Abstract
Many cancer chemopreventive agents have been associated with lower cancer risk by suppressing nuclear factor-κB (NF-κB) signaling pathways, which subsequently leads to attenuated pro-inflammatory mediators and activities. Of the natural compounds, the isothiocyanates (ITCs) found in cruciferous vegetables have received particular attention because of their potential anti-cancer effects. However, limited studies regarding the influence of ITCs structure on NF-κB transactivation and anti-inflammatory action are reported. In the present study, the anti-inflammatory potential of ten structurally divergent synthetic ITCs were evaluated in HT-29-N9 human colon cancer cells and RAW 264.7 murine macrophages. The effect of ITCs on the basal transcriptional activation of NF-κB and the inflammatory response to bacterial lipopolysaccharide (LPS) were assessed. The synthetic ITC analogs suppressed NF-κB-mediated pro-inflammatory gene transcription. Among the ITC analogs, tetrahydrofurfuryl isothiocyanate, methyl-3-isothiocyanatopropionate, 3-morpholinopropyl isothiocyanate and 3,4-methyelendioxybenzyl isothiocyanate showed stronger NF-κB inhibition as compared to the parent compound, phenylethyl isothiocyanate (PEITC). Molecular analysis revealed that several of the pro-inflammatory mediators and cytokines (iNOS, COX-2, IL-1β , IL-6 and TNF-α ,) were reduced by ITCs, and correlated with the downregulation of NF-κB signaling pathways. Immunoblotting showed that ITCs suppressed LPS-induced phosphorylation and degradation of IκBα and decreased nuclear translocation of p65. In parallel, ITCs suppressed the phosphorylation of IκB kinase α /β (IKKα /β ). Taken together, our findings provide the possibility that synthetic ITC analogs might have promising cancer chemopreventive potential, based on their stronger anti-NF-κB and anti-inflammatory activities, than the natural ITCs.
Keywords: Cancer chemopreventive compounds, Isothiocyanates, NF-κB, Anti-inflammatory response, Nrf2
1. Introduction
During the last several decades, numerous studies continue to support the promise that dietary intake of cruciferous vegetables, such as broccoli, cauliflower, watercress, cabbage, Brussels sprouts and other phytochemicals, could be protective against the risk of various types of malignancies [1–4]. The cancer protective effects against chemical-induced carcinogenesis as well as genetically derived tumor models by these cruciferous vegetables are attributed to naturally occurring isothiocyanates (ITCs) [5–8]. Phenylethyl isothiocyanate (PEITC) is one of the best-studied members of the ITC class of compounds that has gained much attention due to its wide-ranging biological functions in vivo and in vitro, particularly cancer chemopreventive activity [6, 9–11]. Indeed, modulation of xenobiotic metabolizing enzyme system and inhibition of cancer cell proliferation via apoptosis induction have been described [1, 12–14]. Recently, PEITC has been shown to have promising anti-inflammatory properties through attenuation of the ubiquitous nuclear factor-κB (NF-κB) signaling pathway [15, 16].
It is well demonstrated that large amounts of the pro-inflammatory mediators, such as nitric oxide (NO) and prostaglandin E2 (PGE2), as well as the pro-inflammatory cytokines, such as interleukin-1β (IL-1β ) and interleukin-6 (IL-6), and tumor necrotic factor-α (TNF-α ), are released at sites of inflammation during chronic inflammation [17], a hallmark phenomenon that is involved in numerous pathological diseases including the development of neoplasms. It is clearly evident that both NO and PGE2 have been implicated in cell proliferation, invasiveness, angiogenesis, inflammation, immune surveillance and modulation of apoptosis [18–23]. Thus, inhibition of NO and PGE2 production has been proposed to be a useful approach for the treatment of various inflammatory diseases as well as potential chemoprevention strategy [18, 24, 25].
Key pro-inflammatory stimuli including mitogens, cytokines, UV irradiation and bacterial lipopolysaccharide (LPS) [26, 27] modulate their effects by inducing the activation of transcription factor NF-κB. In the absence of stimuli, in most cells, NF-κB is associated with inhibitor proteins, IκBs and sequestered in the cytosol. Exposure to stimuli lead to the activation of the upstream IκB kinase complexes (IKKs), resulting in rapid phosphorylation and proteolytic degradation of IκB to releases NF-κB, which then translocates to the nucleus where it regulates gene transcription [26]. NF-κB activates a number of rapid response genes involved in the inflammatory response including iNOS, COX-2, IL-1β , IL-6 and TNF-α . Production of pro-inflammatory mediators and cytokines by these NF-κB response genes may reflect the degree of inflammation and has been suggested to be a measure to assess the effect of chemopreventive agents on the inflammatory process [28].
In the present study, different analogs of phenylethyl isothiocyanate (PEITC), on the basal transcriptional activity of NF-κB in HT-29-N9 human colon cancer cells that were stabilized with luciferase reporter gene and the anti-inflammatory properties of ITCs in RAW 264.7 murine macrophages stimulated with bacterial LPS were examined. Several key parameters associated with LPS-induced inflammation including NO and PGE2 production, iNOS and COX-2 mRNA and protein expression, IL-1β , IL-6 and TNF-α mRNA expression, as well as molecular markers of NF-κB signaling pathways were explored. The results presented in the current investigation showed that synthetic ITCs could suppress NF-κB transactivation. More interestingly, some compounds have inhibitory activities stronger than the parent compound, PEITC. In addition, treatment of LPS-stimulated RAW 264.7 cells with ITCs decreased nitrite and PGE2 production in a dose-dependent manner and these reductions are mediated at the transcription level. Finally, we also found that ITCs exhibited their anti-inflammatory activity via suppression of IκB kinase α /β (IKKα /β ) phosphorylation, IκBα phosphorylation and subsequent p65 NF-κB nuclear translocation.
