Abstract
Stimulation of either the caudal pressor area (CPA) in the most caudal ventrolateral medulla with glutamate, or the nasal mucosa with ammonia vapors, induces an increase in mean arterial blood pressure (MABP). In the present study, we determined if neurons in the CPA serve as a relay for the increase in MABP seen after nasal stimulation. Ammonia vapors stimulated the nasal mucosa of rats anesthetized with either urethane alone or ketamine/xylazine and urethane to induce an increase in MABP, a bradycardia, and an apnea. Bilateral injections (50nl) of glycine (1M) or muscimol (2mM) were placed in the CPA and the nasal mucosa again stimulated. The increases in MABP, the bradycardia and the duration of apnea to nasal stimulation were unchanged after either injection. However, resting MABP and HR were decreased significantly after glycine injections and resting MABP and resting respiratory rate was decreased after muscimol injections. The increase in MABP seen with nasal stimulation also did not change after multiple bilateral injections (3 × 40nl) of ibotenate (5µg/µl) in the CPA, but the bradycardia was eliminated and the duration of apnea was significantly shorter. These results suggest that the increase in MABP induced by nasal stimulation is via routes that do not include neurons in the CPA.
Keywords: CPA, cardiovascular, respiration, diving response, cardiac motor neurons, nasal mucosa
Introduction
Neurons located in the ventrolateral medulla play important roles in the generation and maintenance of cardiorespiratory function. For example, the rostral ventrolateral medulla (RVLM) contains bulbospinal neurons important for the tonic excitation of sympathetic preganglionic neurons in the spinal cord (Kumada et al., 1990; Guyenet, 1990; Dampney, 1994; Sun, 1995), the caudal ventrolateral medulla (CVLM) is important in the baroreceptor reflex (Schreihofer et al., 2002; Schreihofer et al., 2003), and neurons of the ventral respiratory column are important for both rhythm and pattern generation for respiration (Duffin et al., 1995; Rekling et al., 1998; McCrimmon et al., 2000). Preganglionic parasympathetic cardiac motor neurons also exist in the caudal ventrolateral medulla, some extending to the spinomedullary junction (see Panneton et al., ’96, for review). Stimulation of neurons in the most caudal ventrolateral medulla induces an increase in arterial blood pressure (Feldberg et al., 1986; Gordon et al., 1988; Bonham et al., 1989; Iwamoto et al., 1991; Cravo et al., 1991; Zhang et al., 1992; Cravo et al., 1993; Possas et al., 1994; Campos et al., 1999; Natarajan et al., 2000; Sun et al., 2002; de Toledo Bergamaschi et al., 2006; Seyedabadi et al., 2006), while inhibiting neurons in a similar area lowers arterial blood pressure (Feldberg et al., 1986; Possas et al., 1994; Campos Jr et al., 1994; Campos et al., 1999; Natarajan et al., 2000).
A caudal pressor area (CPA) has been defined between the caudal pole of the lateral reticular nucleus (LRN) and the medullary dorsal horn (MDH) near the level of the pyramidal decussation (Gordon et al., 1988; Natarajan et al., 2000; Sun et al., 2002; Sun et al., 2005), but others consider it more caudal and medial (Possas et al., 1994; Campos Jr et al., 1994; Campos et al., 1999; de Toledo Bergamaschi et al., 2006). The pressor effects of CPA stimulation are abolished after inactivation of RVLM neurons (Gordon et al., 1988; Possas et al., 1994; Seyedabadi et al., 2006), prompting the suggestion that neurons in the CPA provide tonic drive to the bulbospinal neurons of the RVLM critical for maintaining sympathetic vasomotor tone (Gordon et al., 1988; Iwamoto et al., 1991; Possas et al., 1994; Campos et al., 1999). However, it has been shown that direct projections from the CPA to the RVLM are sparse (Sun et al., 2005), promoting the idea that the CPA modulates the RVLM via the CVLM (Campos Jr et al., 1994; Natarajan et al., 2000; Horiuchi et al., 2002).
Functional roles for CPA neurons must still be determined, however. It has been reported that relatively large injections of kynurenate into the CPA blocks the cardiorespiratory responses induced by electrical stimulation of nucleus raphe obscurus (Silva et al., 2002), but the CPA does not mediate the pressor response to carotid occlusion (Possas et al., 1994). We investigate herein a role of the CPA for the increase in arterial blood pressure seen in a nasopharyngeal reflex. Stimulation of the upper respiratory tract in mammals, including man, induces increases in sympathetic drive, an apnea and bradycardia (Widdicombe, 1986; Coleridge et al., 1994; Widdicombe et al., 2001). Similar cardiorespiratory responses are seen after stimulation of the nasal mucosa of rodents (Panneton, 1990; Panneton, 1991b; Panneton et al., 1995; Yavari et al., 1996; McCulloch et al., 1997; McCulloch et al., 1999; Rybka et al., 2006), which is innervated in part by the anterior ethmoidal nerve (AEN). We have shown that the AEN has direct projections to the RVLM, CVLM, and CPA in the ventrolateral medulla (Panneton, 1991a; Panneton et al., 2006), and indirect projections via the medullary dorsal horn (MDH) to the solitary complex, A5 area and peribrachial area (Panneton et al., 2000; Panneton et al., 2006); all these areas are known to be important in cardiovascular control.
Thus, the CPA was considered a potential intermediary for the pressor response to nasal stimulation since our studies show: 1) its stimulation elicits an increase in arterial blood pressure (Sun et al., 2002); 2) the CPA is labeled with Fos after nasal stimulation (McCulloch et al., 1997; Rybka et al., 2006); 3) the CPA receives direct projections from the AEN (Panneton, 1991a; Panneton et al., 2006) and is labeled after transsynaptic transfer of Herpes Simplex virus from the AEN (Panneton et al., 2000), 4) the CPA receives secondary projections (Panneton et al., 2006) from the part of the MDH which also receives primary afferent fibers from the AEN (Panneton, 1991a; Panneton et al., 2000; Panneton et al., 2006) and where the cardiorespiratory responses to nasal stimulation are greatly reduced by injections of lidocaine or kynurenate (Panneton et al., 1995); and 5) the CPA projects to brainstem nuclei implicated in cardiovascular control (Sun et al., 2005).
For these reasons, we hypothesized the CPA is important for the increase in arterial blood pressure seen after nasal stimulation. We made injections of glycine, an inhibitory transmitter, of muscimol, a GABA agonist, or of ibotenic acid (IBO), an excitatory neurotoxin, into the CPA in an attempt to block the increase in arterial blood pressure seen after nasal stimulation. However, our results showed that no such injections altered the increase in MABP seen after nasal stimulation. Part of this work has appeared previously in abstract form (Young et al., 1999).
Materials and Methods
Thirty-three adult male Sprague-Dawley rats (~275– 300 g) were used in this study. All protocols were approved by the Animal Care Committee of Saint Louis University and followed the guidelines published in the National Institutes of Health Guide for Care and Handling of Laboratory Animals.
Most rats were anesthetized initially with injections of urethane (IP; 1g/kg); a few rats receiving IBO injections were first anesthetized with a mixture of ketamine hydrochloride (45 mg/kg) and xylazine (35 mg/kg) IP followed by intravenous urethane. A femoral vein was cannulated for the administration of further urethane anesthesia, but such injections were seldom needed. A femoral artery was cannulated for measurement of arterial blood pressure in all rats. The trachea was exposed and transected just caudal to the larynx. A cannula (PE 240) was placed in the distal trachea for spontaneous ventilation, while another was passed through the larynx and into the nasopharynx and attached to suction. Arterial blood pressure and ventilation were sensed and impulses transduced (Gould P23 and PT5, respectively; Grass Instruments) and amplified (Grass 7P122). HR was determined by counting systolic peaks on the blood pressure trace. Signals for arterial blood pressure and ventilation were transmitted to a computer through an A/D interface (1401 plus; Cambridge Electronic Design, CED), stored and analyzed using Spike 2 software (CED).
