Abstract
Periodic supplementation to infants and young children is encouraged in developing countries by the WHO. We investigated vitamin A (VA) in extrahepatic tissues of piglets after supplementation with retinyl acetate to determine long-term storage. 3, 4-Didehydroretinyl acetate (DRA) as a tracer was used to evaluate uptake from chylomicra in 4 h. Sows were fed a VA-depleted diet throughout pregnancy and lactation. Male castrated piglets (n = 28, 11.6 ± 0.5 d) from these sows were weaned onto a VA-free diet for 1 wk, assigned to 4 groups, and dosed orally with 0, 26.2, 52.4, or 105 μmol VA. After 10 d, 5.3 μmol DRA was administered to determine short-term uptake of 3, 4-didehydroretinol (DR). Four hours later, piglets were killed; adrenal glands, kidney, lung, and spleen were collected and analyzed for retinol and DR. Retinol concentrations of kidney and adrenal gland were higher than control, but treated groups did not differ. Retinol concentration was highest in kidney (1.70–2.52 nmol/g), followed by adrenal gland (0.30–0.48 nmol/g), lung (0.15–0.21 nmol/g), and spleen (0.11–0.15 nmol/g). Total retinol in kidney and spleen was different among the groups (P < 0.05). Unesterified retinol was the major VA form; the percent retinol of total VA was lowest in adrenal glands. DR did not differ among the groups. In 4 h, the minimum estimated chylomicron contribution to tissue DR was 63–280% higher than the maximum DR exposure from retinol-binding protein. Constant dietary intake may be important in maintaining VA concentrations in extrahepatic tissues.
Introduction
Vitamin A (VA)3 is essential for growth, epithelial maintenance, vision, and reproduction of humans and animals (1). Preformed VA is found in animal foods (e.g. dairy, eggs, and liver) and provitamin A carotenoids are found in many fruits and vegetables. After absorption, some VA can be distributed to tissues via chylomicra (2) and excess VA is mainly stored in the liver as retinyl esters (3). In the liver, retinyl esters can be hydrolyzed to retinol and released into blood complexed with retinol-binding protein (RBP), which circulates with transthyretin in a molar ratio of 1:1 (4,5) to be transported to target tissues (6). The uptake of retinol from RBP by tissues is mediated by a receptor (7). Recently, it has been proposed that lung and perhaps other tissues require steady input from dietary VA to maintain tissue concentrations (8).
Children with VA deficiency have a higher risk of blindness and dying from infectious disease than VA-sufficient children. More than 250 million children <5 y of age have insufficient VA to maintain optimal health. VA supplements are periodically given to infants and children to improve VA status (9). Supplements have ranged from 25,000 to 50,000 IU (26.2 and 52.4 μmol) retinyl ester for infants at immunization contacts to 100,000 and 200,000 IU (105 and 210 μmol) for infants and preschool children administered biannually. VA supplements may change VA status (10–12) and decrease the mortality rate of infants (13–17) but do not always result in an adequate VA status (18). A lactating sow-piglet model showed that liver VA reserves of young infants improve after direct or maternal supplementation but not dose dependently (19–21). When piglets from sows on a VA-depleted diet were administered 0, 26.2, 52.4, or 105 μmol VA, 26.2 μmol did not result in a mean liver reserve >.0.07 μmol/g liver (the deficiency cutoff) and 24% of piglets were still VA deficient after 52.4 or 105 μmol VA (19).
In contrast to liver, the storage and distribution of VA in extrahepatic tissues of piglets have not been studied after VA supplementation. The distribution of VA in extrahepatic tissues was studied in rat (8,22–24), monkey (25), gilt (26), and seal (27). Extrahepatic tissues are involved in VA metabolism, especially during VA deficiency. Kidney has an auxiliary role during VA deficiency (28) due to increased acyl CoA:retinol acyl transferase activity. Lung contains retinyl esters (1,8,29,30) and can metabolize retinyl palmitate from chylomicron remnants into retinoic acid (31). Greater utilization of retinol in lungs may be necessary for lung development of prenatal rats (32). Spleen can take up VA from perfused chylomicra or emulsions (33). Adrenal glands contain a significant amount of retinoic acid for development (34). In transthyretin-deficient mice, although the concentration of circulating retinol-RBP was low (i.e. 5% of normal), VA status in extrahepatic tissue remained normal (35).
If some tissues receive VA predominantly through chylomicron delivery, continued intake through supplements, fortified foods, or food would be needed to maintain optimal health. However, current practices include periodic supplementation to infants at immunization contacts and to children < 5 y of age biannually (9). Periodic supplementation may not maintain optimal tissue levels in critical organs if chylomicron delivery is necessary. The objectives of this study were to determine the distribution of VA in extrahepatic tissues of weaned piglets after graded VA supplementation 10 d after administration and to evaluate the uptake of newly ingested 3, 4-didehydroretinol (DR) by these tissues 4 h after dosing with 3, 4-didehydroretinyl acetate (DRA). In sows, DR esters peaked in the serum at 3 h after dosing, demonstrating that DR can be used as a chylomicron tracer (36). In piglets given standard 5.3-μmol doses of DRA, DR bound to RBP continued to rise or began to peak at 4 h (37). Furthermore, piglets are a good model because of similar body weight to infants and therefore identical dose sizes can be used as those periodically administered in public health programs (i.e. 25,000–100,000 IU).
