Abstract
Interferon γ (IFN-γ) has pleiotropic biological effects, including intrinsic antiviral activity as well as stimulation and regulation of immune responses. An infectious recombinant human respiratory syncytial virus (rRSV/mIFN-γ) was constructed that encodes murine (m) IFN-γ as a separate gene inserted into the G-F intergenic region. Cultured cells infected with rRSV/mIFN-γ secreted 22 μg mIFN-γ per 106 cells. The replication of rRSV/mIFN-γ, but not that of a control chimeric rRSV containing the chloramphenicol acetyl transferase (CAT) gene as an additional gene, was 63- and 20-fold lower than that of wild-type (wt) RSV in the upper and lower respiratory tract, respectively, of mice. Thus, the attenuation of rRSV/mIFN-γ in vivo could be attributed to the activity of mIFN-γ and not to the presence of the additional gene per se. The mice were completely resistant to subsequent challenge with wt RSV. Despite its growth restriction, infection of mice with rRSV/mIFN-γ induced a level of RSV-specific antibodies that, on day 56, was comparable to or greater than that induced by infection with wt RSV. Mice infected with rRSV/mIFN-γ developed a high level of IFN-γ mRNA and an increased amount of interleukin 12 p40 mRNA in their lungs, whereas other cytokine mRNAs tested were unchanged compared with those induced by wt RSV. Because attenuation of RSV typically is accompanied by a reduction in immunogenicity, expression of IFN-γ by an rRSV represents a method of attenuation in which immunogenicity can be maintained rather than be reduced.
Keywords: vaccine, immunization, paramyxovirus, cytokine
Interferon γ (IFN-γ), a type II interferon, is produced by T cells and natural killer (NK) cells and has diverse biological effects (for review, see refs. 1 and 2). IFN-γ has intrinsic antiviral activity, up-regulates expression of major histocompatibility class I and II molecules, activates macrophages and NK cells, and has an important regulatory role in T helper (Th) cell proliferation. Two subsets of murine Th cells have been distinguished on the basis of the pattern of cytokine secretion: the Th1 subset, whose marker cytokines include interleukin 2 (IL-2) and IFN-γ, and the Th2 subset, whose markers include IL-4, IL-5, IL-6, and IL-10. IFN-γ preferentially inhibits the proliferation of Th2 cells, thus favoring a Th1 response.
Human respiratory syncytial virus (RSV) is the most important viral agent of pediatric respiratory tract disease worldwide. RSV is an enveloped nonsegmented negative-stranded RNA virus of the family Paramyxoviridae (for review, see ref. 3). The RSV genome is 15,222 nt in length and is transcribed into 10 subgenomic mRNAs that encode 11 proteins, including the 4 proteins of the nucleocapsid, N, P, L, and M2–1, and the attachment G and fusion F surface glycoproteins that are the major neutralization and protective antigens. Infectious recombinant (r) RSV can be produced by the intracellular coexpression from transfected plasmids of an RSV antigenomic RNA, which is the positive-sense replicative intermediate of genomic RNA, together with the N, P, M2–1, and L proteins (4).
A vaccine against RSV is not yet available, although there has been considerable progress in developing live-attenuated viruses, vectored antigens, and purified proteins as candidate vaccines (5). There is growing appreciation that certain properties of a RSV vaccine can qualitatively affect the humoral and cellular immune response and can adversely modify the pattern of disease that occurs upon subsequent infection by RSV (6). Immunization with formalin-inactivated RSV in the 1960s was associated with RSV disease enhancement rather than protection (7, 8). In rodents, immunization with formalin-inactivated RSV or with purified RSV glycoprotein induced a Th2-biased response and primed for enhanced pulmonary histopathology upon subsequent RSV challenge (6, 9, 10). This pathological response was abrogated by depletion of CD4+ cells or IL-4 and IL-10 before the challenge (10, 11). Fortunately, infection by RSV induces a response that appears to be biased toward the Th1 subset and is associated with protection rather than enhanced disease upon challenge (5, 6, 12). However, responses in a diverse human population may be difficult to predict, and a vaccine strain that further favors a Th1 response probably is desirable.
A difficulty in the effort to develop a live virus vaccine has been the identification of an attenuated strain that has achieved a satisfactory balance between attenuation and immunogenicity (5), especially because attenuation typically is accompanied by reduced immunogenicity. In this study, we explored the strategy of manipulating the immunogenic properties of a negative-stranded virus by coexpression of a multifunctional cytokine by the same virus. An rRSV, designated rRSV/mIFN-γ, was constructed encoding mIFN-γ as an additional gene to test the possibility that its expression would have one or more of the following effects: attenuation of the virus in vivo, augmentation of the immune response, and enhancement of the Th1 response.