The animals then were placed in a stereotaxic device (Kopf Instruments) and their caudal medullas exposed through a dorsal incision via the atlanto-occipital membrane. The vapors emanating from a cotton ball soaked in a 50% solution of ammonia hydroxide served as the stimulus for all animals. Gentle suction pulled the vapors over the nasal mucosa for 5 sec. Arterial blood pressure and ventilations were recorded for three trials of nasal stimulation separated by 3–5 minutes each; these data were considered as control. After each trial, residual ammonia vapors were removed immediately from the nasal cavity by continued suction through the choanae. The CPA was located with injections of glutamate in initial studies, but stereotaxic coordinates (1.0 mm caudal to the calamus scriptorius, 2.0 mm lateral to the midline, and 1.7 mm ventral to the dorsal surface of the medulla) were used solely in later rats. The carrier was angled 24° in the anterior direction since this angle compensates for the slope of the caudal medulla when the rats are in the stereotaxic apparatus (Sun et al., 2005) and insured orthogonal penetration of the brain with the pipette. Bilateral injections were made via a glass micropipette (OD ~25µm, cemented to a 1µl Hamilton syringe) of glycine (1M; 50nl mixed with red FluoSpheres, Molecular Probes) or muscimol (2mM; 50nl mixed with yellow-green FluoSpheres, Molecular Probes) in 13 rats at a single rostrocaudal level. Approximately 90 sec separated injections on either side. The injections of glycine and muscimol were done in the same rats; glycine injections were completed first, followed by muscimol injections approximately 40 min later. The nasal mucosa was stimulated 1, 3, 5, 10, 15 and 20 min after the glycine/muscimol injections and cardiorespiratory activity recorded. Three bilateral injections (40nl each of 200ng IBO/injection) of IBO (5µg/µl or 0.5%) mixed with red fluorescent beads (Lumafluor) were made into the CPA (AP: −1.0 from calamus scriptorius and 300µ both rostral and caudal to this point; ML: ±2.0mm; DV: 1.6–1.8mm ventral to dorsal surface) in another 12 rats. However, approximately 20 min after the IBO injections the rats stopped breathing and then were ventilated mechanically. Three to 4 h later, the animals again started breathing spontaneously. At least 30 min after the rats resumed spontaneous ventilations, the nasal mucosa was stimulated again with ammonia vapors for at least 3 more trials separated by 5 min.
Resting MABP and HR were calculated for a 5 sec period immediately before either the injections or the nasal stimulus, and are represented as line graphs in Figure 2 and 3. The peak changes of MABP and HR induced by nasal stimulation were determined and are represented as box plots in Figure 3. The duration of apnea was calculated from stimulus onset. The mean±standard error (M±S.E.) of these data and ventilations/min between injections were compared statistically (SPSS v.13.0 software) to similar parameters calculated prior to the glycine/muscimol injections. Three comparisons (Paired–Sample T test) were made statistically for the different time periods. First, changes in resting cardiorespiratory activity after either glycine or muscimol injections was calculated and compared to data obtained prior to the injections (Fig. 2). Secondly, the resting M±S.E. of MABP, HR, and ventilations derived just prior to nasal stimulation (Fig. 3, line graphs) was compared to data induced by nasal stimulation (Fig. 3, box plots). Thirdly, responses induced by nasal stimulation before the injections was compared to those derived by nasal stimulation after injections. Data from the IBO cases used Student-t tests and GB-Stat software. All analyses considered p<0.05 as significant.
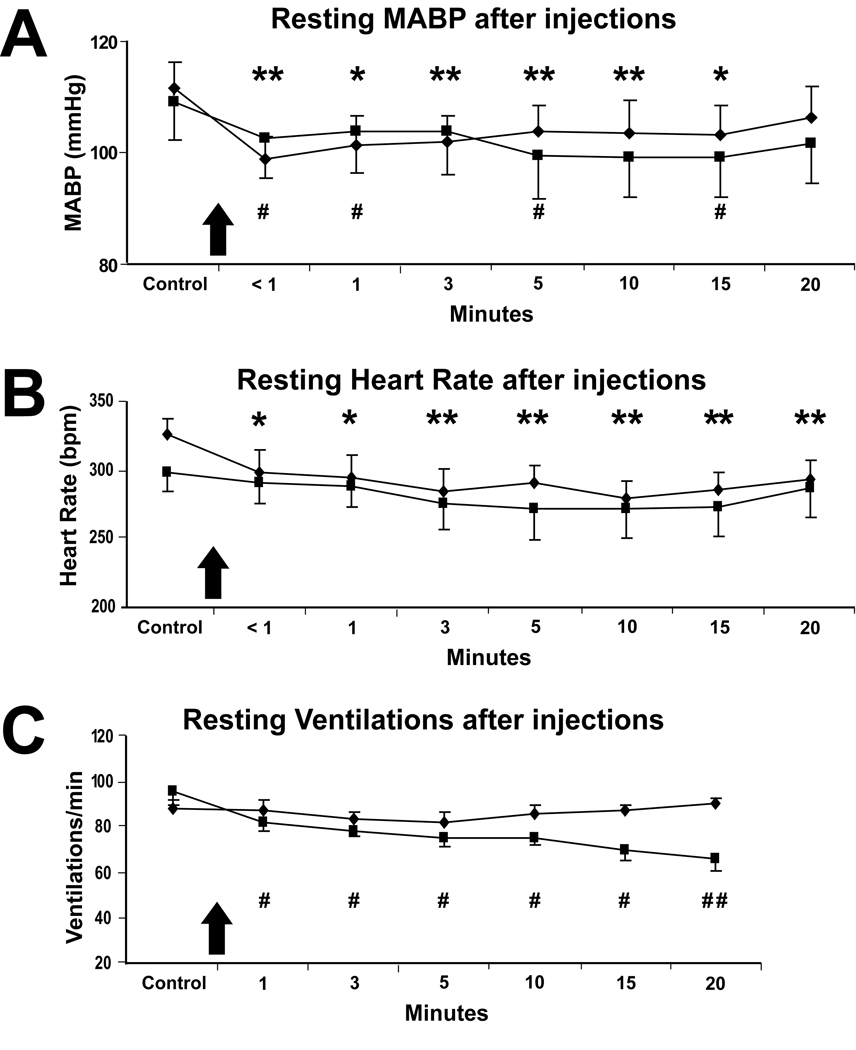
Figure 2.
Line graphs illustrating the effects on resting cardiorespiratory behavior after injections of either glycine (filled diamonds) or muscimol (filled squares) into the CPA of rats (n=7). Resting mean arterial blood pressure (MABP; A), heart rate (HR; B) and ventilations/min (C) are shown before (Control) and after the injections (shown at arrows; 50nl/side) of glycine or muscimol for 20min. Note that after injections of glycine resting MABP and HR both decreased significantly (A, B; asterisks) and after injections of muscimol both resting MABP and resting respiratory rate decreased significantly (A, C; number symbols). Asterisks show significance between control data obtained prior to the injections of glycine, versus data from time points after the injections of glycine, while number symbols show significance between control data obtained prior to the injections of muscimol versus data from time points after the injections of muscimol. #, * p<0.05; ##, ** p<0.01.