Materials and Methods
Animals
This study was approved by the University of Wisconsin-Madison Animal Care and Use Committee. Sows (n = 15), crossbreeds of Large White and Landrace, had 2.4 ± 0.5 parities when assigned to this protocol. They were fed a standardized fortified feed until conception (20) and a feed without added VA during gestation and lactation. Male castrated piglets (n = 28; 11.6 ± 0.5 d old) were selected from 6 sows and weaned to a VA-free feed. At 1 wk, piglets were assigned to 4 treatment groups (n = 7/group) and received oral doses of 0, 7.5, 15, or 30 mg VA (0, 26.2, 52.4, or 105 μmol retinyl acetate) dissolved in corn oil (19) at age 18.6 ± 0.5 d. These doses are the same levels as those periodically given to infants. Piglets were maintained on the VA-free feed for 10 d. On the day they were killed, they were dosed with 1.5 mg (5.3 μmol) DRA and killed 4 h later at age 28.6 ± 0.5 d (Table 1). In addition to the liver and blood (19), the adrenal glands, kidney, lung, and spleen were collected, placed on dry ice, and frozen at −80°C. For comparison to reference values at birth, tissues were collected from 7 piglets that died after birth from other sows fed a normal VA feed (20) and analyzed for retinol and DR content.
TABLE 1.
Characteristics of piglets 10 d after treatment with graded doses of VA (0, 26.2, 52.4, or 105 μmol retinol as retinyl acetate)
| Characteristic | Value |
|---|---|
| Age, d1 | 28.6 ± 0.5 |
| Prior parities of sows, n | 2.25 ± 0.46 |
| Body weight, kg | 7.13 ± 1.36 |
| Adrenal gland weight, g | 1.01 ± 0.24 |
| Kidney weight, g | 38.8 ± 7.8 |
| Lung weight, g | 97.2 ± 26.3 |
| Spleen weight, g | 12.5 ± 3.6 |
Data are means ± SD, n = 28. Characteristics did not differ among treatment groups.
Piglet tissue analysis
Kidney (2 g) and sodium sulfate (6 g) were ground together in a mortar. Purified retinyl butyrate (2.65 nmol) was added to determine extraction efficiency. Dichloromethane was used to extract retinol and retinyl esters and filtered into a 25-mL volumetric flask; 10 mL extract was dried under argon and dissolved in 100 μL 50:50 methanol:dichloromethane; 50 μL was injected. The Waters HPLC system included a SunFire C18 (5 μm, 4.6 × 250 mm) reverse-phase column, 1525 binary pump, 717 autosampler, and 2996 photo-diode array detector. The mobile phase was 85:15 acetonitrile: dichloroethane (v:v) with 0.05% triethylamine as a modifier. The elution was 1 mL/min between 0 and 7 min and ramped to 2 mL/min by 10 min; run time was 40 min. Retinol and retinyl esters were analyzed at 325 nm and DR was analyzed at 350 nm.
Lung (5 g), adrenal gland (0.5 g), or spleen (2 g) were ground with sodium sulfate in a mortar; 0.99, 0.20, and 0.40 nmol retinyl butyrate was added, respectively. The samples were extracted with 20 mL dichloromethane 3 times. The extract was dried in a round-bottom flask using a rotary evaporator. The residue was dissolved in 1 mL dichloromethane (3 times) and transferred to a glass tube (13 × 100 mm). The combined solution was dried under argon and redissolved in 100 μL 50:50 methanol:dichloromethane; 70 μL was injected. HPLC analysis of lung was the same as that for kidney. For adrenal and spleen, a gradient with solvent A [70:30 acetonitrile:water (v:v) (0.05% triethylamine)] and B [85:15 acetonitrile:dichloroethane (v:v) (0.05% triethylamine)] was: 0 min, B = 0%; 5 min, B = 50%; 20 min, B = 100%. The flow rate was 1 mL/min for 20 min and ramped to 2 mL/min by 27 min; run time was 55 min.
Retinol, retinyl esters (linoleate, oleate, palmitate, and stearate), and DR peaks were identified by comparing their retention times with standards, as well as characteristic spectra. Retinol and retinyl esters were quantified using retinyl butyrate and the for retinol (1832) and combined for the retinol concentration (nmol/g tissue) or total retinol in each tissue (nmol/tissue). DR was quantified using its (1455). All values were calculated based on tissue wet weight.
The percent retention of VA in piglet tissues for treatment groups was calculated: total amount of retinol in the treatment group minus the control, divided by the ingested VA amount. Percent retention of DR in piglet tissues was calculated from the recovered 5.3 μmol DRA dose (19). Based on DR concentrations in the serum of the piglets, the minimum chylomicron contribution to the tissues was estimated by determining the maximum exposure to DR on RBP in the 4-h period after supplementation and subtracting it from the total measured DR.
Statistical analysis
SAS software (version 9.1) was used. ANOVA and multiple comparisons with the Student-Newman-Keuls test were used to investigate the effects of VA treatment on retinol and retinyl esters among different tissues (38). α = 0.05 was considered significant. We used Levene's test for homogeneity of variances to test for unequal variances. One-way ANOVA was used to evaluate the effect of different VA doses within each tissue and 2-way ANOVA among tissues (VA treatment × tissue) on retinol concentration, total amount of retinol, retention of ingested VA, DR concentration, and retention of DR. Two-way ANOVA was also used to assess the difference in concentrations affected by treatment and compounds in the same tissue (VA treatment × retinol or retinyl ester). Data were expressed as means ± SD.
