Abstract
A 5T/6T polymorphism in the human MMP-3 promoter affects gene expression and impacts the risk and/or severity of various pathological conditions. Chromatin immunoprecipitation (ChIP) in human fibroblasts homozygous for the 6T site demonstrate that it is bound by NF-κB and ZBP-89 transcription factors in its native chromatin. ChIP in COS-1 cells transfected with plasmids containing the 5T and 6T sites in the context of 2 kb of the MMP-3 promoter showed that NF-κB p50 binds preferentially to the 6T site, while more ZBP-89 binding is detected to the 5T site. Over-expressed ZBP-89 increased transcription from the 5T promoter but not from the 6T, while NF-κB decreased transcription from both promoters, even in the presence of excess ZBP-89. A model is suggested in which the physiological impact of the polymorphism is dependent on the relative levels and activities of these competing factors in various cell types and conditions.
Keywords: stromelysin-1, matrix metalloproteinase, transcription factor, NF-κB, ZBP-89, ZNF148, polymorphism
INTRODUCTION
Matrix metalloproteinases (MMPs) are involved in physiological tissue remodeling as well as a number of pathological processes, including periodontitis, rheumatoid arthritis, atherosclerosis, cancer, angiogenesis, emphysema and osteoporosis [1]. MMP-3 (stromelysin-1) has broad substrate specificity, degrading laminin, fibronectin, and non-fibrillar collagens [2] and can also activate proMMP-1, -8, -9 and –13 [3; 4; 5], inactive plasminogen activator inhibitor I [6] and cleave FasL [7] and E-cadherin [8]. Its role in tissue destruction associated with chronic inflammation is well established.
In a previous study of the MMP-3 promoter, the SIRE site (stromelysin IL-1 responsive element; −1614G(T)TTTTTCCCCCCATCAAAG−1595) was identified as a repressor element and a site of IL-1 induced DNA binding [9]. In normal human fibroblasts, a 5T reporter construct was twice as transcriptionally active as a 6T construct, but both were more active than a mutant construct lacking the site entirely [10]. Several studies have shown that tissue levels of MMP-3 protein are determined by the polymorphism such that more protein is expressed in 5T homozygotes as compared to heterozygotes or 6T homozygotes [11; 12; 13; 14]. In addition, there are now many studies that find an association of either the 5T or 6T polymorphism with susceptibility to or severity of various diseases and conditions [15; 16; 17; 18; 19; 20; 21]. For example, homozygosity for the 6T allele is associated with increased progression of atherosclerosis, while individuals homozygous for the 5T allele have increased risk of aneurysms and myocardial infarction [15; 17; 22]. These studies illustrate the functional significance of the polymorphism in vivo, and suggest that both low and high levels of MMP-3 can have pathological consequences.
Zinc binding protein 89 (ZBP-89) was cloned from a yeast one-hybrid system through its ability to bind to this site [23]. However, over-expressed ZBP-89 was a weak activator of MMP-3 expression, and its binding was dependent on Cs in the site rather than the Ts as expected for the repressor. NF-κB p50 and p65/RelA also bind to the SIRE site and recombinant p50 binds preferentially to the 6T site [24]. However, although ZBP-89, p50 and p65/RelA have all been shown to bind to the site in gelshift assays, their roles in transcriptional regulation of MMP-3 have not been well established.
In the experiments reported here, we show that both NF-κB and ZBP-89 bind to the site in vivo and provide evidence of competitive binding. Over-expressed ZBP-89 activates the 5T promoter, while NF-κB represses both the 5T and the 6T, even in the presence of excess ZBP-89. The results of these studies suggest that the variable impact of this polymorphism is likely related to cell-, tissue- and context-specific differences in the availability and/or activity of these transcription factors.
MATERIALS AND METHODS
Plasmid constructs
The 5TStro construct consists of 2050 bp 5′ flanking region of the human MMP-3 gene in the pGL3Basic luciferase reporter vector [9]. 6TStro was generated by site-directed mutagenesis of 5TStro (Ana-Gen Technologies, Inc., Atlanta, GA). dStro is a deletion mutant lacking the SIRE site. NFκB p50 and p65 expression vectors under control of the RSV promoter were obtained from the NIH AIDS Research Reagents Program. ZBP-89 cDNA was obtained from Dr. Juanita Merchant (University of Michigan) and sub-cloned into the pREP4 mammalian expression vector (Invitrogen). Oligodeoxynucleotides containing single copies of the 5T and 6T SIRE site (5′ G(T)TTTTTCCCCCCATCAAAG 3′) were sub-cloned into the pGL3Enhancer vector (Promega).
