential inhibition of the sodium pump in the vascular smooth muscle and kidney, respectively. Therefore, salt sensitivity is associated with a loss in the responsiveness of renal sodium pump to MBG, while vascular sodium pump exhibits heightened MBG sensitivity.
Acknowledgments
This study has been supported by Intramural Research Program, National Institute on Aging, NIH. The authors gratefully acknowledge critical comments of Edward G. Lakatta, and David E. Anderson, and are grateful for excellent technical assistance by Danielle Joseph, Chad Boily, and Alexandra Newman (Namikas).
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Weinberger MH. Sodium and blood pressure 2003. Curr Opin Cardiol. 2004;19:353–356. doi: 10.1097/01.hco.0000127136.50978.db. [DOI] [PubMed] [Google Scholar]
- 2.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 3.Haddy FJ, Overbeck HW. The role of humoral agents in volume expanded hypertension. Life Sci. 1976;19:935–947. doi: 10.1016/0024-3205(76)90284-8. [DOI] [PubMed] [Google Scholar]
- 4.Blaustein MP. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reasessment and a hypothesis. Am J Physiol. 1977;232:C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- 5.Bagrov AY, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nature Clin Pract Nephrol. 2008;4:378–392. doi: 10.1038/ncpneph0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 7.Bagrov AY, Fedorova OV, Dmitrieva RI, Howald WN, Hunter AP, Kuznetsova EA, Shpen VM. Characterization of a urinary bufodienolide Na+,K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension. 1998;31:1097–1103. doi: 10.1161/01.hyp.31.5.1097. [DOI] [PubMed] [Google Scholar]
- 8.Komiyama Y, Dong XH, Nishimura N, Masaki H, Yoshika M, Masuda M, Takahashi H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin Biochem. 2005;38:36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Fedorova OV, Kolodkin NI, Agalakova NI, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous α-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension. 2001;37:462–426. doi: 10.1161/01.hyp.37.2.462. [DOI] [PubMed] [Google Scholar]
- 10.Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of Dahl rats. Circulation. 2000;102:3009–3014. doi: 10.1161/01.cir.102.24.3009. [DOI] [PubMed] [Google Scholar]
- 11.Fedorova OV, Kolodkin NI, Agalakova NI, Namikas AR, Bzhelyansky A, St-Louis J, Lakatta EG, Bagrov AY. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. J Hypertens. 2005;23:835–842. doi: 10.1097/01.hjh.0000163153.27954.33. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen KD, Iwai J, Heine M, Leitl G, Dahl LK. Genetic influence on the development of renal hypertension in parabiotic rats. Evidence that a humoral hypertensinogenic factor is produced in kidney tissue of hypertension-prone rats. J Exp Med. 1969;130:1353–1365. doi: 10.1084/jem.130.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. An endogenous ligand of α-1 sodium pump, marinobufagenin, is a novel mediator of sodium chloride dependent hypertension. Circulation. 2002;105:1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 14.Periyasamy SM, Liu J, Tanta F, Kabak B, Wakefield B, Malhotra D, Kennedy DJ, Nadoor A, Fedorova OV, Gunning W, Xie Z, Bagrov AY, Shapiro JI. Salt loading induces redistribution of the plasmalemmal Na/K-ATPase in proximal tubule cells. Kidney Int. 2005;67:1868–1877. doi: 10.1111/j.1523-1755.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DE, Fedorova OV, Morrell CH, Longo DL, Kashkin VA, Metzler JD, Bagrov AY, Lakatta EG. Endogenous sodium pump inhibitors and age-associated increases in salt sensitivity of blood pressure in normotensives. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1248–R1254. doi: 10.1152/ajpregu.00782.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedorova OV, Agalakova NI, Morrell CH, Lakatta EG, Bagrov AY. ANP differentially modulates marinobufagenin-induced sodium pump inhibition in kidney and aorta. Hypertension. 2006;48:1160–1168. doi: 10.1161/01.HYP.0000248129.20524.d0. [DOI] [PubMed] [Google Scholar]