Results
Retinol in different tissues
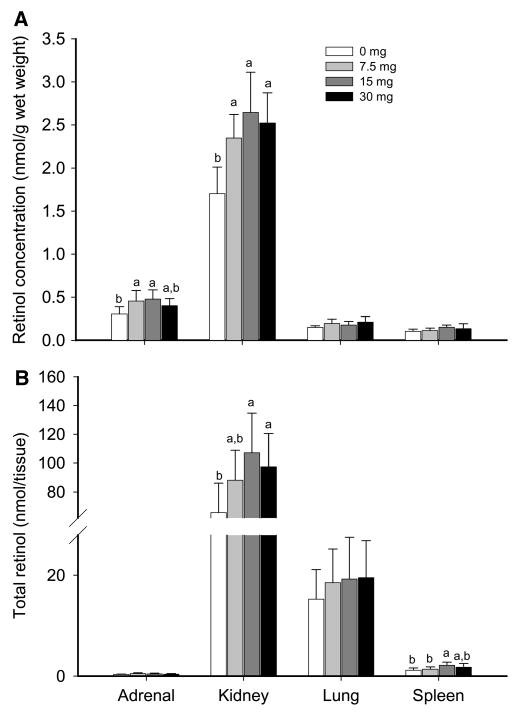
Retinol concentration was the sum of unesterified retinol and retinyl esters. At 10 d after treatment, the retinol concentration of the VA treatment groups (Fig. 1A) was higher than that of the control group for adrenal gland (P < 0.05) and kidney (P < 0.01), but the VA treatment groups did not differ. For lung and spleen, retinol concentration did not differ among groups. The retinol concentration was higher in kidney than in adrenal gland and it was higher in adrenal gland than in lung and spleen (P < 0.05), which did not differ from one another.
FIGURE 1.
Data are means ± SD, n = 7. Within a tissue, labeled means without a common letter differ, P < 0.05.
Total retinol in kidney (Fig. 1B) was higher in the 15 and 30 mg VA groups than in the control group (P < 0.05). For spleen, total retinol in the 15 mg group did not differ from 30 mg but was higher than the control and 7.5 mg groups (P < 0.05). The control, 7.5, and 30 mg groups did not differ. The total amount of retinol in adrenal gland and lung did not differ among groups (Fig. 1B). Kidney contained the highest amount of total retinol (P < 0.01), followed by lung, spleen, and adrenal glands, and spleen and adrenal glands did not differ. The groups given 15- and 30-mg treatments had significantly higher total retinol than controls (P < 0.01), but they did not differ from each other.
Retention of ingested VA in piglet tissues
Retention of ingested VA in the 7.5 mg group was significantly higher than that in the 30 mg group for adrenal gland (P < 0.05); no other differences were found (data not shown). The retention of VA from the supplements was the highest in kidney (0.03–0.09%) and the other tissues did not differ.
Concentration of retinol and retinyl esters in piglet tissues
Unesterified retinol concentration in the adrenal glands and kidney of the control group were lower than that of the VA treatment groups (P < 0.01) and concentrations of retinyl esters in control kidney were lower than that of the 15 mg VA group (P < 0.01) (Table 2). For control and VA treatment groups, the retinol concentration was much higher than that of retinyl esters in each extrahepatic tissue (P < 0.01). Retinol concentrations were highest in kidney, followed by adrenal, lung, and spleen (P < 0.01). Retinyl ester concentrations were the highest in kidney, followed by adrenal (P < 0.01). Retinol was higher than the esters in each extrahepatic tissue (P < 0.05). Unesterified retinol was 66–73% of total retinol in adrenal gland, 86–89% in kidney, 93–96% in lung, and 79–81% in spleen.
TABLE 2.
Retinol and retinyl ester concentrations of adrenal gland, kidney, lung, and spleen of adult pigs, piglets given different doses of VA, and reference piglets that died shortly after birth1,2
| Organ | Form of VA | Adult pig (Ref. 26) | Reference piglets | Treatment group (mg VA)3 | |||
|---|---|---|---|---|---|---|---|
| 0 | 7.5 | 15 | 30 | ||||
| nmol/g wet weight | |||||||
| Liver | Retinol | 405.6 ± 368.3 | 10.8 ± 12.0 | 7.01 ± 2.85b | 13.2 ± 6.44a,b | 19.9 ± 13.7a,b | 26.0 ± 22.1a |
| RE | 1016.34 | 23.6 ± 9.5 | 18.0 ± 6.63c | 53.3 ± 16.9b | 74.6 ± 30.7a,b | 89.7 ± 49.8a | |
| Lung | Retinol | 0.230 ± 0.065 | 0.161 ± 0.047 | 0.144 ± 0.014 | 0.192 ± 0.047 | 0.168 ± 0.038 | 0.199 ± 0.061 |
| RE | 0.2984 | 0.0102 ± 0.0114 | 0.011 ± 0.0043 | 0.0086 ± 0.0037 | 0.012 ± 0.005 | 0.016 ± 0.019 | |
| Kidney | Retinol | 0.960 ± 0.577 | 0.824 ± 0.718 | 1.51 ± 0.280b | 2.079 ± 0.246a | 2.26 ± 0.341a | 2.22 ± 0.393a |
| RE | 1.294 | 0.141 ± 0.052 | 0.185 ± 0.075b | 0.233 ± 0.136a,b | 0.318 ± 0.141a | 0.281 ± 0.084a,b | |
| Adrenal gland | Retinol | 0.347 ± 0.065 | 0.515 ± 0.143 | 0.195 ± 0.037b | 0.323 ± 0.111a | 0.341 ± 0.055a | 0.290 ± 0.049a,b |
| RE | 0.4364 | 0.367 ± 0.308 | 0.106 ± 0.079 | 0.121 ± 0.038 | 0.137 ± 0.078 | 0.111 ± 0.041 | |
| Spleen | Retinol | ND | 0.209 ± 0.027 | 0.086 ± 0.024 | 0.092 ± 0.016 | 0.120 ± 0.014 | 0.108 ± 0.044 |
| RE | ND | 0.057 ± 0.025 | 0.019 ± 0.0038 | 0.023 ± 0.013 | 0.032 ± 0.017 | 0.029 ± 0.013 | |
Data are means ± SD, n = 12 (adult pigs) or 7 (piglets). Means for piglets in a row with superscripts without a common letter differ, P < 0.05.