2. Materials and methods
2.1. Cells and reagents
The HT-29-N9 human colon cancer cells stably transfected with NF-κB-luciferase (Luc) reporter plasmid constructs were generated in our laboratory as previously described [15]. This reporter plasmid has two copies of the κB promoter containing the NF-κB-binding site which were fused to Luc gene. HT-29-N9 cells were maintained in MEM medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in humidified incubator with 5% CO2. The RAW 264.7 cells were obtained from American Type Culture Collections (ATCC, Manassas, VA) and cultured at 37°C in 5% CO2 in DMEM medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
ITC compounds used in this study were purchased from Lancaster Synthesis Inc. (Windham, NH). PEITC and lipopolysaccharide (LPS) derived from E. coli serotype 055:B5 were purchased from Sigma Chemicals Co. (St. Louis, MO). Luciferase assay system and CellTiter 96® aqueous non-radioactive cell proliferation assay were from Promega (Madison, WI). Antibodies specific for detection of COX-2 (catalog no. sc-1745), iNOS (catalog no. sc-650), IκBα (catalog no. sc-847), lamin A (catalog no. sc-20680), β-actin (catalog no. sc-1616) and GADPH (catalog no. sc-25778) were from Santa Cruz Biotechnologies Inc. (Santa Cruz, CA). Antibodies against IKKα (catalog no. 2682), IKKβ (catalog no. 2684), phospho-IKKα /β (catalog no. 2681), p65 (catalog no. 3034) and phospho-IκBα (catalog no. 9241) were from Cell Signaling Technology (Beverly, MA). TRIzol and SuperScript III first-strand cDNA synthesis system were purchased from Invitrogen (Carlsbad, CA). The prostaglandin E2 EIA kit (catalog no. 514010) was obtained from Cayman Chemical Company (Ann Arbor, .MI).
2.2. Cell viability test by MTS assay
Cells were allowed to grow up to 70% confluence before treatment with the indicated concentrations of isothiocyanate compounds. Control cells were supplemented with media containing 0.1% ethanol (vehicle control) for 24 h. Following treatment, cell viability was determined by using the CellTiter 96® aqueous non-radioactive cell proliferation (MTS) assay according to the manufacturer’s instructions. Briefly, cells were treated with combined solution of a tetrazolium compound, MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H- tetrazolium, inner salt], and an electron coupling reagent, phenazine methosulfate (PMS), for additional 2 h at 37°C. The absorbance of the formazan product at 490 nm was measured directly with a μQuant Biomolecular Spectrophotometer from Bio-Tek Instruments Inc. (Winooski, VT).
2.3. Luciferase reporter assay
HT-29-N9 cells were cultured in 6-well plates until 70% confluent followed by treatment with ITCs for 24 h. The NF-κB-luciferase activity was measured using a luciferase assay system according to the manufacturer’s instructions. Briefly, after treatment, cells were washed twice with ice-cold phosphate-buffered saline (PBS) (pH 7.4) and lysed by adding 400 μl of 1x reporter lysis buffer (Promega). After centrifugation at 12,000 g for 15 s at room temperature, 10 ul-aliquot of supernatant was analyzed for luciferase activity by reading with a SIRIUS luminometer (Berthold Detection System, Germany). Normalization of the luciferase activity was done based on the protein concentration, which determined by using BCA protein assay kit from Pierce Biotechnology (Rockford, IL). The luciferase activity was then expressed as fold of induction over the activity of control vehicle-treated cells (0.1% ethanol).
2.4. Measurement of nitrite concentration
RAW 264.7 cells were cultured in 48-well plates until 70% confluent and then were induced with 1 μg/ml of LPS in the presence or absence of selected ITCs at various concentrations (0.1–10 μM). After 24 h treatment, supernatant of spent cell culture media were analyzed for nitrite (NO2−) by the Griess reaction [29]. Briefly, an equal volume of Griess reagent (1% sulfanilamide/0.1% naphtylethyenediamine dihydrochloride in 2.5% H3PO4) was mixed with cell culture supernatants and color development was assessed at λ=550nm with a μ Quant Biomolecular Spectrophotometer from Bio-Tek Instruments Inc. (Winooski, VT). Fresh culture medium was used as the blank in all experiments. The amount of nitrite in the samples was calculated from a sodium nitrite standard curve (0–100 μM) freshly prepared in deionized water.
2.5. Measurement of prostaglandin E2 concentration
RAW 264.7 cells were cultured in 48-well plates and treated with 1 μg/ml of LPS in the presence or absence of selected isothiocyanates at various concentrations (0.5–10 μM) for 24 h. Following treatment, PGE2 concentrations in the culture medium were quantified by using the prostaglandin E2 EIA kit according to the manufacturer’s instructions.
2.6. Protein extraction
To prepare whole-cell lysis extracts, RAW 264.7 cells were cultured in 6-well plates and then treated with 1 μg/ml of LPS in the presence or absence of 5 and 10 μM concentrations selected isothiocyanates. Following 12 h treatment, cells were harvested, washed twice with ice-cold PBS (pH 7.4), and gently lysed with 1x cell lysis buffer (Cell Signaling Technologies, Beverly, MA). Cell lysates were then centrifuged at 10,000 g for 10 min at 4°C. Supernatants were collected and protein concentrations determined using BCA protein assay kit.