Cell culture and transient transfection
COS-1 and human foreskin fibroblast (HFF) cells (ATCC) were maintained in Eagle’s minimal essential medium supplemented with 10% fetal bovine serum and antibiotic/antimicotic (Life Technologies, Inc.). Sequencing of HFF chromosomal DNA revealed the cell line to be homozygous for the 6T version of the MMP-3 promoter. Transient transfection of COS-1 cells was performed in triplicate using Lipofectamine Plus (GibcoBRL), with SV-βgal as a control for transfection efficiency. In co-transfection experiments, pBluescript was used as necessary to equalize amounts of DNA. Cells were harvested 48 hours after transfection and luciferase and β-galactosidase activity were measured using reagents and protocols from Promega and Clontech, respectively.
Chromatin Immunoprecipitation
ChIP assays were performed using the modifications suggested by Nowak et al [25] to improve detection of NFκB binding. Briefly, endogenous proteins were cross-linked to DNA in a two-step process including protein-protein cross-linking with N-hydroxysuccinimade (NHS) ester prior to conventional DNA-protein cross-linking by addition of formaldehyde to the cultured cells. The DNA was then isolated and sheared by sonication. The proteins of interest were immunoprecipitated along with the DNA to which it was bound using antibodies specific for IgG, ZBP-89/ZNF148, p50 and p65 (Santa Cruz), and then the cross-links were reversed. In HFF cells a 1 kb fragment of the endogenous MMP-3 promoter was amplified and quantitated by quantitative real-time PCR using the human MMP-3 (−) 02kb primer set and reagents and protocols from SuperArray Bioscience, with RNA polymerase II binding to the human GAPDH proximal promoter as a positive control. COS-1 cells were transfected with the 5T and 6TStro plasmids 24 hours before performance of ChIP assays. A 340 bp region of the MMP-3 promoter containing the SIRE site was amplified by PCR and analyzed by agarose gel electrophoresis followed by quantitiation using ImageJ software.
RESULTS
Binding of ZBP-89 and NF-κB to 5T and 6T promoters
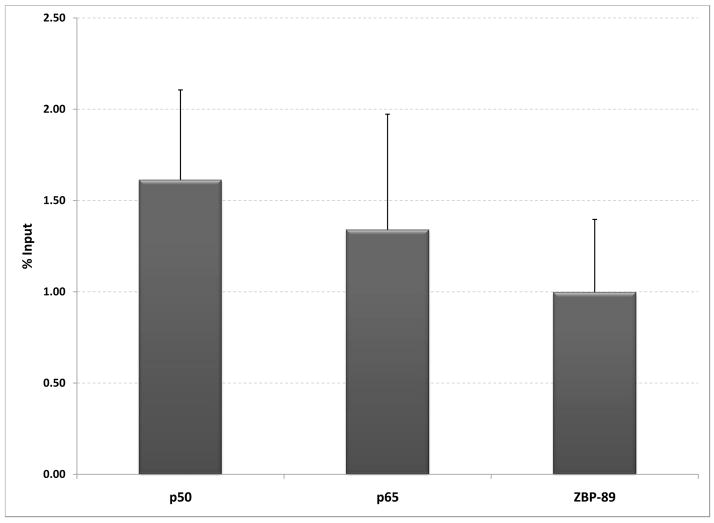
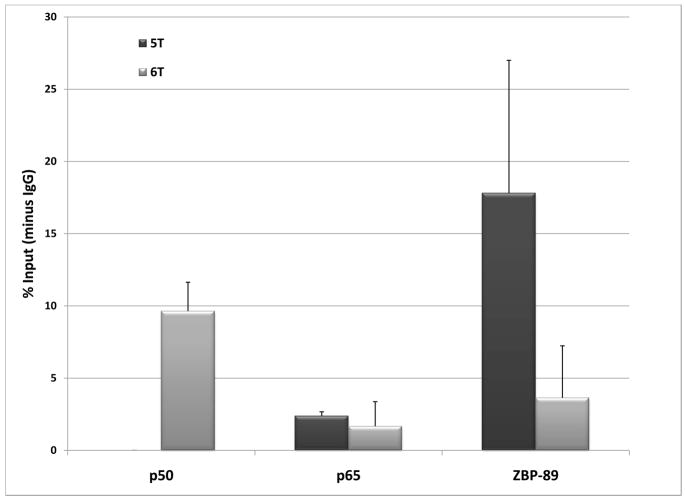