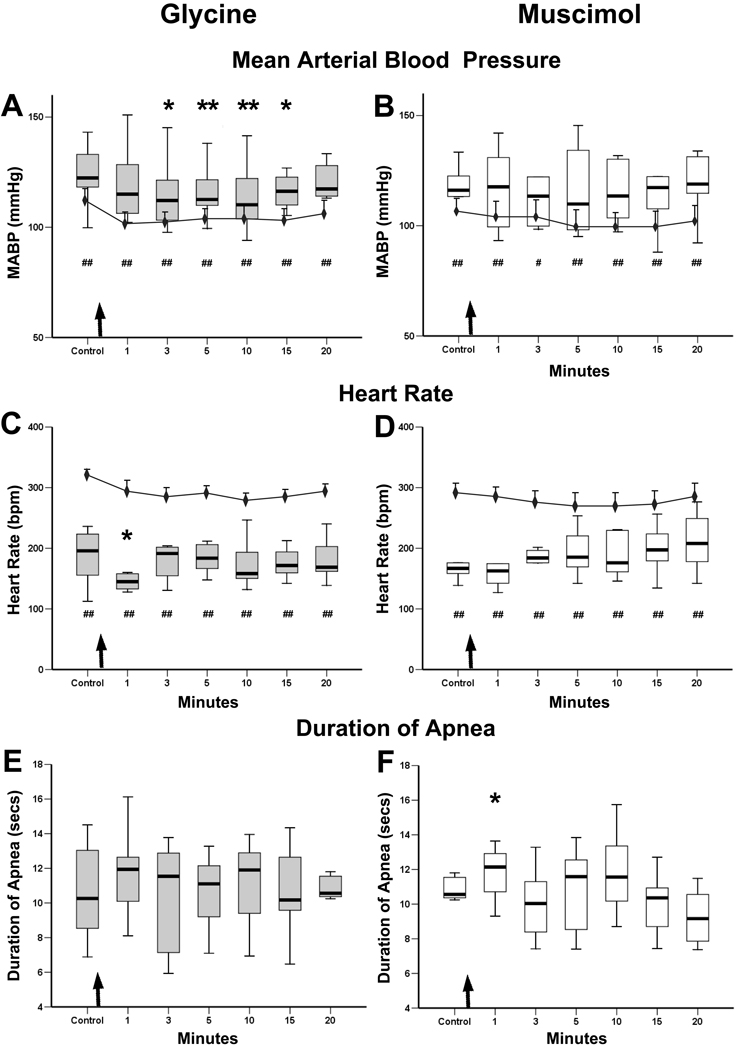
Figure 3.
Graphs showing the cardiorespiratory responses of rats to nasal stimulation after injections of glycine/muscimol into their CPA’s. The responses to stimulating the nasal mucosa with ammonia vapors 1, 3, 5, 10, 15, and 20 minutes after the CPA injections (arrows) are shown as box plots in A–F, while the line graphs in A–D show the baseline levels of MABP (A, B) and HR (C, D) taken just prior to nasal stimulation. The changes in MABP and HR induced by nasal stimulation are compared to levels obtained just prior to stimulation (box plots versus line graph). The significant increase in MABP induced by nasal stimulation (A, B; box plots) was maintained over baseline levels at all time points (number symbols) after either the glycine or muscimol injections, suggesting the injections did not inhibit the increase in MABP from nasal stimulation. However, there was a significant relative rise of MABP to nasal stimulation 3 to 15 minutes post-injection (A, asterisks) after the glycine injections compared to control stimulation.
The significant drop in heart rate induced by nasal stimulation (C, D; box plots) also remained (number symbols) after the glycine/muscimol injections when compared to baseline HR recorded just prior to stimulation (C, D; line graphs), suggesting the injections did not alter changes in HR from nasal stimulation. However, the relative drop was significantly greater (C, asterisk) 1 min after the glycine injections when compared to control values obtained before the injection.
The apnea induced by nasal stimulation after the glycine injections was unchanged when compared to control values obtained prior to the injections (E), but duration of apnea was significantly longer 1 min after injections of muscimol (F, asterisk). #, * p<0.05; ##, ** p<0.01.
After the experiment, the rats were deeply anaesthetized and then perfused transcardially with 0.1 M phosphate–buffered saline, followed by a 4% buffered paraformaldehyde solution (pH 7.3). The brains were removed, and immersed in the same fixative plus 20% sucrose solution. The brainstems were cut transversely at 40–50µm on a freezing microtome, mounted on gelled slides, air dried, stained for Nissl and coverslipped. Some of the sections from brains injected with IBO also were immunohistochemically reacted for NeuN (Chemicon, Inc.) in an effort to show an area devoid of somas, but the neurons still stained normally. The injection sites were located in a microscope and related to the CPA (Sun et al., 2002; Sun et al., 2005). Only those cases with injections centered bilaterally in the CPA were used to calculate the data presented herein.
Results
Effects of Nasal Stimulation Prior to CPA Injections
All rats showed significant increases in MABP, drops in HR and apneas to a 5 sec stimulation of the nasal mucosa with ammonia vapors prior to any injections into their CPA (Fig. 1A). Nasal stimulation with ammonia vapors prior to the glycine injections induced an increase in MABP from 111±5 mmHg to 124±5 mmHg (Fig. 3A, Control), a drop in HR of 321±9 bpm to 186±18 bpm (Fig. 3C, Control), and an apnea which lasted for 11±1 sec (Fig. 3E). Nasal stimulation with ammonia vapors prior to the muscimol injections induced an increase in MABP from 107±6 mmHg to 122±8 mmHg (Fig. 3B, Control), a drop in HR of 290±16 bpm to 174±14 bpm (Fig. 3D, Control), and an apnea which lasted for 10±1 sec (Fig. 3F). Nasal stimulation of rats with ammonia vapors prior to the IBO injections induced MABP to increase from 74±8 mmHg to 108±11 mmHg (Fig. 4A, Before), HR to drop from 264±17 bpm to 72±13 bpm (Fig. 4B, Before), and an apnea lasting for 20±2 sec (Fig. 4C, Before).
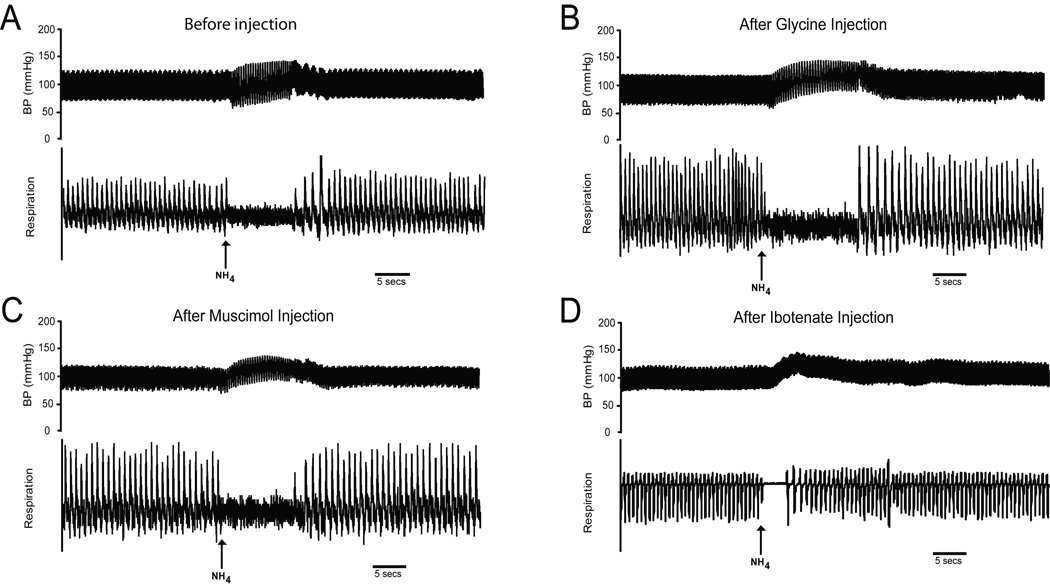
Figure 1.
Traces of arterial blood pressure (upper trace) and ventilations (lower trace) showing typical cardiorespiratory responses in four different rats to nasal stimulation with ammonia vapors (arrows). Vapors from a 50% ammonia solution applied to the nasal mucosa for 5 secs produce an increase in arterial blood pressure, a bradycardia, and an apnea (A). Compare the responses to nasal stimulation after bilateral injections of glycine (B), muscimol (C) or ibotenic acid (D) into the CPA of other rats to those seen prior to such injections (A). Note that the increase in arterial blood pressure and apnea persists after all the injections, but the bradycardia is lost and the duration of apnea shortened after the injections of IBO.