MATERIALS AND METHODS
Plasmid Construction.
RSV gene-start and gene-end signals were attached to the mIFN-γ cDNA by PCR with oligonucleotides TATACCCGGGATGGGGCAAATATGAACGCTACACACTGCAT (positive sense, the XmaI site is in bold, the RSV gene-start sequence is underlined, sequence specific to 5′ terminal part of mIFN-γ gene is italicized, and the initiation codon is shown in bold italics) and ATTACCCGGGAATTTTTAATAACTTCAGCAGCGACTCCTTTTCC (negative sense, the XmaI site is in bold, the RSV gene-end sequence is underlined, sequence specific to 3′ terminal part of mIFN-γ gene is italicized, and the termination codon complement is shown in bold italics). The PCR product was cloned in plasmid pUC19, its sequence was confirmed, and it was then cloned into the XmaI site of the previously described antigenome plasmid D46/1024 (ref. 13, Fig. 1).
Figure 1.
Construction of the rRSV/mIFN-γ chimeric genome. The mIFN-γ cDNA (shaded rectangle, with initiation and termination codons shown on either side in lowercase) was modified to be flanked by RSV gene-start and gene-end signals as an XmaI fragment. This transcription cassette was inserted into an antigenome cDNA that previously had been modified by the insertion of an 8-nt XmaI linker (bold, italicized) into the unique StuI site (the two halves of the StuI site, AGG and CCT, are underlined) present in the G-F intergenic region. XmaI sites are underlined.
RSV-Specific and Ig Isotype-Specific ELISA.
Ninety-six-well plates coated with purified RSV F glycoprotein (4 μg/ml) were incubated with 4-fold dilutions of mouse serum followed by one of the following biotinylated isotype-specific rat anti-mouse antibodies: (i) IgG1-κ against IgG1 heavy chain, (ii) IgG2a-κ, allotype IgK-1A, against IgG2a heavy chain, and (iii) IgM mAb clone LO-MA-7 against IgA heavy chain (Accurate Chemicals). The plates were then incubated with streptavidin linked to alkaline phosphatase (Life Technologies, Gaithersburg, MD) and reacted with p-nitrophenyl phosphate solution (Sigma). The specificity of the reagents for the indicated isotypes was confirmed by ELISA against the following commercially obtained purified murine mAbs [the generous gifts of S. Epstein, the first three are from Cappel and the second three are from Litton Bionetics): IgG1 (MOPC 21), IgG2a (RPC 5), IgG3 (FLOPC 21), IgG2b (MOPC 141), IgA (TEPC 15), and IgM (MOPC 104E)]. Total IgG was detected by incubating F-coated plates with dilutions of the test sera followed by incubation with goat IgG specific to mouse IgG and conjugated to alkaline phosphatase (Cappel), which was then reacted with p-nitrophenyl phosphate. ELISA measurement was with a Vmax kinetic microtiter reader (Molecular Devices).
RESULTS
Construction and Recovery of rRSV Expressing mIFN-γ.
A cDNA clone encoding mIFN-γ was modified to be flanked by RSV gene-start and gene-end transcription signals (Fig. 1). This chimeric transcription cassette was inserted into the G-F intergenic region of the antigenome cDNA D46, which had been modified to contain a unique XmaI site (13). The chimeric RSV antigenome RNA containing the mIFN-γ insert would be 15,729 nt in length and encode 11 mRNAs, with the mIFN-γ gene being eighth in the 3′-to-5′ order. rRSV/mIFN-γ was recovered from transfected cDNA as described previously (4).
rRSV/mIFN-γ formed plaques that were comparable in size to those of a previously described chimeric virus, rRSV/CAT (previously called D46/1024CAT; ref. 13), which is identical to rRSV/mIFN-γ except that its foreign gene encodes chloramphenicol acetyltransferase (CAT) rather than mIFN-γ and its insert length is slightly greater (762 versus 507 nt). The plaque size for each of these chimeric viruses was slightly smaller that of wt RSV, but otherwise the plaque morphology was indistinguishable (not shown).