Previous studies of the binding of these factors in vitro had suggested that recombinant p50 binds preferentially to the 6T site, while ZBP-89 binds to the C rich region and recognizes both 5T and 6T sites [23; 24]. Chromatin immunoprecipitation (ChIP) studies were conducted to determine whether these proteins bind the site in a native chromatin context, and whether these binding preferences exist in the context of the MMP-3 promoter. Sequencing of an HFF cell line revealed it be homozygous for the 6T SIRE site. As shown in Figure 1A, ChIP assays on the native promoter showed binding of all three endogenous proteins. In order to better study the binding preferences of each of the proteins, COS-1 cells were transiently transfected with 5T and 6TStro constructs containing the SIRE site in the context of 2 kb of the human MMP-3 promoter. Figure 1B shows that binding of p50, p65 and ZBP-89 was detected to both versions of the promoter. However, more ZBP-89 binding was detected on the 5T promoter, while more p50 binding was detected on the 6T promoter. Lower levels of p65/RelA binding were detected on both.
Figure 1. Chromatin immunoprecipitation.
A. An HFF cell line was shown be homozygous 6T/6T. Shown are results from 4 independent ChIP assays, using antibodies against p50, p65 and ZBP-89/ZNF148. Results were quantitated by real-time PCR, and normalized to results obtained for RNA polymerase II binding to the GAPDH proximal promoter. The graph shows % input +/− SEM. B. COS-1 cells were transfected with 5T or 6TStro plasmids containing 2 kb of the human MMP-3 promoter and ChIP assays were performed. Immunoprecipitated fragments, or input fractions, were amplified by PCR and run on agarose gels. Quantitation of ethidium bromide stained gels was performed by ImageJ software. The graph shows results from three independent experiments, as % input with results from IgG non-specific immunoprecipitation subtracted, +/− SEM.
The SIRE site functions as an enhancer element in transiently transfected COS-1 cells
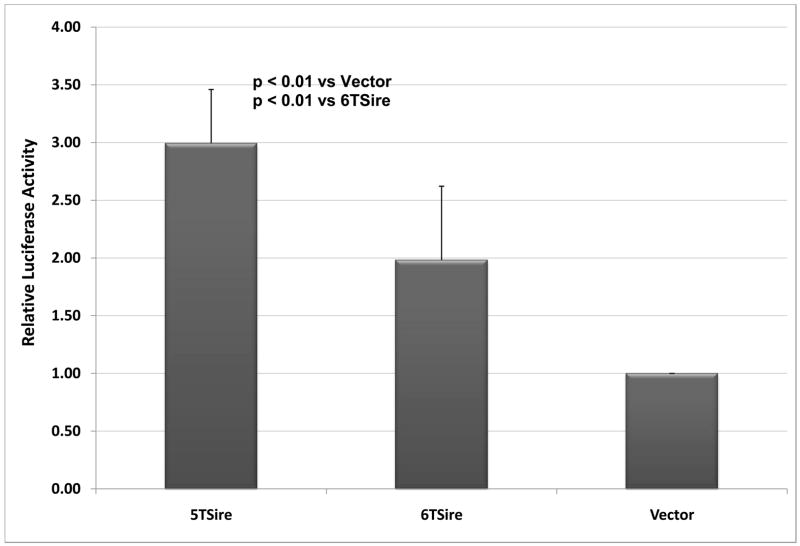
In order to further study the role of the SIRE site in transcriptional regulation, we began by subcloning single copies of 5T and 6T versions of the SIRE site into the pGLEnhancer vector (Promega). These plasmids were transfected into COS-1 cells. Although the 5T site did result in significantly more transcription than the 6T as expected from previous studies, both the 5T and the 6T site were more transcriptionally active than the vector alone (Figure 2A). When the 5T and 6T were compared in the context of 2 kb of MMP-3 promoter, similar results were observed – the 5T promoter was more transcriptionally active than the 6T promoter, but both were more active than a deletion mutant lacking the SIRE site (Figure 1B).
Figure 2. The SIRE site functions as an enhancer element in COS-1 cells.