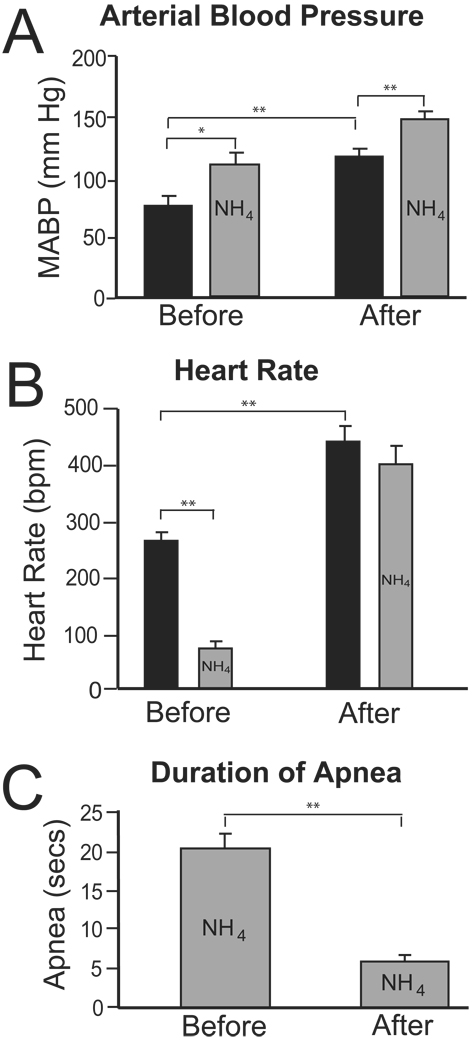
Figure 4.
Bar graphs showing the cardiorespiratory changes of rats (N=7) induced by nasal stimulation with ammonia vapors either before or several hours after injections of ibotenic acid into their CPA’s. In A, note that resting arterial blood pressure (black bars) after IBO injections was significantly higher than that seen before the injections. However, the increase in MABP to nasal stimulation before the ibotenate injections (Before; 34 mmHg) was similar to that after the injections (After; 32 mmHg). In B, resting heart rate (black bars) increased significantly after the IBO injections compared to before the injections, while the significant bradycardia induced by nasal stimulation (gray bars) before the ibotenate injections was lost after the injections. In C, the duration of apnea induced by stimulating the nasal mucosa before the IBO injections was reduced significantly after the injections. *p<0.05, **p<0.01.
Resting Cardiorespiratory Activity After CPA Injections
Only cases with injections centered bilaterally in the CPA (Gordon et al., 1988; Natarajan et al., 2000; Sun et al., 2002) of glycine (7/13), muscimol (6/12), or IBO (7/12) were included in data analysis to minimize effects on juxtaposed neurons. Data was analyzed first to determine the effects of the injections in the CPA on resting cardiorespiratory activity, and secondly, to determine the effects the injections had on changes in cardiorespiratory activity induced by nasal stimulation.
Injections of inhibitory transmitters or their analogs into the CPA depressed resting cardiorespiratory activity. Thus, immediately after bilateral injections of glycine into the CPA (Fig. 2, filled diamonds), resting MABP (Fig. 2A) decreased significantly (p=.001; from 112±5 to 99±4 mmHg) from control values obtained just prior to the injection, and remained significantly lower for 15 min (Fig. 2A, asterisks). Resting HR (Fig. 2B) also immediately decreased significantly (p≤.05; from 325±11 to 298±17 bpm) from values determined just prior to the injections, and remained depressed for the 20min of evaluation (Fig 2B, asterisks). There were no changes in resting ventilations/min (Fig. 2C) after glycine injections.
Bilateral injections of muscimol were made into the CPA (Fig. 2, filled squares) approximately 40 min after the glycine injections. Immediately after injections of muscimol, resting MABP (Fig. 2A) decreased significantly (p<.05; from 109±7 to 103±7 mmHg) from control levels obtained just prior to the injections, and was significantly different 1, 5 and 15 min after these injections (Fig. 2A, number symbols). There were no significant changes in resting HR (Fig. 2B) from control values, but resting ventilations/min were affected significantly (p<.05), and decreased over the 20 minute observation period (Fig. 2C, number symbols).
IBO injections into the CPA often induced an immediate but transient bradycardia (see Sun and Panneton, 2001), and increases in both the depth and rate of ventilation. Nevertheless, spontaneous ventilation stopped after 15–20 min, resuming after 3 to 4 h of mechanical ventilation. Both baseline MABP and HR increased dramatically in all animals in the 3–4 hr following the IBO injections in their CPA’s. Baseline MABP (Fig. 4A, black bars) increased significantly (p<.001; from 74±9 mmHg, Before, to 115±8 mmHg, After), while baseline HR (Fig. 4B, black bars) increased significantly (p73x0003C;.001; from 264±17 bpm, Before, to 441±28 bpm, After), a 67% increase. Resting ventilations were not significantly different when rates before or hours after injections of IBO were compared.
Effects of Nasal Stimulation After CPA Injections
Since our injections of glycine/muscimol mimicked the results of others (Natarajan et al., 2000) on resting cardiorespiratory activity, we determined the changes on cardiorespiratory responses to nasal stimulation induced by these injections. First, the changes in MABP, HR and ventilations induced by stimulating the nasal mucosa with ammonia vapors (box plots in Figs. 3A–D) were compared to baseline cardiorespiratory parameters obtained just prior to the stimulations (line graphs in Figs. 3A–D). Both the increase in MABP as well as the bradycardia induced by nasal stimulation were significantly different than baseline parameters at all times after the injections of either glycine or muscimol (number symbols in Figs. 3A–D). Thus, the injections into the CPA of these substances did not affect the changes induced by nasal stimulation.
We then analyzed the relative rise in MABP and fall in HR induced by nasal stimulation by comparing them before and after the injections. Changes in MABP to nasal stimulation after glycine injections were significantly less than that seen prior to the injections at some time points (Fig. 3A, asterisks), and the drop in HR was greater one minute after the glycine injections (Fig. 3C, asterisk). The duration of apnea resulting from nasal stimulations before glycine injections was unchanged after the injections (Figs. 3E). The rise in MABP and bradycardia induced by nasal stimulation were similar after the muscimol injections (Fig. 3B, D), but the duration of apnea was changed 1 minute after the injections (Fig. 3F, asterisk; p<.05; from 10.3 to 11.4 sec).
Thirty minutes after spontaneous ventilation resumed in the rats injected with IBO, increases in MABP to stimulation of the nasal mucosa remained unchanged (Fig. 4A, gray bars; 34 mmHg, Before, to 32 mmHg, After). However, the bradycardia induced before the injections was no longer present (Fig. 4B, gray bars, After; 441±28 bpm to 402±32 bpm), and the duration of the apnea decreased significantly (Fig. 4C; p<.01; from 20±2 to 6±1 sec).