Northern blot analysis (not shown) of poly(A)+ mRNA isolated from cells infected with rRSV/mIFN-γ or wt RSV demonstrated that the former expressed an mIFN-γ mRNA of the expected size. We and others have shown that foreign sequences placed in nonsegmented negative-stranded RNA viruses are remarkably stable in cell culture (13, 14). Consistent with this, Northern blot and reverse transcription–PCR analysis of the mIFN-γ gene during eight passages of rRSV/mIFN-γ provided no evidence of deletion (data not shown).
Growth of rRSV/mIFN-γ and Production of mIFN-γ in Vitro.
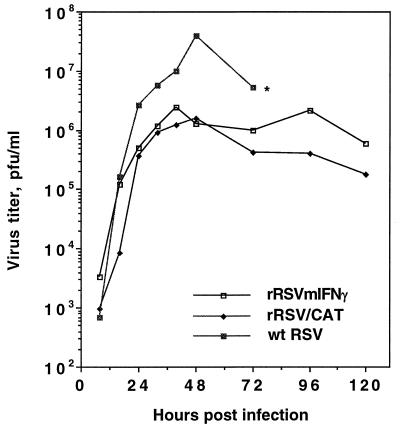
The growth characteristics of rRSV/mIFN-γ, rRSV/CAT, and wt RSV were compared in HEp-2 cells (Fig. 2). rRSV/CAT was chosen as an additional control because it contained a similarly sized insert in the same genome location. The two chimeric viruses grew more slowly and to a lower final titer than did wt RSV. For example, rRSV/mIFN-γ achieved a peak titer of 106.4 pfu (plaque-forming units)/ml at 40 h postinfection, compared with a maximal titer of 107.6 pfu/ml for wt RSV (48 h postinfection), indicating a 16-fold reduction.
Figure 2.
Growth kinetics for rRSV/mIFN-γ, rRSV/CAT, and wt RSV in HEp-2 cells. Cell monolayers were infected with 2 pfu per cell (two replicate wells per virus), and 200 μl aliquots of supernatant were taken at the indicated time, adjusted to contain 100 mM magnesium sulfate and 50 mM Hepes buffer (pH 7.5), flash-frozen, and stored at −70°C until titration. Each aliquot taken was replaced with an equal amount of fresh medium. Each single-step growth curve represents the average of the virus titers from the two infected cell monolayers. The cell monolayer infected with wt RSV was more than 90% destroyed at 72 h postinfection (∗), whereas that infected with rRSV/mIFN-γ or rRSV/CAT was almost intact, reflecting attenuation of the chimeric virus in vitro.
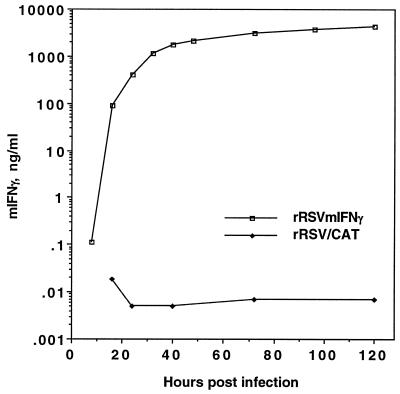
The medium overlaying HEp-2 cells infected with rRSV/mIFN-γ or rRSV/CAT was analyzed for mIFN-γ at different times postinfection (Fig. 3). The concentration of mIFN-γ was 0.1 ng/ml 8 h postinfection, the earliest time tested, 1.8 μg/ml at 40 h, and reached a maximum 4.4 μg/ml at 120 h, which corresponds to 22 μg per 106 cells.
Figure 3.
Kinetics of accumulation of mIFN-γ in culture fluids of HEp-2 cells infected with rRSV/mIFN-γ or rRSV/CAT. Cell monolayers were infected with 2 pfu per cell (two replicate wells per virus), samples were taken at the indicated time points, and the mIFN-γ content was determined in each sample by ELISA using the Quantikine M Mouse IFN-γ Immunoassay (R & D Systems).
Replication, Immunogenicity, and Protective Efficacy of rRSV/mIFN-γ in BALB/c Mice.