A. COS-1 cells were transfected with a pGL3Enhancer vector alone or containing a single copy of the 5T or 6T SIRE site, and B. Cells were transfected with luciferase reporter constructs containing the 5T or 6T site in the context of 2 kb of the human MMP-3 promoter. SV-βgal was co-transfected in each case as a control for transfection efficiency. The graphs show normalized luciferase activity expressed relative to the vector or deletion mutant controls for 5 experiments done in triplicate, +/− SEM. Statistical significance was determined using Student’s t-test.
Effects of ZBP-89 and NF-κB on transcription from 5T and 6T promoters
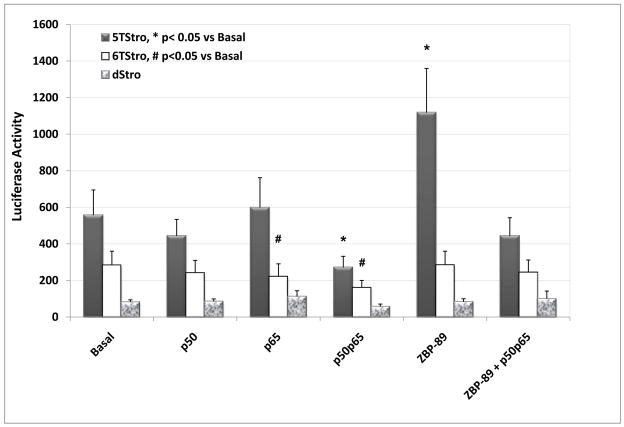
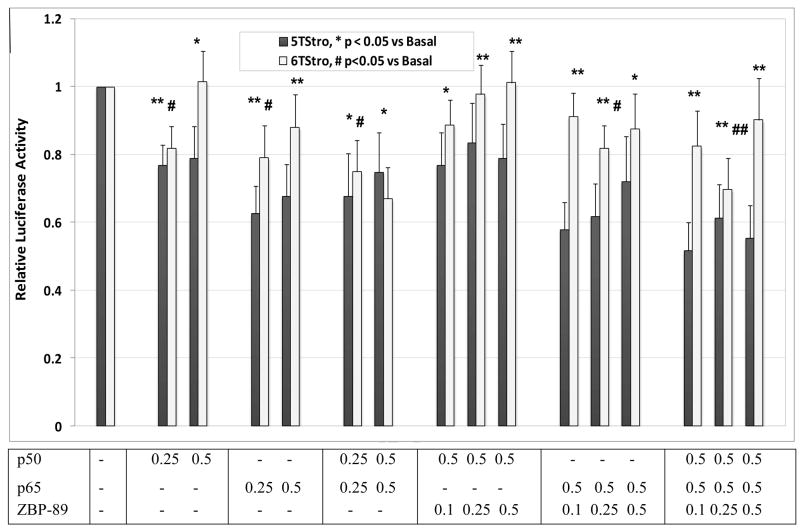
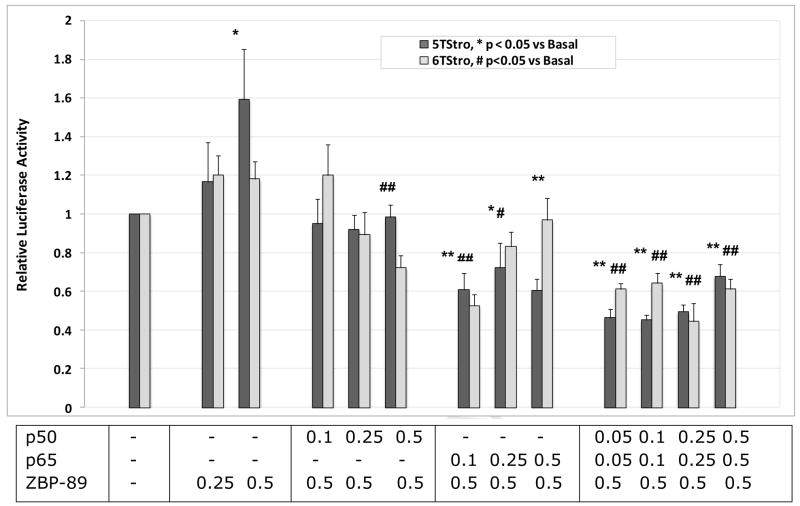
In order to study the effects of the individual transcription factors on transcription from the 5T and 6T promoters, ZBP-89, p50 and p65 were over-expressed separately and together and in different ratios. Results (Figures 3 and 4) showed that ZBP-89 was able to modestly activate transcription from the 5T promoter, but had no significant effect on the 6T promoter or the deletion mutant. NF-κB p50 and p65, both separately and together, were able to inhibit transcription from both promoters, and abrogated the effects of over-expressed ZBP-89 on the 5T promoter. Importantly, none of the three had any effect on transcription from the MMP-3 promoter lacking the SIRE site.