Histological Verification of Injection Sites
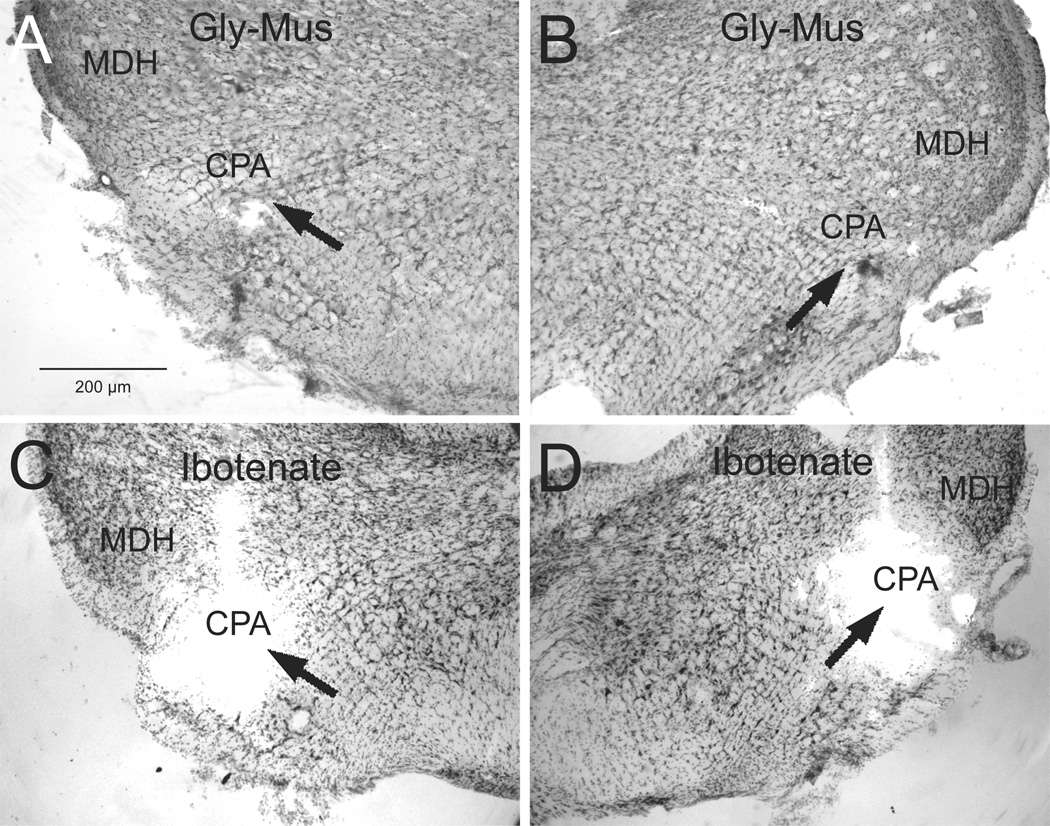
Since the injections were mixed with colored microspheres, the injection sites were readily visualized. Injections centered in the CPA (Gordon et al., 1988; Natarajan et al., 2000; Sun et al., 2002; Sun et al., 2005) were between the caudal pole of the lateral reticular nucleus and the MDH at the level of pyramid decussation. Only cases with injections of glycine, muscimol, or IBO centered in the CPA bilaterally were included in our analysis. Injections of IBO also were made 300µm rostral and caudal from the center of the CPA to ensure its complete destruction (see Sun & Panneton, 2001). Although the yellow and red microspheres mixed in the glycine/muscimol injections were seen prior to staining the sections, they unfortunately dissolved when the sections were processed. However, it is of interest that the fluorescence remained after the IBO injections that used beads purchased from Lumafluor, Inc., (Figs. 5C, D), but was lost in the injections using beads purchased from Molecular Probes (Figs. 5A, B). This may have been due to how the beads are manufactured; the fluorescent molecule is contained internal to a membrane in those from Lumafluor while it is tagged to the membrane itself on those from Molecular Probes. Nevertheless, the point of injection in all cases could still be marked by a cavity in the tissue.
Figure 5.
Photomicrographs of typical injection sites (arrows) for deposits of glycine and muscimol (A, B; case 2218) and ibotenic acid (C, D; case 1559) into the most caudal ventrolateral medulla and centered in the CPA (arrows).Abbreviations: CPA, caudal pressor area; MDH, medullary dorsal horn.Line bar in A for figures A–D.
3. Discussion
Stimulating the nasal mucosa with ammonia vapors induces an increase in MABP, a bradycardia and an apnea. The caudal ventrolateral medulla contains neurons in the CPA modulating blood pressure, preganglionic parasympathetic cardiac motoneurons in the external formation of the nucleus ambiguus, and the caudal ventral respiratory group, and thus is important for cardiorespiratory control. Since neurons in the caudal pressor area may be important in the regulation of blood pressure, we wished to determine if inhibiting/lesioning the CPA would affect the increase in arterial blood pressure induced by nasal stimulation. Our bilateral injections of glycine and muscimol were ineffective in blocking the increase in MABP to nasal stimulation. Such injections also did not block the bradycardia or apnea induced by nasal stimulation. The multiple injections of IBO in the CPA region also had no effect on the increase in MABP to nasal stimulation, but did eliminate the bradycardia and reduced the duration of apnea to nasal stimulation. Since the increase in MABP persisted despite disrupting the CPA, we suggest the circuitry for this behavior utilizes neurons in other parts of the medulla.
Technical considerations
When substances are injected into the brain to either activate or inhibit neurons, much consideration must be placed on the area affected by the injections. Glycine, GABA and glutamate are relatively ubiquitous neurotransmitters in the central nervous system; glycine and GABA are inhibitory while glutamate is excitatory. The concentration and volume of glycine (1M; 50nl) and the GABA agonist muscimol (2mM; 50nl) used in the present study were similar to those used by other investigators of the CPA (Campos et al., 1999; Natarajan et al., 2000; Silva et al., 2002). Glycine and GABA are active for relatively short periods, being measured in minutes (Aprison et al., 1970; Possas et al., 1994; Hupe et al., 1999), whereas muscimol, a GABA agonist, is active for hours (Arikan et al., 2002). It is for this reason that the injections of muscimol were made after the injections of glycine.
IBO is a member of the family of excitants commonly used to ablate neurons without damaging fibers of passage. Chemical lesions are induced when IBO is injected in relatively high concentration (10–25µg/µl) (Hastings et al., 1985; Rugg et al., 1992; Inglis et al., 1997). However, we used a lesser concentration (5µg/µl) in the present study to paralyze neurons acutely in the hopes of disrupting a reflex circuit. The actions of ibotenate and the more potent kainic acid (Guldin et al., 1981) is somewhat paradoxical – first stimulating neurons before silencing them. Either often has been injected into the brainstem in acute experiments to silence neurons (Berger et al., 1982; Cravo et al., 1991; Cravo et al., 1993; Fung et al., 1994; Nattie et al., 1998; St-Jacques et al., 1999; Mutolo et al., 2002).
The fluorescent probe DEAD red has been used in conjunction with injections of IBO into the brainstem (Nattie et al., 1998). These authors calculated a 50nl volume of the excitotoxin affected neurons within a diameter of 643µm. Mutolo and colleagues (2002) found neurons unresponsive in an area less than 800µm from the injection’s center after a 20–30µl injection of kainic acid. We considered our 40nl injections of IBO spread less than 500µm/injection, supporting the theoretical calculations of Nicholson (Nicholson, 1985). Thus, we probably paralyzed a column of neurons approximately 1.2mm in the anterior-posterior plane and about 500µm in the medial-lateral and dorsal-ventral planes, approximately the functional area of the CPA defined by us (Sun et al., 2002; Sun et al., 2005). Many studies utilizing injections of excitatory neurotoxins in chronic animals approximate the effected area using neuroanatomical methods. It is unfortunate that neither the Nissl staining nor immunostaining for NueN indicated neuronal death after survival of only a few hours. However, the IBO injected in the present study may have spread beyond the CPA, since normal respiratory rhythms were disrupted and the animals had to be mechanically ventilated for several hours.
Arterial Blood Pressure
The increase in MABP seen after nasal stimulation with ammonia vapors persisted after injections of glycine, muscimol, or IBO into the CPA, suggesting that the CPA does not mediate such increases in MABP. The injections of glycine immediately induced a significant drop in resting MABP, confirming the results of others (Possas et al., 1994; Campos Jr et al., 1994; Campos et al., 1999; Natarajan et al., 2000). However, the larger relative declines in resting MABP seen by others after injections of GABA (Possas et al., 1994) or glycine (Campos Jr et al., 1994) into the most caudal ventrolateral medulla may have been due to either the larger injection volumes used in these studies, or their more caudal and medial injection sites. Also, injections of kynurenate into the CPA had no effect on resting MABP in one study (Campos Jr et al., 1994) but significantly reduced blood pressure in another (Silva et al., 2002). The smaller declines in resting MABP after injections of muscimol in the present study were similar to those seen by others (Natarajan et al., 2000). Nevertheless, injections of neither glycine nor muscimol into the CPA affected the increase in MABP induced by nasal stimulation.