ND, Not determined; RE, retinyl esters.
Molecular weight of retinol = 286 g/mol.
Means for retinyl palmitate, oleate, and stearate were added together for total RE from reference 26 and therefore the SD could not be determined.
The reference piglets that died just after birth from sows on a standard diet had a liver retinol concentration of 0.035 ± 0.019 μmol/g, which was lower than that of the VA-treated piglets but not the controls. Retinol and retinyl esters in kidney were lower than the study groups and higher than or similar to that in adrenal gland, lung, and spleen (Table 2). Retinol concentrations in this study were compared with adult pigs with normal VA status (26). Liver, adrenal glands, and lung had a higher total retinol concentration and kidney was in the same range as this study, but the percentage as retinyl esters was higher (Table 2).
DR concentration and retention in piglet tissues
DR was not detected in the extrahepatic tissues of the reference piglets that died just after birth but was detected in all tissues of piglets dosed with DRA. The DR concentration in adrenal gland was 0.11 ± 0.05 nmol/g, 0.10 ± 0.05 nmol/g in kidney, 0.07 ± 0.03 nmol/g in lung, and 0.13 ± 0.10 nmol/g in spleen. The DR concentrations did not differ among the groups within a tissue. Spleen contained a higher DR concentration than lung (P < 0.01) but not higher than adrenal gland and kidney. Retention of DR in each tissue did not differ among the 4 groups. The ratio of DR:total retinol (DR:R) was 0.19 ± 0.08 – 0.31 ± 0.13 for adrenal gland, 0.03 ± 0.02 – 0.07 ± 0.04 for kidney, 0.31 ± 0.19 – 0.47 ± 0.30 for lung, and 0.60 ± 0.39 – 1.40 ± 1.27 for spleen. The treatment groups did not differ in ratio (P = 0.075) and no interaction was found between organ and treatment (P = 0.76). However, the DR:R difference among organs was significant (P < 0.0001), which was the highest in spleen, followed by lung and kidney. DR:R in adrenal gland did not differ from kidney or lung. DR:R in kidney was similar to that in serum of these piglets (19).
Estimation of the chylomicron contribution to DR concentrations in the tissues
At 4 h, the mean DR concentration in the serum was 0.0913 μg/L (0.321 nmol/L). The minimum chylomicron contribution to each tissue was estimated by assuming that the DR bound to RBP incrementally increased by 0.00038 ng (0.0013 pmol) DR·mL−1 ·min−1 from time 0 to 4 h, which is reasonable based on prior data in piglets with similar VA status (37). Gastrointestinal [106 ± 9 mL·min−1 ·100 g−1 tissue)] (39) and cerebral blood flow [73–101 mL·min−1 ·100 g−1 tissue)] (40) have been measured in piglets. The value of 100 mL blood−1·min−1 ·100 g−1 tissue) was used for this estimation. We assumed this value is reasonable for the organs studied, because the calculation is corrected for organ weight. The reported range of hematocrits for piglets is 0.26–0.41 (41). The mid-value hematocrit used for this calculation was 0.34; therefore, 66 mL plasma was estimated to pass through 100 g tissue in 1 min. Eq. 1 was used to calculate the amount of DR that passed through 100 g of tissue in 4 h:
Thus, 7.3 ng DR on RBP circulated to 1 g tissue/min (Table 3). Using mean tissue weight, the maximum DR through RBP delivery and the minimum DR amount from chylomicron delivery measured in the tissue were estimated. The minimum chylomicron contribution ranged from 63% higher in lung to 280% higher in spleen than the maximum DR amount that passed through the tissue in 4 h bound to RBP (Table 3).
TABLE 3.
Estimation of the minimum chylomicron contribution to tissue DR within 4 h after 5.3 μmol DRA was administered to piglets on a VA-deficient diet
| Mean organ weight | DR on RBP/g tissue1 | Maximum DR on RBP circulated to tissue2 | DR measured in tissue | Minimum DR from chylomicra3 | |
|---|---|---|---|---|---|
| g | ng | ||||
| Adrenal glands | 1.01 | 7.3 | 7.4 | 29.8 | 22.4 |
| Kidney | 38.8 | 7.3 | 280 | 1190 | 910 |
| Lung | 97.3 | 7.3 | 710 | 1870 | 1160 |
| Spleen | 12.5 | 7.3 | 91 | 441 | 350 |
DR on RBP/g tissue was estimate dusing the following equation: , where 284 ng DR = 1 nmol.
Maximum DR on RBP = (organ weight × 7.3).
Minimum DR from chylomicra = (DR measured in tissue − maximum DR on RBP).