Figure 3. Effects of ZBP-89 and NF-κB on transcription from the MMP-3 promoter.
COS-1 cells were co-transfected with the 5TStro, 6TStro or dStro reporter vectors, along with the expression plasmid(s) for ZBP-89 and/or NF-κB p50 and p65 as indicated, along with SV-βgal as a control for transfection efficiency. pBluescript was added as necessary to equalize amounts of DNA transfected. The graph shows normalized luciferase activity for 5 experiments performed in triplicate, mean +/− SEM. Statistical significance was determined using the Student’s t-test to compare with basal levels for each reporter.
Figure 4. Competition between ZBP-89 and NF-κB.
COS-1 cells were co-transfected with the 5T or 6TStro reporter constructs along with various amounts of transcription factor expression plasmids as indicated, and SV-βgal as a control for transfection efficiency. A. Levels of p50 and p65 are constant, while ZBP-89 amounts increase. B. ZBP-89 is constant, while p50 and p65 increase. pBluescript was added as necessary to equalize amounts of DNA transfected. The graphs show normalized luciferase activity for 4 or 5 experiments performed in triplicate, mean +/− SEM. Statistical significance was determined using the Student’s t-test to compare with basal levels for each reporter.
DISCUSSION
The SIRE site polymorphism in the MMP-3 promoter was previously identified as a repressor element in fibroblasts and endothelial cells [9; 10], with the 6T version functioning as a more effective repressor [10]. ZBP-89 showed no preference for either the 5T or the 6T site in gelshift studies, and activated the MMP-3 promoter when over-expressed [23]. Recombinant NF-κB p50, on the other hand, was shown to bind preferentially to the 6T site [24].
There is a growing body of literature regarding the significance of the polymorphism in determining in vivo levels of MMP-3 protein. In aortic wall [11; 14], dermal [14] and liver [13] tissue samples, as well as fibroblasts isolated from breast tumors [12], protein levels have been shown to correlate with the polymorphism, with 5T homozygotes expressing significantly higher levels than heterozygotes or 6T homozygotes. However, when levels of MMP-3 protein are examined in serum, the results have been more variable. Several studies have found that patients homozygous for the 6T allele have higher serum levels [26; 27; 28], while one has found higher levels in 5T/5T [29] and two have found no difference [30; 31]. A particularly interesting study by Zhu, et al [32], showed that the polymorphism has no significant effect on expression of MMP-3 in THP-1 monocytes unless the cells are stimulated to differentiate, and then the 5T is more active.
Here we show that in transiently transfected COS-1 cells, over-expressed ZBP-89 activates transcription from the 5T promoter only, while NF-κB represses both promoters, even in the presence of excess ZBP-89. It is important to note that while over-expression of both p50 and p65 reduced transcription of both the 5T and 6T promoters, neither had any effect on the mutant promoter (Figure 3). The finding that NF- κB represses MMP-3 transcription is somewhat surprising in light of previous studies that suggest that it is an activator of MMP-3 expression [33; 34]. However, there is no canonical NF-κB site in the human MMP-3 promoter (at least not in the 2 kb used in these studies), and no previous study has demonstrated a direct effect of NF-κB on transcription. While it is possible that there may be an NF-κB site further upstream that could activate transcription in vivo, the results described here strongly suggest that NF-κB acts as a repressor via the SIRE site, and thereby plays an important role in determining the in vivo significance of the polymorphism.
ZBP-89 can either activate or repress transcription, depending on cellular context and competition with other transcription factors [35]. It is ubiquitously expressed, but increased levels have been found in certain tumors [35]. It has been shown to interact with p53 and stabilize it, thus contributing to apoptosis [36]. Its expression is induced by butyrate [37], retinoic acid [38] and TGF-β [35], and we have seen a decrease in mRNA expression in fibroblasts in response to inflammatory cytokines (data not shown). Recent evidence suggests post-translational regulation occurs as well [39].