To prepare cytosol and nuclear extracts, RAW 264.7 cells were cultured in 6-well plates and then treated with 1 μg/ml of LPS in the presence or absence of 10 μM concentrations selected isothiocyanates. Following 30 minute treatment, cells were, washed twice with ice-cold PBS (pH 7.4), and harvested by using NE-PER nuclear and cytoplasmic extraction reagents according to the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL).
2.7. Immunoblotting
15 μg of protein samples were subjected to 10% SDS-polyacrylamide gel electrophoresis and the resolved proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Millipore, Bedford, MA) using a semi-dry transfer system (Fisher Scientific, Pittsburgh, PA). The nonspecific binding of antibodies were blocked by 5% nonfat dried milk in PBST buffer (0.1% Tween 20 in PBS). Immunodetection of COX-2, iNOS, p65, phospho-IκBα , I κ Bα , β-actin, GAPDH and lamin A proteins was carried out using respective primary antibodies (1:1,000 in 3% nonfat dried milk in PBST buffer) and horseradish peroxidase (HRP) conjugated secondary antibodies (1:3,000 in 3% nonfat dried milk in PBST buffer). The immunocomplexes were determined by using the enhanced chemiluminescent system for detecting HRP on immunoblots (Amersham Pharmacia, Piscataway, NJ) and the bands were visualized and quantified by BioRad ChemiDoc XRS system (Hercules, CA). The protein bands were quantified by densitometry and represented as the protein: β-actin, protein: GAPDH or protein: lamin A ratio as and when applicable.
2.8. RNA isolation and reverse transcriptase polymerase chain reaction (RT-PCR) assay
RAW 264.7 cells were treated as described above under Western blot analysis. After 6 h treatment, total RNA was isolated by using a method of TRIzol extraction according to the manufacturer’s instructions. RNA integrity was examined by formaldehyde agarose gel electrophoresis and concentrations were determined by UV spectrophotometry (DU 530 Life Science UV/Visible Spectrophotometer, Fullerton, CA). From each sample, 2 μg of total RNA was then reverse transcribed to single-stranded cDNA by the SuperScript III first-strand cDNA synthesis system. Then PCR analyses were performed on the aliquots of the cDNA preparations to detect COX-2, iNOS, TNF-α , IL-1β , IL-6, and GAPDH (as an internal standard) gene expression using a DNA Engine Dyad® (Waltham, MA). The PCR primers and conditions used in this study were described previously by Khor et al. (2006) [30]. After amplification, PCR products were electrophoresed on 2% agarose gels and visualized using ethidium bromide staining and UV irradiation. The mRNA expression of COX-2, iNOS, TNF-α , IL-1β , IL-6 were quantified by densitometry and represented as the mRNA: GAPDH ratio for each RNA as and when applicable.
2.9. Statistical analyses
The results were presented as means ± standard deviation (S.D.). Data were analyzed by One-way ANOVA followed by a Student’s t test to determine statistical differences between groups using SigmaStat software version 3.11 (Systat Software, Inc., San Jose, CA). The statistical significance of mean differences was based on a p value of < 0.05. Experiments were repeated at least three times.
3. Results
3.1. Suppression of the basal NF-κB transcriptional activity in HT-29-N9 human colon cancer cells by isothiocyanates
The chemical structures of ten structurally divergent synthetic ITCs used in this study are presented in Fig. 1. The cytotoxicity of these ITC analogs in HT-29-N9 cells was initially determined using the MTS assay. It is apparent that none of the tested compounds was cytotoxic at the concentrations tested (data not shown). Next, to examine the structural influence of these ITCs on the basal transcriptional activity of NF-κB, the HT-29-N9 human colon cancer cell line [15], which is stably transfected with NF-κB-luciferase reporter construct, was used as a screening model. Cells were treated with the indicated concentrations of ITCs for 24 h, then the effect of ITC analogs on the NF-κB-luciferase activity was compared.
Fig. 1.
Chemical structures of isothiocyanates used in the current study.
Based on the concentration-dependent modulatory effects of these compounds on the basal NF-κB-luciferase activity, they were classified into three groups; 1) strong NF-κB inhibitors (the maximum inhibitory effect >70% at highest concentration tested), 2) moderate NF-κB inhibitors (maximum inhibitory effect between ~50–65%) and 3) no inhibition effect (Fig. 2, upper panel, middle panel and lower panel, respectively). As shown in Fig. 2, ITC-10 (IC50 = 3.58 ± 2.30 μM), ITC-5 (IC50 = 8.03 ± 1.03 μM) and ITC-4 (IC50 = 8.05 ± 1.70 μM) are strong NF-κB inhibitors. In addition, ITC-8 is also classified as a strong inhibitor because of its high maximum inhibitory effect (IC50 = 16.20 ± 6.18 μM and 73% maximum inhibitory effect obtained at the highest concentration tested). However, ITC-1 (IC50 = 15.36 ± 4.04 μM), ITC-2 (IC50 = 18.85 ± 4.04 μM), ITC-3 (IC50 = 22.99 ± 5.21 μM) and a positive control PEITC (IC50 = 21.24 ± 6.24 μM) showed moderate inhibitory effects over the same concentration range, whereas ITC-6, ITC-7 and ITC-9 could not inhibit the NF-κB transcriptional activity in HT-29-N9 cells. Interestingly, at higher concentrations, ITC-6 and ITC-9 showed increase in the NF-κB-Luc activity. To address whether inhibitory effects of ITCs on NF-κB-transcriptional activity can be translated into anti-inflammatory actions of these compounds, we selected ITC-1, ITC-8 and ITC-9 as representative of each group for further investigation. ITC-8 was chosen instead of ITC-10 as the candidate to represent the strong NF-κB inhibitor because, at higher concentration (>15 μM), ITC-10 was found to have significant toxicity in in vitro [31]. Moreover, ITC-8 was found to have the highest maximum induction activity on the antioxidant response-element (ARE)-mediated luciferase activity among the compounds studied [31].