Discussion
This study uniquely determined extrahepatic retinol concentrations in response to supplemental VA treatment and estimated the chylomicron contribution to tissue concentrations by using an analogue of retinol (i.e. DR) in piglets fed a VA-free diet. The doses used were the same as those provided to infants <1 y of age in developing countries. The retinol concentration in kidney and adrenal glands responded to VA treatment measured 10 d after administration. Kidney contained more VA than adrenal gland, lung, and spleen. Kidney plays an important role in the recycling of retinol-RBP and RBP has been detected in the proximal convoluted tubules in rat kidneys (42). Raccoon dogs, silver foxes (43), and marmoset monkeys (24) have similar VA concentrations in kidney and liver, suggesting a specific function of kidney in VA metabolism and storage of retinol and retinyl esters in addition to excretion of VA in the urine (43). Retinol concentrations in extrahepatic tissues, such as kidney, lung, and adrenal gland, were much lower than that in liver of adult pigs (26) and rats (24,44). Piglets used in this study had liver retinol concentrations of 0.025, 0.066, 0.094, and 0.12 μmol retinol/g liver for the 0, 26.2, 52.4, or 105 μmol VA groups, respectively (19). Most tissues cannot store large amounts of VA relative to liver (45). By comparing tissues within the control group, total retinol concentration of control liver was 82, 15, 165, and 237 times that in adrenal gland, kidney, lung, and spleen, respectively. The retention of ingested VA in the piglet liver was 21.3–38.2% (19), which was much higher than that in the extrahepatic tissues.
The rank of total retinol concentration in tissues agrees with that of Schweigert et al. in adult pigs, which was: liver > kidney > adrenal glands > lung (26). The mean retinol concentrations in VA-sufficient adult pigs (liver stores of 740 nmol/g) were 2.05 nmol/g for kidney, 0.74 nmol/g for adrenal gland, and 0.46 nmol/g for lung (26), which were similar to our data in these piglets. Lespine et al. (44) measured retinol and retinyl ester concentrations in several tissues to verify that total parenteral nutrition with retinyl palmitate allows an adequate supply of VA to peripheral tissues. Orally fed VA-deficient control rats had increased VA content of kidney and lung after treatment. Retinol as a percentage of total VA was higher in kidney than lung; retinol increased in kidney and retinol and retinyl esters increased in lung after refeeding (44). Ten days after VA dosage, we observed a substantial increase in retinol concentration in kidney but not in lung, implicating that chylomicron delivery to lung is an important contribution.
In VA-adequate animals, unesterified retinol in extrahepatic tissues is usually lower than retinyl ester concentration (26,43,46,47) but is a higher percentage (e.g. 38–47%) of total retinol than in liver (26). In these piglets, the distribution of retinol and retinyl esters in extrahepatic tissues differed from those reported in adult pigs (26). Unesterified retinol was the major form, accounting for 66–96% of total retinol concentrations. The VA status of the adult pigs (740 nmol/g liver) and piglets (70 nmol/g liver) were vastly different, resulting in less storage as esters in the piglets. Hydrolysis of esters may have occurred during storage (48) or thawing (49). Although the storage temperature was −80°C, extrahepatic retinyl ester hydrolase is still moderately active at that low temperature (48). This changes the VA profile but does not affect total VA concentrations.
Retention of DR in piglet liver was 23–51%, which was much higher than that in the extrahepatic tissues. More DR was found in the piglet livers for the VA treatment groups than the control group (19) but did not differ in the kidney, adrenal glands, lung, and spleen. Thus, tissue uptake was relatively similar in the 4-h time frame and did not change due to differences in VA status of the piglets. Retinol concentration in kidney and adrenal glands increased in response to VA treatment compared with control, but the 3 treatment groups did not differ. Therefore, the chylomicron contribution, which occurs within a few hours of VA ingestion, may be limited and has a finite capacity not affected by increasing doses. The spleen had a higher DR concentration than the lung, but the lung took up more total DR presumably due to its size. Because retinol concentration did not differ in spleen and lung 10 d after dosing with VA, frequent dietary intake of VA would be needed for maintaining concentrations.
In 4 h, the minimum chylomicron contribution was 63 and 280% higher in lung and spleen, respectively, than the maximum amount of DR that passed through the tissue in 4 h on RBP. Future studies should assess changes with time in tissue concentrations to better assess these findings. A limitation of this study is that the lag time of the processing and absorption of the DR was not assessed. In rats and sows, the rise from 1 to 3.5 h (50) and 0 to 5 h (36) of DR bound to RBP is quite linear, but values between 0 and 1 h have not been assessed. Nonetheless, the DR exposure extrapolated in the first hour of this study represents only 6% of the total in the calculation. Furthermore, because the chylomicron contribution is calculated by difference, a lag in DR exposure to tissue from RBP would increase the chylomicron contribution to the tissue, strengthening our arguments.
Spleen and lung are active in the immune response (51,52) and VA plays an integral role in immunity. Any initial storage of VA that occurred in these tissues may have been utilized before sampling at 10 d after dosage. The spleen is involved in both innate and adaptive immune processes in humans (51). The lung has constant exposure with the environment and is therefore one of the first defenses to inhaled antigens (52). The DR:R was much higher in these 2 tissues than the kidney or serum. We interpret this to mean that DR was rapidly taken up by these tissues from the chylomicra and initial stores of VA from the VA dose given 10 d prior were low. This is in agreement with the hypothesis set forth by Ross and Li (8). If these 2 essential immune system organs need a constant supply of VA for proper function from the diet or supplements, current practices of biannual supplementation to children may not be optimal. Although few dispute the benefits of VA supplementation to preschool children in the prevention of mortality and overt VA deficiency (53), perhaps food-based approaches to the global VA problem need to be considered (54) alongside of supplementation programs to mitigate morbidity and maintain optimal health.