The ChIP experiments, both in HFF and COS-1 cells, are consistent with the hypothesis that p50 homodimers bind preferentially to the 6T site, while ZBP-89 binds preferentially to the 5T site, and p65 (presumably as part of a p50/p65 heterodimer) binds both to a lesser extent. The fact that both p50 and p65 could inhibit transcription of both the 5T and 6T promoters, even in the presence of excess ZBP-89, suggests that the factors are competing for binding to both sites, and that ZBP-89 binding to the 6T site is limited by the higher affinity of NF-κB to this site.
Since neither the 5T nor the 6T site was able to compete very effectively for NF-κB binding to a consensus site in gelshift studies [24], it is likely that the SIRE site functions as a repressor element most effectively when cellular levels of NF-κB are high, and as an enhancer element when levels of ZBP-89 are high enough to compete effectively. Since ZBP-89 expression has been linked to cellular proliferation [35], it is likely that endogenous levels are higher in COS-1 cells relative to normal fibroblasts, which might help to explain the difference in SIRE site function between this and previous studies. While it is clear that the physiological impact of the polymorphism depends on the relative concentrations and activities of each of these transcription factors under different conditions, further studies are needed to determine the potential roles of auxiliary factors and/or chromatin structure in the function of this site.
Acknowledgments
We gratefully acknowledge the gift of ZBP-89 full-length cDNA from Dr. Juanita Merchant (University of Michigan), and thank Bill Laidlaw and Dr. Dianzheng Zhang for technical assistance. The work was supported by grant R15DE016277 from the NIH/NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryan ME, Golub LM. Modulation of matrix metalloproteinase activities in periodontitis as a treatment strategy. Periodontol. 2000;24:226–38. doi: 10.1034/j.1600-0757.2000.2240111.x. [DOI] [PubMed] [Google Scholar]
- 2.Parsons SL, Watson SA, Brown PD, Collins HM, Steele RJ. Matrix metalloproteinases. Br J Surg. 1997;84:160–6. [PubMed] [Google Scholar]
- 3.Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJ. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987;248:265–8. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–4. [PubMed] [Google Scholar]
- 5.Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin) Biochemistry. 1990;29:10261–70. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- 6.Lijnen HR, Arza B, Van Hoef B, Collen D, Declerck PJ. Inactivation of plasminogen activator inhibitor-1 by specific proteolysis with stromelysin-1 (MMP-3) J Biol Chem. 2000;275:37645–50. doi: 10.1074/jbc.M006475200. [DOI] [PubMed] [Google Scholar]
- 7.Matsuno H, Yudoh K, Watanabe Y, Nakazawa F, Aono H, Kimura T. Stromelysin-1 (MMP-3) in synovial fluid of patients with rheumatoid arthritis has potential to cleave membrane bound Fas ligand. J Rheumatol. 2001;28:22–8. [PubMed] [Google Scholar]
- 8.Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Borghaei RC, Sullivan C, Mochan E. Identification of a cytokine-induced repressor of interleukin-1 stimulated expression of stromelysin 1 (MMP-3) J Biol Chem. 1999;274:2126–31. doi: 10.1074/jbc.274.4.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J Biol Chem. 1996;271:13055–60. doi: 10.1074/jbc.271.22.13055. [DOI] [PubMed] [Google Scholar]
- 11.Deguara J, Burnand KG, Berg J, Green P, Lewis CM, Chinien G, Waltham M, Taylor P, Stern RF, Solomon E, Smith A. An increased frequency of the 5A allele in the promoter region of the MMP3 gene is associated with abdominal aortic aneurysms. Hum Mol Genet. 2007;16:3002–7. doi: 10.1093/hmg/ddm258. [DOI] [PubMed] [Google Scholar]