Fig. 2.
Concentration-dependent inhibition of NF-κB-luciferase activity in HT-29-N9 cells by isothiocyanates. Cells were treated with various concentrations of different isothiocyanates for 24 h and lysates of the cells were measured for luciferase activity. Each column and bar represents the mean ± S.D. of three independent assays. *Significant difference from control (0.1% ethanol) at P < 0.05.
3.2. Inhibition of nitrite production, expression of iNOS mRNA and protein in LPS-stimulated RAW 264.7 murine macrophages by isothiocyanates
We next examined whether the suppression effect of ITCs on NF-κB-transcriptional activity is translated into inhibition of the pro-inflammatory enzyme iNOS activity. We used LPS-stimulated RAW 264.7 cells [32] as a model system and quantified nitrite concentration as a measure of NO production catalyzed by iNOS enzyme. Effect of LPS in enhancing the NO production in RAW264.7 cells was time-dependent (data not shown). At 24 h, LPS (1 μg/ml) significantly induced accumulation of nitrite in culture media, with levels reaching 26.35 ± 0.48 μM as compared to control cells 1.13 ± 0.53 μM (~23-fold higher). As shown in Fig. 3A, treatment of PEITC, ITC-1 or ITC-8 inhibited LPS-induced NO production in a dose-dependent manner (IC50 values were 1.28 ± 0.41, 2.03 ± 0.73, 2.57 ± 0.99 μM, respectively) and the maximum inhibitory effect, at 10 μM, was >75%. Interestingly, at the higher concentration, ITC-9 showed modest suppression effect on LPS-induced NO production (23% inhibition on LPS-induced NO production) as compared to minimal inhibition of NF-κB-Luc activity in HT-29-N9 cells described above. Since the cytotoxic effect was not observed in RAW 264.7 cells after ITCs treatment (data not shown), this result implied that ITCs inhibited nitrite release without causing cell death.
Fig. 3.
Inhibition of nitric oxide production in LPS-activated RAW 264.7 cells by isothiocyanates (A). Cells were treated with LPS (1 μg/ml) for 24 h in the presence of different concentrations of PEITC or ITC-1 or ITC-8 or ITC-9. The culture supernatants were subsequently analyzed for nitrite production. The data were percentage of control values (LPS-stimulated cells in the absence of ITCs). Each point represents the mean ± S.D. of three independent assays. *Significant difference from control (0.1% ethanol) at P < 0.05. Suppression of iNOS protein (B) and mRNA (C) expression by ITCs. The protein expression of iNOS and GAPDH was monitored after 12 h treatment with vehicle control (0.1% ethanol) or LPS (1 μg/ml) or combination of LPS/PEITC or LPS/ITC-1 or LPS/ITC-8 or LPS/ITC-9 using immunoblotting. The mRNA expression of iNOS and GAPDH was quantified by RT-PCR analysis after 6 h incubation. Cells were treated as described in (B).
Because nitrite formation was significantly suppressed by ITCs, a reduction in the de novo protein synthesis could possibly be involved in the inhibition of iNOS activity, thus we next examined the effect of ITCs on the expression level of iNOS protein. As determined by using immunoblotting, LPS increased iNOS protein expression when compared to un-stimulated control cells in a time-dependent pattern (data not shown). Importanly, at 12 h, 10 μM of PEITC, ITC-1 or ITC-8 completely abolished the LPS-stimulated iNOS protein expression (Fig. 3B). Parallel to the results obtained from nitrite measurements, these inhibitory effects of the ITCs were dose-dependent. Interestingly, ITC-9 could also decrease the LPS-stimulated iNOS protein expression to a certain degree.
Since the expression of iNOS is regulated at the level of transcription, RT-PCR was performed to study the effect of ITCs on iNOS mRNA expression. The response of iNOS mRNA to LPS stimulation was observed at 6 h after treatment. Consistent with the NO production and iNOS protein expression, the iNOS mRNA expression was decreased with increasing concentration of ITCs, however the alteration of iNOS mRNA level by ITC-9 was not observed. These results indicated that the reductions in the expression of iNOS protein and mRNA contributed to the inhibitory effect of ITCs on LPS-induced NO production (with the exception of ITC-9).
3.3. Inhibition of Prostaglandin E2 (PGE2) production, expression of COX-2 mRNA and protein in LPS-stimulated RAW 264.7 cells by isothiocyanates
To examine whether ITCs could affect LPS-stimulated PGE2 synthesis, a measure of pro-inflammatory enzyme COX-2 activity, RAW 264.7 cells were treated under the same condition as described above in Fig. 3A and the concentrations of PGE2 in culture media were measured as described in Materials and methods. LPS stimulation (1 μg/ml; 24 h) yielded a high amount of PGE2 biosynthesis with levels reaching 14,596 ± 200 pg/ml as compared to cells in quiescent state 258 ± 57 pg/ml (~57-fold higher). The significant suppression observed in cells treated with 10μM of ITC-1, ITC-8 or PEITC was 62%, 63%, 59%, respectively (Fig. 4A). These ITCs also showed dose-dependent reduction of PGE2 biosynthesis as compared to the vehicle control (not shown). The IC50 values in suppressing LPS-stimulated PGE2 synthesis of ITC-1, ITC-8 and PEITC were 8.71 ± 2.36, 7.68 ± 1.52, 5.27 ± 1.07 μM, respectively. At the same concentration range, ITC-9 did not have any suppression effect on LPS-induced PGE2 production. The inhibitory actions of ITCs were not caused by their cytotoxicity since the concentration that inhibits PGE2 production does not affect cell viability.