There was a marked rise in resting MABP after the IBO injections, but the relative increase in MABP either before (34 mmHg) or after (32 mmHg) nasal stimulation was not significantly different. The rise in resting MABP could be from spread of the IBO into the CVLM, increased peripheral resistance, cardiac output, or destruction of the CPA. The neurons of the CPA are found among fiber tracts running longitudinally through the brainstem (Sun et al., 2005), and the IBO could have followed these fibers and spread rostrally into the CVLM. Inhibiting/silencing neurons in the CVLM either with injections of muscimol (Willette et al., 1984), glutamate receptor antagonists (Guyenet et al., 1987; Blessing, 1989; Jung et al., 1991; Silva et al., 2002), kainic acid (Cravo et al., 1991; Cravo et al., 1993; Mutolo et al., 2002), or with electrolytic lesions (Murugaian et al., 1989), also induces an elevation of MABP. It is of interest, however, that the increases in MABP noted after bilateral lesions of the CVLM (Murugaian et al., 1989; Cravo et al., 1991; Mutolo et al., 2002) were temporary, and fell to near normal levels within 30 minutes. The hypertension seen after injections of IBO into the CPA in the present study, however, was still strong after 5–6 hours, suggesting our injections did not affect the CVLM.
Either stimulating the CPA or disinhibiting it with bicuculline induces an increase in sympathetic nerve activity (Natarajan et al., 2000; Seyedabadi et al., 2006) and increases in MABP and HR, while inhibiting the CPA induces an opposite reaction. If we assume that the IBO injections eventually silenced CPA neurons, we might expect MABP to fall, yet this was not the case. It is possible that the IBO was still exciting neurons in the CPA after 5–6 hr in the present study, or that there was loss of a disproportionate number of inhibitory neurons. Increasing either stroke volume or heart rate elevates cardiac output, and either could increase MABP. Although stroke volume was not measured in the present study, heart rate increased dramatically after IBO injections into the CPA, and this could have influenced the increase in MABP. Moreover, a nearby medullo-cervical pressor area, the MCPA, recently has been described extending from the caudal ventrolateral medulla into the cervical spinal cord (Seyedabadi et al., 2006). Our injections may have inhibited both the CPA and MCPA (Seyedabadi et al., 2006).
Heart Rate
Resting heart rate was reduced significantly immediately after injections of glycine into the CPA in the present study, confirming the results of others (Possas et al., 1994; Natarajan et al., 2000). Nevertheless, the bradycardia induced by nasal stimulation remained relatively unchanged after injections of either glycine or muscimol into the CPA. An abrupt bradycardia sometimes was induced immediately with IBO injections centered in the CPA (see Fig. 1 in Sun and Panneton, 2001), possibly due to the initial excitatory actions of IBO on caudal preganglionic parasympathetic cardiac motor neurons. However, the bradycardia induced by nasal stimulation was inhibited completely hours after IBO injections. The loss of bradycardia after the multiple IBO injections may have induced greater spread of this neurotoxin than the glycine/muscimol injections, possibly silencing more chronotropic cardiac motor neurons. Since even resting HR increased by over 67% after IBO injections, a rate limiting vagal tone possibly was decreased or eliminated.
Ventilation
There was no change in normal respiratory rate or the duration of apnea elicited by nasal stimulation after glycine injections in the present study. After the injections of muscimol however, there was a significant drop in resting respiratory rate for at least 20 minutes. It is unknown if this was due to either the activation/inactivation of specific receptors, or the longer half-life, and further spread, of muscimol. There also was no change in resting respiratory rate after IBO injections, but the duration of apnea induced by nasal stimulation was significantly reduced.
The caudal part of the ventral respiratory column is just dorsomedial to the CPA. Neurons of the caudal ventral respiratory column are active mostly in expiration (Feldman, 1986; Bongianni et al., 1994; Bongianni et al., 2002; Bongianni et al., 2005). In this regard there commonly was a short apnea both after injections of glutamate to locate the CPA and after the IBO injections in the present study, confirming previous observations (Bongianni et al., 1994; Seyedabadi et al., 2006). However, normal respiratory rhythm eventually returned, validating that the caudal ventral respiratory group does not influence rhythm generation (Bongianni et al., 1994; Bongianni et al., 2002; Bongianni et al., 2005). Stimulation of the nasal mucosa with ammonia vapors elicits an apnea which usually lasts longer than stimulus application (Panneton, 1990; Panneton, 1991b; Panneton et al., 1995; Yavari et al., 1996;McCulloch et al., 1999; present study). The duration of apnea induced by nasal stimulation was unchanged after glycine injections, but was increased 1 min after muscimol injections. Interestingly, the duration of the apnea induced by nasal stimulation prior to the IBO injections was reduced significantly after the injections, such that the apnea now lasted only as long as the stimulus. We postulate that nearby respiratory neurons were disrupted with the IBO injections, and may be important in prolonging apnea after nasal stimulation has ceased.
Summary
Stimulating the nasal mucosa with either water or irritating vapors induces an increase in arterial blood pressure, a bradycardia, and an apnea (Drummond et al., 1979; Doyle et al., 1988; Panneton, 1990; Panneton, 1991b; Gieroba et al., 1994; McCulloch et al., 1995; Panneton et al., 1995; Yavari et al., 1996; Yu et al., 1997; McCulloch et al., 1997; Nalivaiko et al., 2003; Panneton et al., 2003; Rybka et al., 2006). Anesthetizing the nasal mucosa abolishes these responses to nasal stimulation (Drummond et al., 1979; McCulloch et al., 1995; Yavari et al., 1996). Since both the AEN and the portion of the MDH receiving primary afferent fibers from this nerve project directly to the CPA (Panneton, 1991a; Panneton et al., 2000; Panneton et al., 2006), we tested whether the CPA mediates the increase in arterial blood pressure after nasal stimulation.
We conclude that CPA neurons play a minimal role in producing the increase in arterial blood pressure induced by nasal stimulation. Thus, other brainstem areas receiving either direct projections from the AEN (Panneton, 1991a; Panneton et al., 2006) or indirect projections from the ventral MDH (Panneton et al., 2000; Panneton et al., 2006) should be considered. The RVLM or CVLM receive such primary afferent fibers directly while the parabrachial complex and A5 area receive indirect projections; all are implicated as important in cardiovascular regulation. These areas must be tested to determine if they are important mediators of MABP during nasal stimulation. Our data also implies that the use of IBO to functionally paralyze neurons of acute animals should be interpreted cautiously, and perhaps is better used in chronic animals where the effective death of neurons can be visualized.
The neural circuitry for cardiovascular and respiratory function is very complex and highly integrated, and any disruption of the responses to nasal stimulation induced by injections in the CPA may merely implicate disruption of normal homeostatic connections. It should also be noted that these cardiorespiratory responses to nasal stimulation are similar to those of underwater submersion in mammals, collectively called the diving response. Thus knowledge of the circuit driving increases in MABP to nasal stimulation may help determine the circuitry driving vasoconstriction for the diving response.