- 12.Holliday DL, Hughes S, Shaw JA, Walker RA, Jones JL. Intrinsic genetic characteristics determine tumor-modifying capacity of fibroblasts: matrix metalloproteinase-3 5A/5A genotype enhances breast cancer cell invasion. Breast Cancer Res. 2007;9:R67. doi: 10.1186/bcr1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtinghagen R, Bahr MJ, Wehmeier M, Michels D, Haberkorn CI, Arndt B, Flemming P, Manns MP, Boeker KH. Expression and coordinated regulation of matrix metalloproteinases in chronic hepatitis C and HCV-induced liver cirrhosis. Clin Sci (Lond) 2003 doi: 10.1042/CS20030098. [DOI] [PubMed] [Google Scholar]
- 14.Medley TL, Kingwell BA, Gatzka CD, Pillay P, Cole TJ. Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ Res. 2003;92:1254–61. doi: 10.1161/01.RES.0000076891.24317.CA. [DOI] [PubMed] [Google Scholar]
- 15.Abilleira S, Bevan S, Markus HS. The role of genetic variants of matrix metalloproteinases in coronary and carotid atherosclerosis. J Med Genet. 2006;43:897–901. doi: 10.1136/jmg.2006.040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astolfi CM, Shinohara AL, da Silva RA, Santos MC, Line SR, de Souza AP. Genetic polymorphisms in the MMP-1 and MMP-3 gene may contribute to chronic periodontitis in a Brazilian population. J Clin Periodontol. 2006;33:699–703. doi: 10.1111/j.1600-051X.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu PY, Li YH, Tsai WC, Tsai LM, Chao TH, Wu HL, Chen JH. Stromelysin-1 promoter 5A/6A polymorphism is an independent genetic prognostic risk factor and interacts with smoking cessation after index premature myocardial infarction. J Thromb Haemost. 2005;3:1998–2005. doi: 10.1111/j.1538-7836.2005.01515.x. [DOI] [PubMed] [Google Scholar]
- 18.Mora B, Bonamico M, Ferri M, Megiorni F, Osborn J, Pizzuti A, Mazzilli MC. Association of the matrix metalloproteinase-3 (MMP-3) promoter polymorphism with celiac disease in male subjects. Hum Immunol. 2005;66:716–20. doi: 10.1016/j.humimm.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto K, Mimura K, Murawaki Y, Yuasa I. Association of functional gene polymorphisms of matrix metalloproteinase (MMP)-1, MMP-3 and MMP-9 with the progression of chronic liver disease. J Gastroenterol Hepatol. 2005;20:1102–8. doi: 10.1111/j.1440-1746.2005.03860.x. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M, Haro H, Wakabayashi Y, Kawa-uchi T, Komori H, Shinomiya K. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 2001;83:491–5. doi: 10.1302/0301-620x.83b4.11617. [DOI] [PubMed] [Google Scholar]
- 21.Vairaktaris E, Yapijakis C, Vasiliou S, Derka S, Nkenke E, Serefoglou Z, Vorris E, Vylliotis A, Ragos V, Neukam FW, Patsouris E. Association of -1171 promoter polymorphism of matrix metalloproteinase-3 with increased risk for oral cancer. Anticancer Res. 2007;27:4095–100. [PubMed] [Google Scholar]
- 22.Beyzade S, Zhang S, Wong YK, Day IN, Eriksson P, Ye S. Influences of matrix metalloproteinase-3 gene variation on extent of coronary atherosclerosis and risk of myocardial infarction. J Am Coll Cardiol. 2003;41:2130–7. doi: 10.1016/s0735-1097(03)00482-0. [DOI] [PubMed] [Google Scholar]
- 23.Ye S, Whatling C, Watkins H, Henney A. Human stromelysin gene promoter activity is modulated by transcription factor ZBP-89. FEBS Lett. 1999;450:268–72. doi: 10.1016/s0014-5793(99)00509-8. [DOI] [PubMed] [Google Scholar]
- 24.Borghaei RC, Rawlings PL, Jr, Javadi M, Woloshin J. NF-kappaB binds to a polymorphic repressor element in the MMP-3 promoter. Biochem Biophys Res Commun. 2004;316:182–8. doi: 10.1016/j.bbrc.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Nowak DE, Tian B, Brasier AR. Two-step cross-linking method for identification of NF-kappaB gene network by chromatin immunoprecipitation. Biotechniques. 2005;39:715–25. doi: 10.2144/000112014. [DOI] [PubMed] [Google Scholar]
- 26.Mattey DL, Nixon NB, Dawes PT, Ollier WE, Hajeer AH. Association of matrix metalloproteinase 3 promoter genotype with disease outcome in rheumatoid arthritis. Genes Immun. 2004;5:147–9. doi: 10.1038/sj.gene.6364050. [DOI] [PubMed] [Google Scholar]