Fig. 4.
Inhibition of PGE2 production in LPS-activated RAW 264.7 cells by isothiocyanates (A). PGE2 concentrations were determined using the prostaglandin E2 EIA kit. Each column and bar represents the mean ± S.D. of three independent assays. *Significant difference from control (0.1% ethanol) at P < 0.05. Suppression of COX-2 protein (B) and mRNA (C) expression by isothiocyanates. Cells were treated as previously described (Fig. 3B–C).
Since ITC-1, ITC-8 and PEITC were shown to effectively suppress LPS-induced PGE2 synthesis, their effects on LPS-induced COX-2 protein and mRNA expression were further investigated. As shown in Fig. 4B–C, these ITCs dose-dependently suppressed COX-2 expression at both protein and mRNA level. Although, ITC-9 had modest suppression effect on LPS-induced COX-2 protein expression, however, it did not change the level of LPS-induced COX-2 mRNA expression drastically. Given that these ITCs significantly inhibited LPS-induced PGE2 production and COX-2 mRNA/protein expression, it may suggest that the inhibition of ITCs on PGE2 production could be mainly through transcriptional mechanisms.
3.4. Suppression effects of isothiocyanates on the mRNA expression of pro-inflammatory cytokine IL-1β , IL-6 and TNF-α in LPS-stimulated RAW 264.7 cells
As described above, ITCs potently inhibited LPS-induced production of pro-inflammatory enzyme iNOS and COX-2. We further investigated their effects on LPS-induced pro-inflammatory cytokine IL-1β , IL-6 and TNF-α expression by RT-PCR. In response to LPS, the expression of IL-1β , IL-6 and TNF-α was markedly upregulated and treatment with ITCs significantly inhibited their induction by LPS (with the exception of ITC-9, Fig. 5). Comparing the effect of ITCs on cytokine mRNA expression, we observed a similar pattern of inhibitory effects of ITCs on LPS-induced iNOS and COX-2 expression. These data suggested that ITCs may elicit their overall anti-inflammatory effects through the same transcription factor or pathway including NF-κ B that regulates the transcription levels of these pro-inflammatory enzymes and cytokines.
Fig. 5.
Suppression of IL-1β , IL-6 and TNF-α mRNA expression by isothiocyanates in LPS-activated RAW 264.7 cells. Cells were treated as described in Fig. 3c.
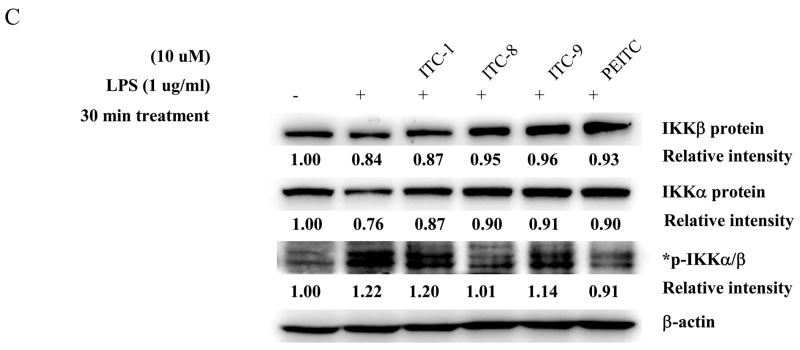
3.5. Suppression of phosphorylation of Iκ B kinase α /β (IKK α /β ), phosphorylation and degradation of IκBα protein, and nuclear translocation of p65 in LPS-stimulated RAW 264.7 cells by isothiocyanates
To further investigate the molecular mechanism involved in ITC-mediated inhibitions of iNOS, COX-2, IL-1β , IL-6 and TNF-α transcription as described above, we next focused on the NF-κB signaling pathway, which is known to be responsible for the transactivation of these genes. One of the major mechanisms involving the transcriptional activation of NF-κB is through the phosphorylation of IκB kinase α /β (IKKα /β ) and subsequent phosphorylation of IκBα protein, allowing the release of p65 NF-κB and its translocation to the nucleus. As shown in Fig. 6, the increased phosphorylation of IκBα occurred concomitantly with its decreased degradation after treatment with LPS (1 μg/ml) in the positive control (Fig. 6A). Treatments with ITCs decreased both the LPS-induced phosphorylation and degradation of I κ Bα protein marginally. Furthermore, the p65 nuclear translocation was notably observed after LPS stimulation (Fig. 6B). Consistently, treatment with ITCs significantly attenuated LPS-induced p65 nuclear translocation, except for ITC-9, which is classified as a non-NF-κB inhibitor (Fig. 6B). In addition, our data affirmed that LPS-induced phosphorylation of IκBα was mediated through the activation of the upstream IκB kinase complexes (IKKs). In parallel with the inhibitory effect of ITCs on LPS-induced phosphorylation and degradation of IκBα protein, ITCs caused a slight down-modulation of phosphorylated IKKα /β complexes (Fig. 6C). These data confirm that stimulation of NF-κB pathway would be required for LPS-induced pro-inflammatory mediators release and ITCs could selectively suppress this IKKα /β-mediated phosphorylation of IκBα and NF-κB pathway leading to down-modulation of inflammatory response. However, it is worth noticing that NF-κB pathway may be only one pathway among several modified by ITCs because phosphorylation of IKKα /β is also regulated by other upstream factors such as MAPKs pathways, including ERK, JNK and p38 and we did found the upregulation of these kinases after LPS stimulation (30 minute treatment). Yet, these ITCs did not alter the phosphorylation levels of these kinases, suggesting that ITCs could modulate the phosphorylation level of IKKα /β through other upstream factors or pathways.