Acknowledgments
We thank Jordan Mills in nutritional sciences for his help in developing the methods for VA analysis. We are grateful for the continued support of Thomas Crenshaw, professor of animal science, in the orchestration of our swine studies.
Footnotes
Supported by NIHNIDDK 61973, and USDA National Research Initiative grants 2003-35200-13754 and 2007-35200-17729.
Author disclosures: T. Sun, R. L. Surles, and S. A. Tanumihardjo, no conflicts of interest.
Abbreviations used: DR, 3, 4-didehydroretinol; DRA, 3, 4-didehydroretinyl acetate; DR:R, ratio of 3, 4-didehydroretinol to total retinol; RBP, retinol-binding protein; VA, vitamin A.
Literature Cited
- 1.Gerlach T, Biesalski HK, Weiser H, Haeussermann B, Baessler KH. Vitamin A in parenteral nutrition: uptake and distribution of retinyl esters after intravenous application. Am J Clin Nutr. 1989;50:1029–38. doi: 10.1093/ajcn/50.5.1029. [DOI] [PubMed] [Google Scholar]
- 2.Paik J, Vogel S, Quadro L, Piantedosi R, Gottesman M, Lai K, Hamberger L, Vieira MdM, Blaner WS. Vitamin A: overlapping delivery pathways to tissues from the circulation. J Nutr. 2004;134:S276–80. doi: 10.1093/jn/134.1.276S. [DOI] [PubMed] [Google Scholar]
- 3.Goodman DS, Huang S, Shiratori T. Time distribution and metabolism of newly absorbed vitamin A in the rat. J Lipid Res. 1965;6:390–6. [PubMed] [Google Scholar]
- 4.Muto Y, Goodman DS. Vitamin A transport in rat plasma. Isolation and characterization of retinol-binding protein. J Biol Chem. 1972;247:2533–41. [PubMed] [Google Scholar]
- 5.Rask L, Anundi H, Bohme J, Eriksson U, Fredriksson A, Nilsson SF, Ronne H, Vahlquist A, Peterson PA. The retinol-binding protein. Scand J Clin Lab Invest Suppl. 1980;154:45–61. [PubMed] [Google Scholar]
- 6.Muto Y, Milch PO, Goodman DS, Smith JE. Regulation of retinol-binding protein metabolism by vitamin A status in rat. J Biol Chem. 1972;247:2542–50. [PubMed] [Google Scholar]
- 7.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge W, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007 January 25; doi: 10.1126/science.1136244. Epub at www.sciencexpress.org. [DOI] [PubMed]
- 8.Ross AC, Li N. Lung retinyl ester is low in young adult rats fed a vitamin A-deficient diet after weaning, despite neonatal vitamin A supplementation and maintenance of normal plasma retinol. J Nutr. 2007;137:2213–8. doi: 10.1093/jn/137.10.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross DA. Recommendations for vitamin A supplementation. J Nutr. 2002;132:S2902–6. doi: 10.1093/jn/132.9.2902S. [DOI] [PubMed] [Google Scholar]
- 10.Wieringa FT, Dijkhuizen MA, West CE, Thurnham DI, Muhilal, Van der Meer JWM. Redistribution of vitamin A after iron supplementation in Indonesian infants. Am J Clin Nutr. 2003;77:651–7. doi: 10.1093/ajcn/77.3.651. [DOI] [PubMed] [Google Scholar]
- 11.Bahl R, Bhandari N, Wahed MA, Kumar GT, Bhan MK. Vitamin A supplementation of women postpartum and of their infants at immunization alters breast milk retinol and infant vitamin A status. J Nutr. 2002;132:3243–8. doi: 10.1093/jn/132.11.3243. [DOI] [PubMed] [Google Scholar]
- 12.Rahman MM, Mahalanabis D, Wahed MA, Islam MA, Habte D. Administration of 25,000 IU vitamin A doses at routine immunization in young infants. Eur J Clin Nutr. 1995;49:439–45. [PubMed] [Google Scholar]
- 13.Malaba LC, Iliff PJ, Nathoo KJ, Marinda E, Moulton LH, Zijenah LS, Zvandasara P, Ward BJ, Humphrey JH. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am J Clin Nutr. 2005;81:454–60. doi: 10.1093/ajcn.81.2.454. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, Hidayat S, Tielsch J, West KP, et al. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr. 1996;128:489–96. doi: 10.1016/s0022-3476(96)70359-1. [DOI] [PubMed] [Google Scholar]
- 15.Arthur P, Bahl R, Bhan MK, Kirkwood BR, Martines J, Moulton LH, Penny ME, Ram M, Underwood B, et al. Randomised trial to assess benefits and safety of vitamin A supplementation linked to immunisation in early infancy. Lancet. 1998;352:1257–63. [PubMed] [Google Scholar]