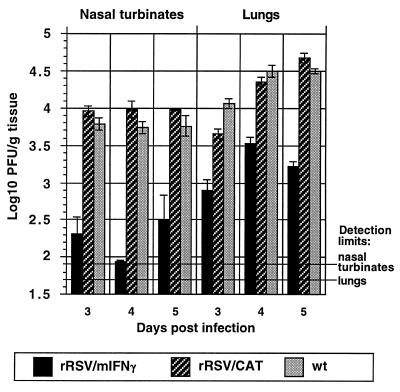
To evaluate replication of rRSV/mIFN-γ in vivo, mice were infected intranasally with 106 pfu of rRSV/mIFN-γ, rRSV/CAT, or wt RSV. Animals were sacrificed on day 3, 4, or 5 postinfection, and the concentration of the virus in the upper (nasal turbinates) and lower (lungs) respiratory tract was determined by plaque assay. Replication of rRSV/mIFN-γ was reduced relative to wt RSV by up to 63- and 20-fold in the upper and lower respiratory tracts, respectively (Fig. 4). In contrast, replication of rRSV/CAT was not significantly different from that of wt RSV, showing that the presence of an additional foreign gene of comparable size per se did not attenuate RSV replication in mice.
Figure 4.
Kinetics of virus replication in the upper and lower respiratory tract of BALB/c mice inoculated intranasally with 106 pfu of rRSV/mIFN-γ, rRSV/CAT, or wt RSV. Five mice from each group were sacrificed on the indicated day, the nasal turbinates and lung tissues were removed and homogenized, and the concentration of infectious virus was determined by plaque assay of individual tissue specimens. Mean log10 titer per gram tissue with SD is shown. The limit of virus detection in the upper and lower respiratory tract is indicated.
Serum samples were collected on days 0, 28, and 56 from mice infected with rRSV/mIFN-γ, rRSV/CAT, or wt RSV and were analyzed by an RSV-specific and antibody isotype-specific ELISA and by an RSV-neutralization assay (Table 1). The levels of IgA antibodies induced by the viruses were not significantly different. There was a significant increase (4-fold) of the total IgG specific to RSV F protein in mice vaccinated with rRSV/mIFN-γ compared with animals vaccinated with wt RSV or rRSV/CAT on day 56, but not on day 28. The titer of IgG1 antibodies was not significantly different between viruses on day 28, but on day 56 the mean titer of IgG1 from mice immunized with rRSV/mIFN-γ was higher than that of mice immunized with wt RSV (reciprocal 12.1 log2 versus 9.3 log2; P < 0.05) or rRSV/CAT. In contrast, the mean titer of IgG2a on day 56 was decreased for mice immunized with rRSV/mIFN-γ compared with wt RSV (9.6 log2 versus 11.6 log2; P < 0.001). Neutralizing antibody titers of mice infected with RSV/mIFN-γ compared with wt RSV and rRSV/CAT were marginally lower on day 28 but were modestly higher on day 56 (12.3 versus 11.2, log2; P < 0.2).
Table 1.
RSV serum antibody titers (reciprocal mean log2 ± SE) on days 0 (before immunization), 28, and 56
| Virus | ELISA antibodies to RSV F protein
|
RSV-neutralizing antibodies
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgA
|
IgG1
|
IgG2a
|
Total IgG
|
||||||||||||
| 0 | 28 | 56 | 0 | 28 | 56 | 0 | 28 | 56 | 0 | 28 | 56 | 0 | 28 | 56 | |
| rRSV/m1FN-γ | 7.3 | 10.8 ± 0.5* | 10.3 ± 0.6* | <5.3 | 7.3 ± 1.3* | 12.1 ± 1.0† | <5.3 | 10.8 ± 0.5 | 9.6 ± 0.3‡ | <5.3 | 11.3 ± 0.4 | 12.6 ± 0.6§ | <3.3 | 9.1 ± 0.3¶ | 12.3 ± 0.6¶ |
| rRSV/CAT | 7.3 | 12.1 ± 0.7 | 7.3 ± 1.3 | <5.3 | 10.3 ± 0.5 | 6.3 ± 1.2 | <5.3 | 10.1 ± 0.4 | 10.1 ± 0.4 | <5.3 | 10.8 ± 0.3 | 10.3 ± 0.4 | <3.3 | 9.9 ± 1.2 | 11.7 ± 0.7 |
| wt RSV | 7.3 | 11.8 ± 0.8 | 9.1 ± 1.1 | <5.3 | 10.3 ± 1.2 | 9.3 ± 0.9 | <5.3 | 11.1 ± 0.3 | 11.6 ± 0.3 | <5.3 | 11.8 ± 0.3 | 10.6 ± 0.4 | <3.3 | 9.8 ± 0.3 | 11.2 ± 0.5 |
| Placebo | 7.3 | 7.8 ± 0.3 | 4.1 ± 0.4 | <5.3 | <5.3 | <5.3 | <5.3 | <5.3 | <5.3 | <5.3 | 7.32 ± 0.0 | <5.3 | <3.3 | <3.3 | <3.3 |
Eight mice per group were used. Antibody titers on day 56 were determined in a separate assay.