Fig. 6.
Effects of isothiocyanates on I κ Bα and phosphorylated-IκBα protein (A), p65 localization (B) and IKKα , IKKβ and phosphorylated-IKKα /β protein (C) in LPS-activated RAW 264.7 cells. The level of protein expression was monitored after 30 min treatment using immunoblotting. *The two bands that are located at the central of the lanes were included in the quantification.
4. Discussion
As various agents are able to activate NF-κB and inhibition of NF-κB activity was found to be beneficial in cancer prevention and treatment, the current study was conducted to screen structurally divergent PEITC analogs for potential anti-inflammatory activity. In most cells, NF-κB is sequestered in the cytosol, associated with inhibitor proteins, IkBs, however, NF-κB is constitutively activated in many cancers such as human breast, colon and ovarian carcinoma [33]. Recently, our group have found that ITC such as sulforaphane and PEITC suppress the high basal activity of NF-κB in human prostate cancer PC-3 cells [34], therefore, we were motivated to further investigate ability of various ITCs in suppressing NF-κB as it was shown that inhibition of NF-κB activity in carcinoma cell lines could dramatically reduce cell growth and metastatic properties in vivo [35, 36] and also enhanced the anti-tumor therapy of a chemo-resistant tumor in vivo [37]. Given the important roles of NF-κB in carcinogenesis, transformation, apoptosis, and chemoprevention, the construct was done in the cells to elucidate the chemopreventive mechanisms of these compounds in modulating NF-κB.
The in vitro and in vivo cancer chemopreventive potential of the naturally occurring ITCs have gained increasing popularity recently [1–4, 38]. Among these, PEITC, one of the most extensively studied members, has been shown to possess a great ability to modulate the activity of xenobiotic metabolizing enzymes and induce apoptosis in cancer cells [13, 14, 39, 40]. Recently, its anti-inflammatory activity has been suggested to be an alternative protective mechanism against carcinogenesis [15, 41]. In the current investigation, we found that several structurally divergent synthetic ITCs have superior NF-κB inhibition and anti-inflammatory actions compared to the well-known PEITC.
Transcription factor NF-κB plays a critical role in several signal transduction pathways involved in various cancers as well as chronic inflammatory diseases [17]. Activation of NF-κB can protect cancer cells from apoptotic stimuli, apparently through the induction of survival genes. Hence, agents that are able to inhibit NF-κB transcriptional regulation and modulate the inflammatory response may have therapeutic use and chemopreventive value. Recently, there is a growing interest amongst researchers in targeting NF-κB signaling pathway in the fight against carcinogenesis [42–44]. Considering limited studies concerning the structure-activity relationship of ITCs on NF-κB transactivation, we examined the effect of structurally divergent synthetic ITCs on the basal transcriptional activity of NF-κB. In our current study, based on the degree of NF-κB-Luc inhibitory effect, ITCs were classified into three groups. Of these, tetrahydrofurfuryl isothiocyanate (ITC-4), methyl-3-isothiocyanatopropionate (ITC-5), 3-morpholinopropyl isothiocyanate (ITC-8) and 3,4-methyelendioxybenzyl isothiocyanate (ITC-10) are the strongest NF-κB inhibitors, more potent than PEITC. These data suggested that some of the synthetic analogs of PEITC with wide range of functional groups could inhibit NF-κB transactivation, while the best candidate with such inhibitory activity found to be the one maintaining its aromatic ring, ITC-10 (6.2-fold increased as compared to PEITC). However, the aromatic ring is not essential for this inhibitory activity, as observed in ITC-3. Though there is a heterocyclic furan ring to take the place of the aromatic dioxybenzyl group in ITC-10, ITC-3 has a poorer activity as compared to PEITC. Moreover, when there is a tetrahydrofurfuryl replacement in ITC-3, forming a non-aromatic compound, ITC-4, the inhibitory NF-κB transactivation activity was somehow regained, and interestingly, ITC-4 has 2.6-fold increased potency as compared to PEITC. The fact that ITC-5 and ITC-8 also possessed strong inhibitory activity and being classified as strong NF-κB inhibitors, again suggested that the aromaticity is not essential for the inhibitory activity. Moreover, such aromaticity could drastically reduce the activity, as observed in ITC-9 when a benzoic moiety is connected directly to the isothiocyanate N=C=S group, ITC-9 was found to be one of the poorest NF-κB inhibitors. At this juncture, our collective data indicated that various synthetic ITCs with different functional groups have different degrees of NF-κB inhibitory activity. We have also identified that among the tested analogs, ITC-4, ITC-5, ITC-8 and ITC-10 were found to have stronger NF-κB inhibitory activity (2.6 to 6.2-fold increase) as compared to their parent naturally occurring compound, PEITC. Such finding suggests that the putative receptor site interacting with ITCs, could be relatively nonspecific and is able to interact with structurally diverse ITCs.
To have therapeutic use and chemopreventive value, the inhibition of NF-κB activity of ITCs needs to be translated into anti-inflammatory actions. Overproduction of NO and PGE2, a hallmark of inflammation, in a RAW 264.7 murine macrophage model [32], has been used as target mediators to determine the anti-inflammatory action of ITCs. In agreement with the results obtained from NF-κB-luciferase activity assay, we observed that the overall inhibitory effects of ITCs (ITC-1, ITC-8 and PEITC) on NF-κB-transcriptional activity were well-correlated to their anti-inflammatory effects assessed by the above markers, suggesting that HT-29-N9 could be used as a model for screening NF-κB inhibitors.