- 16.Rahmathullah L, Tielsch JM, Thulasiraj RD, Katz J, Coles C, Devi S, John R, Prakash K, Sadanand AV, et al. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomised trial in southern India. BMJ. 2003;327:254–7. doi: 10.1136/bmj.327.7409.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West KP, Jr, Katz J, Shrestha SR, Leclerq SC, Khatry SK, Pradhan EK, Adhikari R, Wu LSF, Pokhrel RP, et al. Mortality of infants less than 6 mo of age supplemented with vitamin A: a randomized, double-masked trial in Nepal. Am J Clin Nutr. 1995;62:143–8. doi: 10.1093/ajcn/62.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Idindili B, Masanja H, Urassa H, Bunini W, van Jaarsveld P, Aponte J, Kahigwa E, Mshinda H, Ross D, et al. Randomized controlled safety and efficacy trial of 2 vitamin A supplementation schedules in Tanzanian infants. Am J Clin Nutr. 2007;85:1312–9. doi: 10.1093/ajcn/85.5.1312. [DOI] [PubMed] [Google Scholar]
- 19.Surles RL, Mills JP, Valentine AR, Tanumihardjo SA. One-time graded doses of vitamin A to weanling piglets enhance hepatic retinol but do not always prevent a deficient vitamin A status. Am J Clin Nutr. 2007;86:1045–53. doi: 10.1093/ajcn/86.4.1045. [DOI] [PubMed] [Google Scholar]
- 20.Penniston KL, Valentine AR, Tanumihardjo SA. A theoretical increase in infants' hepatic vitamin A is realized using a supplemented lactating sow model. J Nutr. 2003;133:1139–42. doi: 10.1093/jn/133.4.1139. [DOI] [PubMed] [Google Scholar]
- 21.Valentine AR, Tanumihardjo SA. One-time vitamin A supplementation of lactating sows enhances hepatic retinol in their offspring independent of dose size. Am J Clin Nutr. 2005;81:427–33. doi: 10.1093/ajcn.81.2.427. [DOI] [PubMed] [Google Scholar]
- 22.Cifelli CJ, Ross AC. All-trans-retinoic acid distribution and metabolism in vitamin A marginal rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G195–202. doi: 10.1152/ajpgi.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cifelli CJ, Green JB, Green MH. Dietary retinoic acid alters vitamin A kinetics in both the whole body and in specific organs of rats with low vitamin A status. J Nutr. 2005;135:746–52. doi: 10.1093/jn/135.4.746. [DOI] [PubMed] [Google Scholar]
- 24.Wallingford JC, Underwood BA. Vitamin A status needed to maintain vitamin A concentrations in nonhepatic tissues of the pregnant rat. J Nutr. 1987;117:1410–5. doi: 10.1093/jn/117.8.1410. [DOI] [PubMed] [Google Scholar]
- 25.Mills JP, Penniston KL, Tanumihardjo SA. Extra-hepatic vitamin A concentrations in captive rhesus (Macaca mulatta) and marmoset (Callithrix jacchus) monkeys fed excess vitamin A. Int J Vitam Nutr Res. 2005;75:126–32. doi: 10.1024/0300-9831.75.2.126. [DOI] [PubMed] [Google Scholar]
- 26.Schweigert FJ, Buchholz I, Schuhmacher A, Gropp J. Effect of dietary beta-carotene on the accumulation of beta-carotene and vitamin A in plasma and tissues of gilts. Reprod Nutr Dev. 2001;41:47–55. doi: 10.1051/rnd:2001111. [DOI] [PubMed] [Google Scholar]
- 27.Schweigert FJ, Luppertz M, Stobo WT. Fasting and lactation effect fat-soluble vitamin A and E levels in blood and their distribution in tissue of grey seals (Halichoerus grypus) Comp Biochem Physiol A Mol Integr Physiol. 2002;131:901–8. doi: 10.1016/s1095-6433(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 28.Jurek MA, Powers RH, Gilbert LG, Aust SD. The effect of TCDD on acyl CoA-retinol acyltransferase activity and vitamin A accumulation in the kidney of male Sprague-Dawley rats. J Biochem Toxicol. 1990;5:155–60. doi: 10.1002/jbt.2570050304. [DOI] [PubMed] [Google Scholar]
- 29.Bhat PV, Lacroix A. Separation and estimation of retinyl fatty acyl esters in tissues of normal rat by high-performance liquid chromatography. J Chromatogr. 1983;272:269–78. doi: 10.1016/s0378-4347(00)86129-0. [DOI] [PubMed] [Google Scholar]
- 30.Napoli JL, McCormick AM, Omeara B, Dratz EA. Vitamin A metabolism: alpha-tocopherol modulates tissue retinol levels in vivo, and retinyl palmitate hydrolysis in vitro. Arch Biochem Biophys. 1984;230:194–202. doi: 10.1016/0003-9861(84)90100-0. [DOI] [PubMed] [Google Scholar]
- 31.Chytil F. The lungs and vitamin A. Am J Physiol. 1992;262:L517–27. doi: 10.1152/ajplung.1992.262.5.L517. [DOI] [PubMed] [Google Scholar]
- 32.Geevarghese SK, Chytil F. Depletion of retinyl esters in the lungs coincides with lung prenatal morphological maturation. Biochem Biophys Res Commun. 1994;200:529–35. doi: 10.1006/bbrc.1994.1480. [DOI] [PubMed] [Google Scholar]
- 33.Hultin M, Carneheim C, Rosenqvist K, Olivecrona T. Intravenous lipid emulsions: removal mechanisms as compared to chylomicrons. J Lipid Res. 1995;36:2174–84. [PubMed] [Google Scholar]
- 34.Haselbeck RJ, Ang HL, Deltour L, Duester G. Retinoic acid and alcohol/retinol dehydrogenase in the mouse adrenal gland: a potential endocrine source of retinoic acid during development. Endocrinology. 1997;138:3035–41. doi: 10.1210/endo.138.7.5274. [DOI] [PubMed] [Google Scholar]