Difference relative to the wt RSV control is not statistically significant (Student’s t test) because of a high variability of individual samples.
Statistical significance calculated by Student’s t test compared with wt RSV control:
P < 0.05;
P < 0.001;
P < 0.002;
P < 0.2.
To evaluate protective efficacy, five mice from the groups described above were challenged on day 56 by the intranasal instillation of 106 pfu per animal of wt RSV. Four days later, the mice were sacrificed and nasal turbinates and lungs were harvested for virus quantitation. Challenge virus was not detectable in animals that had been infected previously with rRSV/mIFN-γ, and only a very low level of replication was observed in the upper respiratory tract in animals previously infected with wt RSV (data not shown).
Pulmonary Cytokine mRNAs.
The levels of mRNAs encoding selected cytokines were determined in the lungs of mice infected with rRSV/mIFN-γ or wt RSV to determine whether the level of mIFN-γ mRNA synthesis was increased and whether its synthesis affected the level of other Th1 or Th2 cytokine mRNAs. Five mice each from groups infected with rRSV/mIFN-γ, wt RSV, or placebo were sacrificed on days 1 and 4 after infection or days 1 and 4 after challenge with wt RSV on day 28 (days 29 and 32). Total lung RNA was isolated and analyzed for selected cytokine mRNAs by a commercial ribonuclease protection assay (Fig. 5). This direct assay reflects the concentration of an mRNA at the site of interest at a given time and precludes possible artifacts because of in vitro manipulation of harvested cells. The mRNA levels were determined for the Th1 marker cytokines IL-2 and IFN-γ, the Th2 marker cytokines IL-4, IL-6, and IL-10, and the IL-12 p40 protein, which is the inducible component of the IL-12 heterodimer.
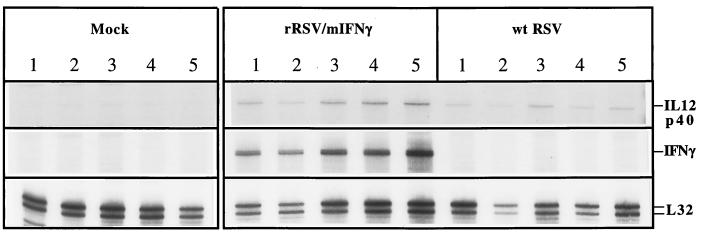
Figure 5.
Detection of pulmonary mIFN-γ, IL-12 p40, and L-32 (housekeeping gene) mRNAs by ribonuclease protection assay. Mice (five animals per group) were inoculated intranasally with medium alone (mock) or 106 pfu per animal of rRSV/mIFN-γ or wt RSV, and on day 4 after immunization the lungs were harvested and total RNA was purified. RNA was hybridized with radioactive RNA probes synthesized using mCK-2B template set (PharMingen RiboQuant Multi-Probe RNase Protection Assay System), treated with ribonuclease A, purified, and electrophoresed in a 5% denaturing acrylamide gel. Autoradiographs are shown for the region of the gel containing protected species corresponding to the indicated mRNAs. Different exposure times were used for each of the three mRNAs. Normal mouse RNA and yeast were used as additional negative controls (not shown).
Fig. 5 shows an autoradiograph of an assay of IFN-γ and IL-12 p40 mRNAs in lungs of five individual animals harvested 4 days after immunization with the indicated virus. Increased accumulation of mIFN-γ was seen in the rRSV/mIFN-γ-infected animals, and a slight, but statistically significant, increase in IL-12 p40 mRNA was seen in the rRSV/mIFN-γ-infected animals compared with those infected with wt RSV. The results from this and other gels were quantitated with a PhosphorImager (Molecular Dynamics), and the mean value for each set of five mice was expressed as a percentage of the mouse L-32 housekeeping gene mRNA in the same gel lane (Fig. 6).
Figure 6.