- 27.Samnegard A, Silveira A, Lundman P, Boquist S, Odeberg J, Hulthe J, McPheat W, Tornvall P, Bergstrand L, Ericsson CG, Hamsten A, Eriksson P. Serum matrix metalloproteinase-3 concentration is influenced by MMP-3 -1612 5A/6A promoter genotype and associated with myocardial infarction. J Intern Med. 2005;258:411–9. doi: 10.1111/j.1365-2796.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 28.White AJ, Duffy SJ, Walton AS, Ng JF, Rice GE, Mukherjee S, Shaw JA, Jennings GL, Dart AM, Kingwell BA. Matrix metalloproteinase-3 and coronary remodelling: implications for unstable coronary disease. Cardiovasc Res. 2007;75:813–20. doi: 10.1016/j.cardiores.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Oceandy D, Yusoff R, Baudoin FM, Neyses L, Ray SG. Promoter polymorphism of the matrix metalloproteinase 3 gene is associated with regurgitation and left ventricular remodelling in mitral valve prolapse patients. Eur J Heart Fail. 2007;9:1010–7. doi: 10.1016/j.ejheart.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Meijer MJ, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, Hommes DW, Verspaget HW. Role of matrix metalloproteinase, tissue inhibitor of metalloproteinase and tumor necrosis factor-alpha single nucleotide gene polymorphisms in inflammatory bowel disease. World J Gastroenterol. 2007;13:2960–6. doi: 10.3748/wjg.v13.i21.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanni S, Melandri G, Hanemaaijer R, Cervi V, Tomasi L, Altimari A, Van Lent N, Tricoci P, Bacchi L, Branzi A. Matrix metalloproteinases in premature coronary atherosclerosis: influence of inhibitors, inflammation, and genetic polymorphisms. Transl Res. 2007;149:137–44. doi: 10.1016/j.trsl.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Zhu C, Odeberg J, Hamsten A, Eriksson P. Allele-specific MMP-3 transcription under in vivo conditions. Biochem Biophys Res Commun. 2006;348:1150–6. doi: 10.1016/j.bbrc.2006.07.174. [DOI] [PubMed] [Google Scholar]
- 33.Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc Res. 2001;50:556–65. doi: 10.1016/s0008-6363(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 34.Bondeson J, Lauder S, Wainwright S, Amos N, Evans A, Hughes C, Feldmann M, Caterson B. Adenoviral gene transfer of the endogenous inhibitor IkappaBalpha into human osteoarthritis synovial fibroblasts demonstrates that several matrix metalloproteinases and aggrecanases are nuclear factor-kappaB-dependent. J Rheumatol. 2007;34:523–33. [PubMed] [Google Scholar]
- 35.Bai L, Logsdon C, Merchant JL. Regulation of epithelial cell growth by ZBP-89: potential relevance in pancreatic cancer. Int J Gastrointest Cancer. 2002;31:79–88. doi: 10.1385/IJGC:31:1-3:79. [DOI] [PubMed] [Google Scholar]
- 36.Bai L, Merchant JL. ZBP-89 promotes growth arrest through stabilization of p53. Mol Cell Biol. 2001;21:4670–83. doi: 10.1128/MCB.21.14.4670-4683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai L, Merchant JL. Transcription factor ZBP-89 cooperates with histone acetyltransferase p300 during butyrate activation of p21waf1 transcription in human cells. J Biol Chem. 2000;275:30725–33. doi: 10.1074/jbc.M004249200. [DOI] [PubMed] [Google Scholar]
- 38.Dawson MI, Park JH, Chen G, Chao W, Dousman L, Waleh N, Hobbs PD, Jong L, Toll L, Zhang X, Gu J, Agadir A, Merchant JL, Bai L, Verma AK, Thacher SM, Chandraratna RA, Shroot B, Hill DL. Retinoic acid (RA) receptor transcriptional activation correlates with inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced ornithine decarboxylase (ODC) activity by retinoids: a potential role for trans-RA-induced ZBP-89 in ODC inhibition. Int J Cancer. 2001;91:8–21. doi: 10.1002/1097-0215(20010101)91:1<8::aid-ijc1007>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Chupreta S, Brevig H, Bai L, Merchant JL, Iniguez-Lluhi JA. Sumoylation-dependent control of homotypic and heterotypic synergy by the Kruppel-type zinc finger protein ZBP-89. J Biol Chem. 2007;282:36155–66. doi: 10.1074/jbc.M708130200. [DOI] [PubMed] [Google Scholar]