We had found that the suppressive effect of ITCs on LPS-induced production of NO and PGE2 was mediated at the transcription level. In addition, pro-inflammatory cytokine TNF-α , IL-1β and IL-6 mRNA expression stimulated by LPS treatment was attenuated by ITCs. These data are in agreement to that previously found for PEITC [16, 45]. Noticeably, these cytokines are critical molecules in immune responses and inflammatory networking in carcinogenesis, suggesting this inhibitory effect of ITCs can be a partial molecular basis for their anti-inflammatory and chemopreventive properties.
Focusing on NF-κB signaling pathway, a critical step in LPS-induced NF-κB nuclear accumulation and transcriptional activity is its dissociation from IκB protein. Exposure to stimuli leads to the activation of the upstream IκB kinase complexes (IKKs), resulting in rapid phosphorylation and proteolytic degradation of IκB protein, which makes free NF-κB translocate to the nucleus where it regulates gene transcription [26]. Consistently, we found that ITCs inhibited LPS-induced IKKα /β phosphorylation, IkBα phosphorylation and degradation and subsequently, decrease LPS-induced p65 nuclear accumulation. It is clearly evident that ITCs inhibited NF-κB transactivation via stabilization of IκBα and lower p65 nuclear translocation. Since ITC analogs could decrease phosphorylation of IκB kinase complexes (IKKs), therefore, the upstream factors such as MAPKs pathways, including ERK, JNK and p38, may be a target of these ITCs to impair NF-κB signaling. Surprisingly, our results showed that ITCs did not alter these MAPKs pathways (not shown). Therefore future study exploring the upstream targets of these ITCs is needed.
Inflammatory responses are mediated by several transcription factors and cellular signaling events. In addition to NF-κB, another early transcription factor AP1, which regulates expression of a number of proinflammatory genes either alone or by coupling with NF-κB [46], may also be involved in anti-inflammatory activity of ITCs. We have recently reported that the ITCs activate the Nrf2/ARE signaling pathway [31], leading to the upregulation of anti-oxidative stress/cellular defense enzymes such as heme oxygenase 1 (HO-1). Interestingly, our present results showed that there is a "general trend" of correlation between ITC induction of HO-1 and NF-κB signaling. It is possible that there is a “cross-talk” between Nrf2/ARE and NF-κB signaling pathways in response to inflammation. In addition, HO-1 induction by synthetic chalcone has been reported to suppress the inflammatory response to LPS [47], therefore, induction of HO-1 by these ITCs could be partially contributing to the overall anti-inflammatory properties of ITCs.
In conclusion, our data indicate that the inhibitory effect of synthetic ITC analogs on NF-κB-mediated pro-inflammatory gene transcription is quite specific and depends on the chemical structure. A subtle change in the ITC structure can have a significant impact on its inhibitory effect. Amongst the ITCs, tetrahydrofuran (ITC-4), isothiocyanatopropionate (ITC-5), morpholine (ITC-8) and methylenedioxybenzene (ITC-10) functional groups are suggested to have superior NF-κB inhibition than others. These ITC analogs strongly inhibit the generation of NO and PGE2 in LPS-stimulated RAW 264.7 cells via interruption of the NF-κB pathway. Moreover, they also suppress the expression of pro-inflammatory cytokines TNF-α , IL-1β and IL-6. Our findings suggest the synthetic ITC analogs may have superior chemopreventive effects than natural ITCs and also have therapeutic use as anti-inflammatory agents.
Acknowledgments
The source of financial support: This work was supported by the National Institute of Health (R01-CA73674 to A.-N.T.K.), and the Post doctoral fellowship scholarship was funded by the Thailand Center of Excellence for Life Sciences (TCELS), Thailand (to A. Prawan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Poppel G, Verhoeven DT, Verhagen H, Goldbohm RA. Brassica vegetables and cancer prevention. Epidemiology and mechanisms. Adv Exp Med Biol. 1999;472:159–168. doi: 10.1007/978-1-4757-3230-6_14. [DOI] [PubMed] [Google Scholar]
- 2.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 3.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- 4.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- 5.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. Faseb J. 2006;20:506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khor TO, Keum YS, Lin W, Kim JH, Hu R, Shen G, Xu C, Gopalakrishnan A, Reddy B, Zheng X, Conney AH, Kong AN. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 2006;66:613–621. doi: 10.1158/0008-5472.CAN-05-2708. [DOI] [PubMed] [Google Scholar]
- 7.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Hu R, Khor TO, Shen G, Jeong WS, Hebbar V, Chen C, Xu C, Reddy B, Chada K, Kong AN. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis. 2006;27:2038–2046. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]
- 9.Stoner GD, Morrissey DT, Heur YH, Daniel EM, Galati AJ, Wagner SA. Inhibitory effects of phenethyl isothiocyanate on N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus. Cancer Res. 1991;51:2063–2068. [PubMed] [Google Scholar]
- 10.Yang YM, Conaway CC, Chiao JW, Wang CX, Amin S, Whysner J, Dai W, Reinhardt J, Chung FL. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002;62:2–7. [PubMed] [Google Scholar]