Levels of cytokine mRNAs in the lungs of mice after primary infection with rRSV/mIFN-γ or wt RSV and after challenge with wt RSV. Mice were immunized intranasally with 106 pfu per animal of rRSV/mIFN-γ, wt RSV, or medium alone. On day 1 or 4 after immunization five animals from each group were sacrificed and pulmonary RNA was isolated. Additional immunized animals were challenged on day 28 with 106 pfu of wt RSV, and total lung RNA was isolated individually from five animals from each group on day 29 or 32. The accumulation of mRNA for selected cytokines was measured by the ribonuclease protection assay described in the legend to Fig. 5 (PharMingen; template sets mCK-1 and mCK-2B). Radioactivity for each mRNA was quantified by PhosphorImager analysis, backgrounds were subtracted, and each value was calculated as a percentage of radioactivity relative to the L-32 housekeeping gene mRNA and displayed as an average of five animals with the SD. Samples that lacked detectable mRNA for the indicated cytokine are marked with asterisks.
On day 1 or 4 postinfection with wt RSV we observed: (i) the expression of the Th1 cytokine IFN-γ mRNA, but not that of IL-2; (ii) an increase in the level of IL-12 p40 mRNA; and (iii) the expression of the Th2 cytokine IL-6 and IL-10 mRNAs. Infection with rRSV/mIFN-γ induced a cytokine profile similar to that induced by wt RSV except that the levels of IFN-γ mRNA were higher on both days 1 and 4, but especially on day 4, and the level of IL-12 p40 mRNA also was higher on days 1 and 4. Thus, apart from the quantitative differences in IFN-γ and IL-12 p40, wt RSV and rRSV/mIFN-γ induced a similar profile of Th1 and Th2 cytokines. After virus challenge (days 29 and 32), the level of IFN-γ, IL-2, IL-10, and IL-12 p40, but not IL-6, also was increased in mice immunized with wt RSV or with rRSV/mIFN-γ compared with mock-immunized mice.
As an additional control, another group of animals in the same experiment was immunized parenterally with formalin-inactivated RSV (11) and subjected to the same harvest and challenge schedule. IL-4 mRNA was not detected in this or any other group after the initial immunization or infection (not shown). Upon challenge, IL-4 mRNA was increased greatly in the group that had received the formalin-treated vaccine, but was not detectable in the other groups (not shown).
DISCUSSION
A chimeric virus, rRSV/mIFN-γ, was constructed that expresses the mIFN-γ gene as a separate mRNA from an additional transcriptional unit placed eighth in the gene order, between the G and F genes. This virus directed the synthesis of high levels of mIFN-γ in cell culture. Growth of rRSV/mIFN-γ in cell culture was reduced 16-fold compared with wt RSV. However, the magnitude of this effect was comparable to that observed for rRSV/CAT, which contains the CAT gene in the same genome location. Thus, we attribute the growth restriction in vitro to the presence of the foreign gene rather than to its encoded product. That the expression of mIFN-γ did not inhibit viral growth in human HEp-2 cells is not surprising because human IFN-γ and mIFN-γ share only 40% amino acid sequence identity.
Replication of rRSV/mIFN-γ in BALB/c mice was reduced 63- and 20-fold in the upper and lower respiratory tract, respectively, compared with wt RSV. In contrast, rRSV/CAT assayed in parallel was not restricted compared with wt RSV, indicating that the attenuation of rRSV/mIFN-γ in vivo was not a result of the presence of the additional gene per se, but rather was a consequence of expression of mIFN-γ. Because the growth restriction in vivo operated early in infection, it seems likely that it was due to effects of the expressed mIFN-γ on innate immunity, such as induction of oligoadenylate synthetase and the resulting antiviral cascade or possibly the activation of NK cells and macrophages, rather than to effects on adaptive immunity. That the growth of rRSV/mIFN-γ was restricted only 63-fold or less suggests that IFN-γ is not the major effector of resistance to RSV. For another respiratory virus, influenza A virus, expression of IFN-γ by the host was not needed for an efficient immune response, although its absence resulted in a Th2-biased antibody and cytokine response (15).
The question of whether the coexpression of IFN-γ during RSV infection could further bias T cell proliferation in favor of a Th1 response was addressed by analyzing the pattern of cytokine mRNA and RSV-specific antibody isotypes. Infection with wt RSV was associated with increases in mRNA for the Th1 marker IFN-γ but not IL-2, for the Th2 markers IL-6 and IL-10, and for IL-12 p40, which is produced primarily by monocytes and macrophages. Infection with rRSV/mIFN-γ resulted in an increased level of IFN-γ mRNA and a slightly increased (less than 2-fold) level of IL-12 p40 mRNA over that observed with wt RSV. The increase in mIFN-γ mRNA presumably was due, at least in part, to that expressed by the recombinant virus. The increase in the IL-12 p40 mRNA probably was a result of IFN-γ-mediated activation of its monocyte/macrophage source, although this was not observed previously in vitro (16). Animals infected with rRSV/mIFN-γ did not exhibit differences from wt RSV in the level of mRNAs for the other Th1 marker, IL-2, or for the Th2 markers IL-6 or IL-10. There were modest increases in total IgG and IgG1 RSV-specific antibodies in mice immunized with rRSV/mIFN-γ compared with wt RSV, with the latter antibodies being a marker for a Th2 response (17). There also was a modest decrease in IgG2a, a marker for a Th1 response (17). Thus, neither the cytokine nor the antibody response was consistent with an increased bias toward Th1 markers either upon the initial infection with rRSV/mIFN-γ or after challenge with wt RSV.