Acknowledgment
The authors thank Rajko Juric for his excellent histology and the expertise of Ms. Dana Oliver for help with the statistical analysis. We appreciate Dr. Michael Anch, Jay Aguara, Vivianne Greenwood and Brent Harvey for a critical reading of this manuscript. This work was supported by NIH grant R01 HL64772 to WMP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aprison MH, Davidoff RA, Werman R. Glycine: its metabolic and possible roles in nervous tissue. In: Lajha A, editor. Handbook of Neurochemistry. New York, NY: Plenum Press; 1970. pp. 381–397. [Google Scholar]
- Arikan R, Blake NMJ, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J. Neurosci. Methods. 2002;118:51–57. doi: 10.1016/s0165-0270(02)00143-7. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Cooney KA. Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. J. Appl. Physiol. 1982;52:131–140. doi: 10.1152/jappl.1982.52.1.131. [DOI] [PubMed] [Google Scholar]
- Blessing WW. Baroreceptor-vasomotor reflex after N-methyl-D-aspartate receptor blockade in rabbit caudal ventrolateral medulla. J. Physiol. (Lond.) 1989;416:67–78. doi: 10.1113/jphysiol.1989.sp017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongianni F, Corda M, Fontana GA, Pantaleo T. Chemical activation of caudal medullary expiratory neurones alters the pattern of breathing in the cat. J. Physiol. (Lond.) 1994;474:497–507. doi: 10.1113/jphysiol.1994.sp020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Carfi M. Respiratory responses to ionotropic glutamate receptor antagonists in the ventral respiratory group of the rabbit. Pflugers Arch. 2002;444:602–609. doi: 10.1007/s00424-002-0874-1. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Nardone F, Pantaleo T. Ionotropic glutamate receptors mediate excitatory drive to caudal medullary expiratory neurons in the rabbit. Brain Res. 2005;1056:145–157. doi: 10.1016/j.brainres.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Jeske I. Cardiorespiratory effects of DL-homocysteic acid in caudal ventrolateral medulla. Am. J. Physiol. 1989;256:H688–H696. doi: 10.1152/ajpheart.1989.256.3.H688. [DOI] [PubMed] [Google Scholar]
- Campos RR, Jr, Possas OS, Cravo SL, Lopes OU, Guertzenstein PG. Putative pathways involved in cardiovascular responses evoked from the caudal pressor area. Braz. J. Med. Biol. Res. 1994;27:2467–2479. [PubMed] [Google Scholar]
- Campos RR, McAllen RM. Tonic drive to sympathetic premotor neurons of rostral ventrolateral medulla from caudal pressor area neurons. Am. J. Physiol. 1999;276:R1209–R1213. doi: 10.1152/ajpregu.1999.276.4.R1209. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG. Reflexes evoked from tracheobronchial tree and lungs. In: Cherniak NS, Widdicombe JG, editors. Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda, MD: Amer. Physiol. Soc.; 1994. pp. 395–430. [Google Scholar]
- Cravo SL, Morrison SF. The caudal ventrolateral medulla is a source of tonic sympathoinhibition. Brain Res. 1993;621:133–136. doi: 10.1016/0006-8993(93)90308-a. [DOI] [PubMed] [Google Scholar]
- Cravo SL, Morrison SF, Reis DJ. Differentiation of two cardiovascular regions within caudal ventrolateral medulla. Am. J. Physiol. 1991;261:R985–R994. doi: 10.1152/ajpregu.1991.261.4.R985. [DOI] [PubMed] [Google Scholar]
- Dampney RAL. The subretrofacial vasomotor nucleus: Anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog. Neurobiol. 1994;42:197–227. doi: 10.1016/0301-0082(94)90064-7. [DOI] [PubMed] [Google Scholar]
- de Toledo Bergamaschi C, de Arruda Carillo B, Futuro Neto HA, de Campos RR. Differential baroreceptor modulation mediated by the ventrolateral medulla. Auton. Neurosci. 2006:126–127. 156–162. doi: 10.1016/j.autneu.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Doyle RE, Panneton WM, Vogler GA, Romeo JP, Watson BJ, Higgins B. The muskrat in biomedical research. J. Lab. Anim. Sci. 1988;38:667–674. [PubMed] [Google Scholar]
- Drummond PC, Jones DR. The initiation and maintenance of bradycardia in a diving mammal, the muskrat, Ondatra zibethica. J. Physiol. (Lond.) 1979;290:253–271. doi: 10.1113/jphysiol.1979.sp012770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J, Ezure K, Lipski J. Breathing rhythm generation: Focus on the rostral ventrolateral medulla. News Physiol. Sci. 1995;10:133–140. [Google Scholar]
- Feldberg W, Guertzenstein PG. Blood pressure effects of leptazol applied to the ventral surface of the brainstem of cats. J. Physiol. (Lond.) 1986;372:445–456. doi: 10.1113/jphysiol.1986.sp016019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL. Neurophysiology of breathing in mammals. In: Bloom FE, editor. Handbook of Physiology. The Nervous System: Intrinsic regulatory systems of the brain. Bethesda, MD: Am. Physiol. Soc.; 1986. pp. 463–524. [Google Scholar]
- Fung M-L, Wang W, St John WM. Medullary loci critical for expression of gasping in adult rats. J. Physiol. (Lond.) 1994;480:597–611. doi: 10.1113/jphysiol.1994.sp020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieroba ZJ, Yu Y-H, Blessing WW. Vasoconstriction induced by inhalation of irritant vapour is associated with appearance of Fos protein in C1 catecholamine neurons in rabbit medulla oblongata. Brain Res. 1994;636:157–161. doi: 10.1016/0006-8993(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, McCann LA. Pressor response evoked by microinjections of L-glutamate into the caudal ventrolateral medulla of the rat. Brain Res. 1988;457:251–258. doi: 10.1016/0006-8993(88)90693-2. [DOI] [PubMed] [Google Scholar]
- Guldin WO, Markowitsch HJ. No detectable remote lesions following massive intrastriatal injections of ibotenic acid. Brain Res. 1981;225:446–451. doi: 10.1016/0006-8993(81)90852-0. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. Role of the ventrolateteral medulla oblongata in blood pressure regulation. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Function. New York: Oxford University Press; 1990. pp. 145–167. [Google Scholar]
- Guyenet PG, Filtz TM, Donaldson SR. Role of excitatory amino acids in rat vagal and sympathetic baroreflexes. Brain Res. 1987;407:272–284. doi: 10.1016/0006-8993(87)91105-x. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Winn P, Dunnett SB. Neurotoxic amino acid lesions of the lateral hypothalamus: a parametric comparison of the effects of ibotenate, N-methyl-D, L-aspartate, and quisqualate in the rat. Brain Res. 1985;360:248–256. doi: 10.1016/0006-8993(85)91240-5. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Dampney RAL. Evidence for tonic disinhibition of RVLM sympathoexcitatory neurons from the caudal pressor area. Auton. Neurosci. 2002;99:102–110. doi: 10.1016/s1566-0702(02)00114-5. [DOI] [PubMed] [Google Scholar]
- Hupe JM, Chouvet G, Bullier J. Spatial and temporal parameters of cortical inactivation by GABA. J. Neurosci. Methods. 1999;86:129–143. doi: 10.1016/s0165-0270(98)00162-9. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Semba K. Discriminable excitotoxic effects of ibotenic acid, AMPA, NMDA and quinolinic acid in the rat laterodorsal tegmental nucleus. Brain Res. 1997;755:17–27. doi: 10.1016/s0006-8993(97)00101-7. [DOI] [PubMed] [Google Scholar]
- Iwamoto GA, Brtva RD, Waldrop TG. Cardiorespiratory responses to chemical stimulation of the caudalmost ventrolateral medulla in the cat. Neurosci. Let. 1991;129:86–90. doi: 10.1016/0304-3940(91)90726-a. [DOI] [PubMed] [Google Scholar]
- Jung R, Bruce EN, Katona PG. Cardiorespiratory responses to glutaminergic antagonists in the caudal ventrolateral medulla of rats. Brain Res. 1991;564:286–295. doi: 10.1016/0006-8993(91)91465-d. [DOI] [PubMed] [Google Scholar]
- Kumada M, Terui N, Kuwaki T. Arterial baroreceptor reflex: Its central and peripheral neural mechanisms. Prog. Neurobiol. 1990;35:331–361. doi: 10.1016/0301-0082(90)90036-g. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Ramirez JM, Alford S, Zuperku EJ. Unraveling the mechanism for respiratory rhythm generation. Bioessays. 2000;22:6–9. doi: 10.1002/(SICI)1521-1878(200001)22:1<6::AID-BIES3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McCulloch PF, Panneton WM. Fos immunohistochemical determination of brainstem neuronal activation in the muskrat after nasal stimulation. Neuroscience. 1997;78:913–925. doi: 10.1016/s0306-4522(96)00633-1. [DOI] [PubMed] [Google Scholar]