- 11.Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555:191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Yuan X, Pan Z, Shen G, Kim JH, Yu S, Khor TO, Li W, Ma J, Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;5:1918–1926. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- 13.Hu R, Kim BR, Chen C, Hebbar V, Kong AN. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis. 2003;24:1361–1367. doi: 10.1093/carcin/bgg092. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Xu C, Keum YS, Reddy B, Conney A, Kong AN. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with beta-phenylethyl isothiocyanate and curcumin. Carcinogenesis. 2006;27:475–482. doi: 10.1093/carcin/bgi272. [DOI] [PubMed] [Google Scholar]
- 15.Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res. 2004;21:661–670. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 16.Rose P, Won YK, Ong CN, Whiteman M. Beta-phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide. 2005;12:237–243. doi: 10.1016/j.niox.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 18.Goossens L, Pommery N, Henichart JP. COX-2/5-LOX dual acting anti-inflammatory drugs in cancer chemotherapy. Curr Top Med Chem. 2007;7:283–296. doi: 10.2174/156802607779941369. [DOI] [PubMed] [Google Scholar]
- 19.Meric JB, Rottey S, Olaussen K, Soria JC, Khayat D, Rixe O, Spano JP. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol. 2006;59:51–64. doi: 10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Rao CV. Nitric oxide signaling in colon cancer chemoprevention. Mutat Res. 2004;555:107–119. doi: 10.1016/j.mrfmmm.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 22.Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G626–634. doi: 10.1152/ajpgi.2001.281.3.G626. [DOI] [PubMed] [Google Scholar]
- 23.Kroncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. 1998;113:147–156. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu KK. Transcription-based COX-2 inhibition: a therapeutic strategy. Thromb Haemost. 2006;96:417–422. [PubMed] [Google Scholar]
- 25.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 26.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 27.Muller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993;187:233–256. doi: 10.1016/S0171-2985(11)80342-6. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Nims RW, Cook JC, Krishna MC, Christodoulou D, Poore CM, Miles AM, Grisham MB, Wink DA. Colorimetric assays for nitric oxide and nitrogen oxide species formed from nitric oxide stock solutions and donor compounds. Methods Enzymol. 1996;268:93–105. doi: 10.1016/s0076-6879(96)68012-4. [DOI] [PubMed] [Google Scholar]
- 30.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 31.Prawan A, Keum YS, Khor TO, Yu S, Nair S, Li W, Hu L, Kong AN. Structural influence of isothiocyanates on the antioxidant response element (ARE)-mediated heme oxygenase-1 (HO-1) expression. Pharm Res. 2008;25:836–844. doi: 10.1007/s11095-007-9370-9. [DOI] [PubMed] [Google Scholar]
- 32.Naureckiene S, Edris W, Ajit SK, Katz AH, Sreekumar K, Rogers KE, Kennedy JD, Jones PG. Use of a murine cell line for identification of human nitric oxide synthase inhibitors. J Pharmacol Toxicol Methods. 2007;55:303–313. doi: 10.1016/j.vascn.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Dejardin E, Deregowski V, Chapelier M, Jacobs N, Gielen J, Merville MP, Bours V. Regulation of NF-kappaB activity by I kappaB-related proteins in adenocarcinoma cells. Oncogene. 1999;18:2567–2577. doi: 10.1038/sj.onc.1202599. [DOI] [PubMed] [Google Scholar]
- 34.Xu C, Shen G, Chen C, Gélinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 35.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 36.Duffey DC, Chen Z, Dong G, Ondrey FG, Wolf JS, Brown K, Siebenlist U, Van Waes C. Expression of a dominant-negative mutant inhibitor-kBa of nuclear factor-kB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Res. 1999;59:3468–3474. [PubMed] [Google Scholar]
- 37.Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 38.Keum YS, Jeong WS, Kong AN. Chemopreventive functions of isothiocyanates. Drug News Perspect. 2005;18:445–451. doi: 10.1358/dnp.2005.18.7.939350. [DOI] [PubMed] [Google Scholar]
- 39.Chen YR, Han J, Kori R, Kong AN, Tan TH. Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J Biol Chem. 2002;277:39334–39342. doi: 10.1074/jbc.M202070200. [DOI] [PubMed] [Google Scholar]
- 40.Yu R, Jiao JJ, Duh JL, Tan TH, Kong AN. Phenethyl isothiocyanate, a natural chemopreventive agent, activates c-Jun N-terminal kinase 1. Cancer Res. 1996;56:2954–2959. [PubMed] [Google Scholar]
- 41.Chen YH, Dai HJ, Chang HP. Suppression of inducible nitric oxide production by indole and isothiocyanate derivatives from Brassica plants in stimulated macrophages. Planta Med. 2003;69:696–700. doi: 10.1055/s-2003-42790. [DOI] [PubMed] [Google Scholar]
- 42.Calzado MA, Bacher S, Schmitz ML. NF-kappaB inhibitors for the treatment of inflammatory diseases and cancer. Curr Med Chem. 2007;14:367–376. doi: 10.2174/092986707779941113. [DOI] [PubMed] [Google Scholar]
- 43.Umezawa K. Inhibition of tumor growth by NF-kappaB inhibitors. Cancer Sci. 2006;97:990–995. doi: 10.1111/j.1349-7006.2006.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Uto T, Fujii M, Hou DX. 6-(Methylsulfinyl)hexyl isothiocyanate suppresses inducible nitric oxide synthase expression through the inhibition of Janus kinase 2-mediated JNK pathway in lipopolysaccharide-activated murine macrophages. Biochem Pharmacol. 2005;70:1211–1221. doi: 10.1016/j.bcp.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 46.Adcock IM. Transcription factors as activators of gene transcription: AP-1 and NF-kappa B. Monaldi Arch Chest Dis. 1997;52:178–186. [PubMed] [Google Scholar]
- 47.Lee SH, Seo GS, Kim JY, Jin XY, Kim HD, Sohn DH. Heme oxygenase 1 mediates anti-inflammatory effects of 2',4',6'-tris(methoxymethoxy) chalcone. Eur J Pharmacol. 2006;532:178–186. doi: 10.1016/j.ejphar.2006.01.005. [DOI] [PubMed] [Google Scholar]