Mice immunized with wt RSV or rRSV/mIFN-γ were highly resistant to RSV challenge. Despite its growth restriction in vivo, rRSV/mIFN-γ induced titers of total IgG against RSV F protein and RSV-neutralizing serum antibodies that were higher than that induced by wt RSV. Our previous results (18) showed that chimpanzees vaccinated with RSV cpts248/404, a candidate live-attenuated virus vaccine, developed lower titers of RSV-neutralizing antibodies compared with wt RSV-immunized animals (7.9 log2 versus 11.1 log2, a 9.2-fold difference), suggesting a correlation between the level of RSV replication and its immunogenicity. That the antibody response to rRSV/IFN-γ was increased moderately overall despite its reduced level of virus replication is encouraging for the development of a live-attenuated RSV vaccine.
We have shown previously (19) that rodents such as mice or cotton rats mount extremely efficient immune responses to RSV antigens, whereas immunogenicity is usually much less when evaluated in nonhuman primates or human volunteers. This might be especially important for young infants, whose antibody response to RSV has been shown to be reduced (20). Thus, differences in antigenicity that could be very significant in humans often are not detected in rodents because of their greater immunoresponsiveness to RSV antigen. Furthermore, replication of RSV in rodents is very restricted, such that only a small percentage of pulmonary cells are infected and disease typically does not occur. It is very likely that the effect of IFN-γ on attenuation, immunogenicity, or reactogenicity will be greater in a fully permissive host. To evaluate this, we presently are constructing an rRSV that contains human rather than murine IFN-γ. Evaluation of this virus in chimpanzees, which is the animal that most resembles humans with regard to RSV replication, disease, and immunogenicity, should yield a more realistic picture of the effects of the coexpressed IFN-γ. One possible complication, namely, that expression of the human cytokine in infected cultured primate cells will hamper the preparation of vaccine lots, presumably could be obviated by using cells from a different species.
A number of cytokine genes have been inserted into recombinant DNA viruses, mostly vaccinia virus, revealing effects on attenuation, pathogenicity, and immunogenicity (refs. 21 and 22; for review, see ref. 23). In a poxvirus, expression of IFN-γ or type 1 IFN attenuated the virus for the host, but this attenuation was accompanied by a decreased humoral immune response (24–26). Expression of IFN-γ by a simian immunodeficiency virus (SIV) lacking the nef gene resulted in further attenuation of the SIV mutant for monkeys, but the cytokine insert was very unstable after several weeks of replication, and the attenuation was accompanied by a decrease in the humoral immune response to SIV glycoprotein (27). In the present work, we have extended this strategy to a vaccine development program for a negative-stranded virus. The results demonstrate that it is possible to attenuate the virus while maintaining immunogenicity, a result previously attained only in the case of expression of IL-2 by a vaccinia virus vector (28). The present results are encouraging and indicate that the coexpression of IFN-γ represents a new type of attenuation of nonsegmented negative-stranded RNA viruses, one that reduces virus growth without compromising immunogenicity.
Acknowledgments
We thank Ena Camargo, Myron Hill, Cai-Yen Firestone, and Chris J. Cho for technical assistance.