- 35.Wei SH, Episkopou V, Piantedosi R, Maeda S, Shimada K, Gottesman ME, Blaner WS. Studies on the metabolism of retinol and retinol-binding protein in transthyretin-deficient mice produced by homologous recombination. J Biol Chem. 1995;270:866–70. doi: 10.1074/jbc.270.2.866. [DOI] [PubMed] [Google Scholar]
- 36.Surles RL, Li J, Tanumihardjo SA. The modified-relative-dose-response values in serum and milk are positively correlated over time in lactating sows with adequate vitamin A status. J Nutr. 2006;136:939–45. doi: 10.1093/jn/136.4.939. [DOI] [PubMed] [Google Scholar]
- 37.Valentine AR, Tanumihardjo SA. Adjustments to the modified relative dose response (MRDR) test for assessment of vitamin A status minimize the blood volume used in piglets. J Nutr. 2004;134:1186–92. doi: 10.1093/jn/134.5.1186. [DOI] [PubMed] [Google Scholar]
- 38.Zar JH. Biostatistical analysis. Upper Saddle River (NJ): Prentice Hall; 1996. [Google Scholar]
- 39.Nowicki P, Stonestreet B, Hansen N, Yao A, Oh W. Gastrointestinal blood flow and oxygen consumption in awake newborn piglets: effect of feeding. Am J Physiol. 1983;245:G697–702. doi: 10.1152/ajpgi.1983.245.5.G697. [DOI] [PubMed] [Google Scholar]
- 40.Chemtob S, Beharry K, Rex J, Varma D, Aranda J. Prostanoids determine the range of cerebral blood flow autoregulation of newborn piglets. Stroke. 1990;21:777–84. doi: 10.1161/01.str.21.5.777. [DOI] [PubMed] [Google Scholar]
- 41.Friendship R, Henry S. Cardiovascular system, hematology and clinical chemistry. In: Leman A, Straw B, Mengeling W, D'Allaire S, Taylor D, editors. Diseases of swine. 7th. Ames (IA): Iowa State University Press; 1992. [Google Scholar]
- 42.Kato M, Kato K, Goodman DS. Immunocytochemical studies on the localization of plasma and of cellular retinol-binding proteins and of transthyretin (prealbumin) in rat liver and kidney. J Cell Biol. 1984;98:1696–704. doi: 10.1083/jcb.98.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raila J, Buchholz I, Aupperle H, Raila G, Schoon HA, Schweigert FJ. The distribution of vitamin A and retinol-binding protein in the blood plasma, urine, liver and kidneys of carnivores. Vet Res. 2000;31:541–51. doi: 10.1051/vetres:2000138. [DOI] [PubMed] [Google Scholar]
- 44.Lespine A, Periquet B, Garcia J, Ghisolfi J, Thouvenot JP. Retinol and retinyl ester concentrations in rat tissues during total parenteral nutrition. J Nutr Biochem. 1998;9:316–23. [Google Scholar]
- 45.Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science. 1990;250:399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt CK, Brouwer A, Nau H. Chromatographic analysis of endogenous retinoids in tissues and serum. Anal Biochem. 2003;315:36–48. doi: 10.1016/s0003-2697(02)00662-0. [DOI] [PubMed] [Google Scholar]
- 47.Kerti A, Buchholz I, Schweigert FJ. Content of retinol and retinyl esters in blood plasma, liver, kidney and reproductive organs of Japanese quails. Acta Vet Hung. 2002;50:435–43. doi: 10.1556/AVet.50.2002.4.6. [DOI] [PubMed] [Google Scholar]
- 48.Azais-Braesco V, Dodeman I, Delpal S, Alexandre-Gouabau MC, Partier A, Borel P, Grolier P. Vitamin A contained in the lipid droplets of rat liver stellate cells is substrate for acid retinyl ester hydrolase. Biochim Biophys Acta. 1995;1259:271–6. doi: 10.1016/0005-2760(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 49.Yeung DL, Veenbaig MJ. Effect of freezing and thawing during storage on retinyl ester:retinol ratio in rat liver. Can J Physiol Pharmacol. 1972;50:48–51. doi: 10.1139/y72-009. [DOI] [PubMed] [Google Scholar]
- 50.Tanumihardjo SA, Olson JA. A modified relative dose-response assay employing 3, 4-didehydroretinol (vitamin A2) in rats. J Nutr. 1988;118:598–603. doi: 10.1093/jn/118.5.598. [DOI] [PubMed] [Google Scholar]
- 51.Wluka A, Olszewski WL. Innate and adaptive processes in the spleen. Ann Transplant. 2006;11:22–9. [PubMed] [Google Scholar]
- 52.Cook DN, Bottomly K. Innate immune control of pulmonary dendritic cell trafficking. Proc Am Thorac Soc. 2007;4:234–9. doi: 10.1513/pats.200701-026AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaton GH, Martorell R, L'Abbé KA. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries: Summary report. University of Toronto; Toronto, Canada: 1993. [Google Scholar]
- 54.Tanumihardjo SA. Food-based approaches for ensuring adequate vitamin A nutrition. Comp Rev Food Sci Food Safety. 2008 In press. [Google Scholar]