- McCulloch PF, Panneton WM, Guyenet PG. The rostral ventrolateral medulla mediates the sympathoactivation produced by chemical stimulation of the nasal mucosa. J. Physiol. (Lond.) 1999;516:471–484. doi: 10.1111/j.1469-7793.1999.0471v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch PF, Paterson IA, West NH. An intact glutamatergic trigeminal pathway is essential for the cardiac response to simulated diving. Am. J. Physiol. 1995;269:R669–R677. doi: 10.1152/ajpregu.1995.269.3.R669. [DOI] [PubMed] [Google Scholar]
- Murugaian J, Sundaram K, Krieger A, Sapru H. Electrolytic lesions in the depressor area of the ventrolateral medulla of the rat abolish depressor responses to the aortic nerve stimulation. Brain Res. 1989;499:371–377. doi: 10.1016/0006-8993(89)90787-7. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Carfi M, Pantaleo T. Respiratory changes induced by kainic acid lesions in rostral ventral respiratory group of rabbits. Am. J. Physiol. 2002;283:R227–R242. doi: 10.1152/ajpregu.00579.2001. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, De Pasquale CG, Blessing WW. Electrocardiographic changes associated with the nasopharyngeal reflex in conscious rabbits: vago-sympathetic co-activation. Auton. Neurosci. 2003;105:101–104. doi: 10.1016/S1566-0702(03)00048-1. [DOI] [PubMed] [Google Scholar]
- Natarajan M, Morrison SF. Sympathoexcitatory CVLM neurons mediate responses to caudal pressor area stimulation. Am. J. Physiol. 2000;279:R364–R374. doi: 10.1152/ajpregu.2000.279.2.R364. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Erlichman JS, Li AH. Brain stem lesion size determined by DEAD red or conjugation of neurotoxin to fluorescent beads. J. Appl. Physiol. 1998;85:2370–2375. doi: 10.1152/jappl.1998.85.6.2370. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Panneton WM. Controlled bradycardia induced by nasal stimulation in the muskrat, Ondatra zibethicus. J. Auton. Nerv. Syst. 1990;30:253–264. doi: 10.1016/0165-1838(90)90257-j. [DOI] [PubMed] [Google Scholar]
- Panneton WM. Primary afferent projections from the upper respiratory tract in the muskrat. J. Comp. Neurol. 1991a;308:51–65. doi: 10.1002/cne.903080106. [DOI] [PubMed] [Google Scholar]
- Panneton WM. Trigeminal mediation of the diving response in the muskrat. Brain Res. 1991b;560:321–325. doi: 10.1016/0006-8993(91)91251-u. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Gan Q. Cardiovascular changes induced in freely diving, swimming and nasally stimulated rats. Neurosci. Abstr. 2003:28. [Google Scholar]
- Panneton WM, Gan Q, Juric R. Brainstem projections from recipient zones of the anterior ethmoidal nerve in the medullary dorsal horn. Neuroscience. 2006;141:889–906. doi: 10.1016/j.neuroscience.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Panneton WM, McCulloch PF, Sun W. Trigemino-autonomic connections in the muskrat: the neural substrate for the diving response. Brain Res. 2000;874:48–65. doi: 10.1016/s0006-8993(00)02549-x. [DOI] [PubMed] [Google Scholar]
- Panneton WM, McCulloch PF, Tan Y, Tan Y, Yavari P. Brainstem origin of preganglionic motoneurons in the muskrat. Brain Res. 1996;738:342–346. doi: 10.1016/s0006-8993(96)01048-7. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Yavari P. A medullary dorsal horn relay for the cardiorespiratory responses evoked by stimulation of the nasal mucosa in the muskrat, Ondatra zibethicus: Evidence for excitatory amino acid transmission. Brain Res. 1995;691:37–45. doi: 10.1016/0006-8993(95)00597-j. [DOI] [PubMed] [Google Scholar]
- Possas OS, Campos RR, Jr, Cravo SL, Lopes OU, Guertzenstein PG. A fall in arterial blood pressure produced by inhibition of the caudalmost ventrolateral medulla: The caudal pressor area. J. Auton. Nerv. Syst. 1994;49:235–245. doi: 10.1016/0165-1838(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Prebötzinger complex and pacemaker neurons: Hypothesized site and kernel for respiratory rhythm generation. Ann. Rev. Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Rugg EL, Dunbar JS, Latimer M, Winn P. Excitotoxic lesions of the pedunculopontine tegmental nucleus of the rat. I. Comparison of the effects of various excitotoxins, with particular reference to the loss of immunohistochemically identified cholinergic neurons. Brain Res. 1992;589:181–193. doi: 10.1016/0006-8993(92)91277-l. [DOI] [PubMed] [Google Scholar]
- Rybka EJ, McCulloch PF. The anterior ethmoidal nerve is necessary for the initiation of the nasopharyngeal response in the rat. Brain Res. 2006;1075:122–132. doi: 10.1016/j.brainres.2005.12.112. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. The baroreflex and beyond: Control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin. Exp. Pharmacol. Physiol. 2002;29:514–521. doi: 10.1046/j.1440-1681.2002.03665.x. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Baro-activated neurons with pulse-modulated activity in the rat caudal ventrolateral medulla express GAD67 mRNA. J. Neurophysiol. 2003;89:1265–1277. doi: 10.1152/jn.00737.2002. [DOI] [PubMed] [Google Scholar]
- Seyedabadi M, Li Q, Padley JR, Pilowsky PM, Goodchild AK. A novel pressor area at the medullo-cervical junction that is not dependent on the RVLM: efferent pathways and chemical mediators. J Neurosci. 2006;26:5420–5427. doi: 10.1523/JNEUROSCI.1190-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NF, Pires JGP, Dantas MA, Futuro Neto HA. Excitatory amino acid receptor blockade with the caudal pressor area and rostral ventrolateral medulla alters cardiovascular responses to nucleus raphe obscurus stimulation in rats. Braz. J. Med. Biol. Res. 2002;35:1237–1245. doi: 10.1590/s0100-879x2002001000019. [DOI] [PubMed] [Google Scholar]
- St-Jacques R, St-John WM. Transient, reversible apnoea following ablation of the pre- Bötzinger complex in rats. J. Physiol. (Lond.) 1999;520:303–314. doi: 10.1111/j.1469-7793.1999.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Prog. Neurobiol. 1995;47:157–233. doi: 10.1016/0301-0082(95)00026-8. [DOI] [PubMed] [Google Scholar]
- Sun W, Panneton WM. Negative chronotropism of the heart is inhibited with lesions of the caudal medulla in the rat. Brain Res. 2001;908:208–212. doi: 10.1016/s0006-8993(01)02614-2. [DOI] [PubMed] [Google Scholar]
- Sun W, Panneton WM. The caudal pressor area of the rat: its precise location and projections to the ventrolateral medulla. Am. J. Physiol. 2002;283:R768–R778. doi: 10.1152/ajpregu.00184.2002. [DOI] [PubMed] [Google Scholar]
- Sun W, Panneton WM. Defining projections from the caudal pressor area of the caudal ventrolateral medulla. J. Comp. Neurol. 2005;482:273–293. doi: 10.1002/cne.20434. [DOI] [PubMed] [Google Scholar]
- Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environ. Health Perspect. 2001;109:579–584. doi: 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Reflexes from the upper respiratory tract. In: Cherniak NS, Widdicombe JG, editors. Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda, MD: American Physiological Society; 1986. pp. 363–394. [Google Scholar]
- Willette RN, Punnen S, Krieger AJ, Sapru HN. Interdependence of rostral and caudal ventrolateral medullary areas in the control of blood pressure. Brain Res. 1984;321:169–174. doi: 10.1016/0006-8993(84)90696-6. [DOI] [PubMed] [Google Scholar]
- Yavari P, McCulloch PF, Panneton WM. Trigeminally-mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J. Auton. Nerv. Syst. 1996;61:195–200. doi: 10.1016/s0165-1838(96)00072-0. [DOI] [PubMed] [Google Scholar]
- Young PA, Sun W, Panneton WM. Cardiorespiratory responses to nasal stimulation after ibotenic acid injections into the caudal pressor area (CPA) Neurosci. Abstr. 1999;25:1174. [Google Scholar]
- Yu YH, Blessing WW. Cerebral blood flow in rabbits during the nasopharyngeal reflex elicited by inhalation of noxious vapor. J. Auton. Nerv. Syst. 1997;66:149–153. doi: 10.1016/s0165-1838(97)00080-5. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Davis PJ, Carrive P, Bandler R. Vocalization and marked pressor effect evoked from the region of the nucleus retroambigualis in the caudal ventrolateral medulla of the cat. Neurosci. Lett. 1992;140:103–107. doi: 10.1016/0304-3940(92)90692-z. [DOI] [PubMed] [Google Scholar]