- 17.Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signaling in NaCl-loaded Dahl-S rats. J Hypertens. 2005;23:1515–1523. doi: 10.1097/01.hjh.0000174969.79836.8b. [DOI] [PubMed] [Google Scholar]
- 18.Fedorova OV, Zhuravin IA, Agalakova NI, Yamova LA, Talan MI, Lakatta EG, Bagrov AY. Intrahippocampal microinjection of an exquisitely low dose of ouabain mimics NaCl loading and stimulates a bufadienolide Na/K-ATPase inhibitor. J Hypertens. 2007;25:1834–1844. doi: 10.1097/HJH.0b013e328200497a. [DOI] [PubMed] [Google Scholar]
- 19.Fedorova OV, Anderson DE, Lakatta EG, Bagrov AY. Interaction of NaCl and behavioral stress on endogenous sodium pump ligands in rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R352–R358. doi: 10.1152/ajpregu.2001.281.1.R352. [DOI] [PubMed] [Google Scholar]
- 20.Fotis H, Tatjanenko LV, Vasilets LA. Phosphorylation of the α-subunits of the Na,ATPase from mammalian kidneys and Xenopus oocytes by cGMP-dependent protein kinase results in stimulation of ATPase activity. Eur J Biochem. 1999;260:904–910. doi: 10.1046/j.1432-1327.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 21.Scavone C, Scanlon C, McKee M, Nathanson JA. Atrial natriuretic peptide modulates sodium and potassium activated adenosine triphosphothase through a mechanism involving cyclic GMP and cyclic GMP-dependent protein kinase. J Pharmacol Exper Ther. 1995;272:1036–1043. [PubMed] [Google Scholar]
- 22.Gupta G, Azam M, Yang L, Danziger RS. The β2 subunit inhibits stimulation of the α1/β1 form of soluble guanylyl cyclase by nitric oxide. Potential relevance to regulation of blood pressure. J Clin Invest. 1997;100:1488–1492. doi: 10.1172/JCI119670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasser JM, Sullivan JC, Elmarakby AA, Kemp BE, Pollock DM, Pollock JS. Reduced NOS3 phosphorylation mediates reduced NO/cGMP signaling in mesenteric arteries of deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 2004;43:1080–1085. doi: 10.1161/01.HYP.0000122804.32680.c9. [DOI] [PubMed] [Google Scholar]
- 24.Simchon S, Manger W, Blumberg G, Brensilver J, Cortell S. Impaired renal vasodilation and urinary cGMP excretion in Dahl salt-sensitive rats. Hypertension. 1996;27:653–657. doi: 10.1161/01.hyp.27.3.653. [DOI] [PubMed] [Google Scholar]
- 25.Simchon S, Manger WM, Carlin RD, Peeters LL, Rodriguez J, Batista D, Brown T, Merchant NB, Jan KM, Chien S. Salt-induced hypertension in Dahl salt-sensitive rats. Hemodynamics and renal responses. Hypertension. 1989;13:612–621. doi: 10.1161/01.hyp.13.6.612. [DOI] [PubMed] [Google Scholar]
- 26.Itoh H, Nakao K, Mukoyama M, Yamada T, Hosoda K, Shirakami G, Morii N, Sugawara A, Saito Y, Shiono S, Arai H, Ikuyo Yoshida I, Imura H. Chronic blockade of endogenous atrial natriuretic polypeptide (ANP) by monoclonal antibody against ANP accelerates the development of hypertension in spontaneously hypertensive and deoxycorticosterone acetate-salt-hypertensive rats. J Clin Invest. 1989;84:145–154. doi: 10.1172/JCI114134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melo LG, Veress AT, Chong CK, Pang SC, Flynn TG, Sonnenberg H. Salt-sensitive hypertension in ANP knockout mice: potential role of abnormal plasma renin activity. Am J Physiol. 1998;274:R255–R261. doi: 10.1152/ajpregu.1998.274.1.R255. [DOI] [PubMed] [Google Scholar]
- 28.Quaschning T, D’Uscio LV, Shaw S, Gröne HJ, Ruschitzka F, Lüscher TF. Vasopeptidase inhibition restores renovascular endothelial dysfunction in salt-induced hypertension. J Am Soc Nephrol. 2001;12:2280–2287. doi: 10.1681/ASN.V12112280. [DOI] [PubMed] [Google Scholar]
- 29.Aviv R, Gurbanov K, Hoffman A, Blumberg S, Winaver J. Urinary neutral endopeptidase 24.11 activity: modulation by chronic salt loading. Kidney Int. 1995;47:855–860. doi: 10.1038/ki.1995.128. [DOI] [PubMed] [Google Scholar]
- 30.Lee J. Reciprocal regulation of angiotensin converting enzyme and neutral endopeptidase in rats with experimental hypertension. Physiol Res. 2004;53:365–368. [PubMed] [Google Scholar]