ABBREVIATIONS
- RSV
respiratory syncytial virus
- rRSV
recombinant RSV
- mIFN-γ
murine interferon γ
- CAT
chloramphenicol acetyl- transferase
- rRSV/CAT or RSV/mIFN-γ
recombinant RSV containing the CAT or mIFN-γ gene, respectively
- pfu
plaque-forming units
- NK
natural killer
- Th
T helper
- wt
wild type
References
- 1.De Mayer E, De Mayer-Guingard J. In: Cytokines. Mire-Sluis A R, Thorpe R, editors. San Diego: Academic; 1998. pp. 391–400. [Google Scholar]
- 2.De Mayer E, De Mayer-Guingard J. In: The Cytokine Handbook. Thomson A, editor. San Diego: Academic; 1998. pp. 491–516. [Google Scholar]
- 3.Collins P L, McIntosh K, Chanock R M. In: Virology. Fields B N, Knipe D M, Howley P M, editors. New York: Lippincott–Raven; 1996. pp. 1313–1351. [Google Scholar]
- 4.Collins P L, Hill M G, Camargo E, Grosfeld H, Chanock R M, Murphy B R. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowe J E, Jr, Collins P L, Chanock R M, Murphy B R. In: New Generation Vaccine. Levine M M, Woodrow G C, Kaper G B, Cobon J S, editors. New York: Dekker; 1997. pp. 711–725. [Google Scholar]
- 6.Graham B S, Henderson G S, Tang Y-W, Lu X, Neuzil K M, Colley D G. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 7.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 8.Kapikian A Z, Mitchell R H, Chanock R M, Shvedoff R A, Stewart C E. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 9.Connors M, Collins P L, Firestone C-Y, Sotnikov A V, Waitze A, Davis A R, Hung P P, Chanock R M, Murphy B R. Vaccine. 1992;10:475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 10.Connors M, Giese N A, Kulkarni A B, Firestone C-Y, Morse H C, Murphy B R. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connors M, Kulkarni A B, Firestone C-Y, Holmes K L, Morse H C, Sotnikov A V, Murphy B R. J Virol. 1992;66:7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussell T, Spender L S, Georgiou A, O’Garra A, Openshaw P J M. J Gen Virol. 1996;77:2447–2455. doi: 10.1099/0022-1317-77-10-2447. [DOI] [PubMed] [Google Scholar]
- 13.Bukreyev A, Camargo E, Collins P. J Virol. 1996;70:6634–6641. doi: 10.1128/jvi.70.10.6634-6641.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnell M J, Buonocore L, Kretzschmar E, Johnson E, Rose J K. Proc Natl Acad Sci USA. 1996;93:11359–11365. doi: 10.1073/pnas.93.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham M B, Dalton D K, Giltinan D, Braciale V L, Stewart T A, Braciale T J. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snapper C M, Finkelman F D. In: Fundamental Immunology. Paul W E, editor. New York: Raven; 1993. pp. 837–863. [Google Scholar]
- 18.Crowe J E, Jr, Bui P T, Davis A R, Chanock R M, Murphy B R. Vaccine. 1994;12:783–790. doi: 10.1016/0264-410x(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 19.Collins P L, Purcell R H, London W T, Lawrence L A, Chanock R M, Murphy B R. Vaccine. 1990;8:164–168. doi: 10.1016/0264-410x(90)90141-8. [DOI] [PubMed] [Google Scholar]
- 20.Murphy B R, Alling D W, Snyder M H, Walsh E E, Prince G A, Chanock R M, Hemming V G, Rodriguez W Z, Kim H W, Graham B S, et al. J Clin Microbiol. 1986;24:894–898. doi: 10.1128/jcm.24.5.894-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramshaw I A, Andrew M E, Phillips S M, Boyle D B, Coupar B E. Nature (London) 1987;329:545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- 22.Flexner C, Hugin A, Moss B. Nature (London) 1987;330:259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- 23.Rolf M S, Ramshaw I A. Curr Opin Immunol. 1997;9:517–524. doi: 10.1016/s0952-7915(97)80104-5. [DOI] [PubMed] [Google Scholar]
- 24.Leong K H, Ramsay A J, Boyle D B, Ramshaw I A. J Virol. 1994;68:8125–8130. doi: 10.1128/jvi.68.12.8125-8130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bembridge G P, Lopez J A, Cook R, Melero J A, Taylor G. J Virol. 1998;72:4080–4087. doi: 10.1128/jvi.72.5.4080-4087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karaca K, Sharma J M, Winslow B J, Junker D E, Reddy S, Cochran M, McMillen J. Vaccine. 1998;16:1496–1503. doi: 10.1016/s0264-410x(97)00295-8. [DOI] [PubMed] [Google Scholar]
- 27.Giavedoni L, Ahmad S, Jones L, Yilma T. J Virol. 1997;71:866–872. doi: 10.1128/jvi.71.2.866-872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flexner C, Moss B, London W T, Murphy B, R. Vaccine. 1990;8:17–21. doi: 10.1016/0264-410x(90)90171-h. [DOI] [PubMed] [Google Scholar]