Abstract
The control of energy homeostasis in women is correlated with the anorectic effects of oestrogen, which can attenuate body weight gain and reduce food intake in rodent models. This review will investigate the multiple signalling pathways and cellular targets that oestrogen utilises to control energy homeostasis in the hypothalamus. Oestrogen affects all of the hypothalamic nuclei that control energy homeostasis. Oestrogen controls the activity of hypothalamic neurones through gene regulation and neuronal excitability. Oestrogen’s primary cellular pathway is the control of gene transcription through the classical ERs (ERα and ERβ) with ERα having the primary role in energy homeostasis. Oestrogen also controls energy homeostasis through membrane-mediated events via membrane-associated ERs or a novel, putative membrane ER that is coupled to G-proteins. Therefore, oestrogen has at least two receptors with multiple signalling and transcriptional pathways to activate during immediate and long-term anorectic effects. Ultimately, it is the interactions of all the receptor-mediated processes in hypothalamus and other areas of the CNS that will determine the anorectic effects of oestrogen and its control of energy homeostasis.
Introduction
It is well known that oestrogen is involved in the regulation of appetite, energy expenditure, body weight and adipose tissue deposition/distribution in females (1–3). Food intake in human females varies across the menstrual cycle with daily food intake lowest during the peri-ovulatory period when oestrogen levels are at maximum (4). Menopausal women tend to gain body fat, which appears to be a consequence of the decline in endogenous oestrogens (5–7). In animal models, ovariectomy induces an increase in food intake and decreases ambulatory and wheel running activities, which is reversed with oestrogen replacement (8–12). Therefore, hypo-oestrogenic states are associated with decreased activity and an increase in body weight in females. The anorectic effects of oestrogen are thought to be mediated through the CNS actions based on the findings that direct injections of oestradiol into the paraventricular nucleus or arcuate/ventromedial nucleus are the most effective to reduce food intake, body weight and increase wheel running activity especially in females (8,9,13).
In rodents and primates, energy homeostasis (defined by food intake, body weight, metabolic rates, etc.) is altered by the phasic changes in oestrogen levels during the oestrous cycle. A strong link between the reproductive cycle in females and the central control of energy homeostasis and feeding behaviour in the hypothalamus has been previously reviewed (4,14,15). Briefly, during the oestrous cycle, female guinea pigs, which have a true luteal phase unlike rats and mice, eat less during and immediately after the pro-oestrous phase where oestrogen levels are at their peak (13) and during the oestrous cycle in rats, energy expenditure and respiratory quotients, all measures of metabolic rates, decrease during the oestrous phase of the cycle (16). Female rodents may eat about 25% less during a significant portion of the oestrous cycle when oestrogen is at peak levels (4). The decrease in food intake is mostly due to a decrease in meal size (meal frequency may actually increase) (11). In rats, oestrogen inhibits meal size during the light cycle through a constant or tonic effect while having a phasic effect on both meal size and meal number during the dark cycle (17). The role of oestrogen in energy homeostasis has been further substantiated through studies of mutant mice with targeted disruption of the aromatase (ArKO) gene, the product of which converts androgens into oestrogens. In the ArKO genotype, females are severely obese and the obesity is reversed with oestrogen treatment (18).
The greater tendency for post-menopausal women towards obesity and the alterations of weight gain and feeding behaviour in rodent models clearly indicate that gonadal steroids, especially oestrogen, have a significant role in the CNS control of energy homeostasis. The cellular mechanisms and hypothalamic circuits involved in the CNS effects of oestrogen on energy homeostasis are only partially understood. Oestrogen is known to attenuate weight gain post-ovariectomy in multiple rodent models primarily through an ERα-dependent mechanism although there is evidence suggesting a role for another receptor-mediated mechanism (12,19). Furthermore, the multiple hypothalamic neuronal circuits that control feeding and metabolism are known to express oestrogen receptors (ER) (20–24). This review will discuss the potential transcriptional and rapid response mechanisms oestrogen has to control energy homeostasis and feeding including classical ERs and novel membrane receptors. While the nucleus of the solitary tract (NTS) in the brain stem is also involved in the control of feeding behaviour, this discussion is limited to the hypothalamic control of energy homeostasis and feeding because of the importance of oestradiol in the control of multiple hypothalamic and homeostatic functions.
Hypothalamic control of energy homeostasis
The control of energy homeostasis and feeding behaviour has been extensively reviewed (25–29) and will be described briefly herein. Only those nuclei and neuronal circuits involved in feeding and metabolism relevant to oestrogen signalling mechanisms will be discussed. These nuclei while distinct are not completely isolated with numerous reciprocal neural connections between them. The arcuate nucleus contains neurones that are vital to the control of energy homeostasis and are a primary targets for many peripheral signals including glucose and leptin (25–27). The two types of arcuate neurones that are relevant to the oestrogenic effects on energy homeostasis are the pro-opiomelanocortin (POMC) and neuropeptide Y (NPY) neurones (12,30–33). These two distinct neuronal populations function as a complementary/opposing circuit in the central control of energy homeostasis. POMC neurones decrease food intake (anorectic) primarily by the activity of two transcripts, α-melanocyte-stimulating hormone (α-MSH) and cocaine- and amphetamine-regulated transcripts (CART). NPY neurones increase food intake (orexigenic) via the actions of NPY and agouti gene-related protein (AgRP), which is an antagonist of theα-MSH receptor (MC3/4) involved in the control of energy homeostasis (25–27).
Another hypothalamic area involved in the control of feeding is the lateral hypothalamus, which is thought to be the downstream target of arcuate POMC/NPY neurones. Stimulation of the lateral hypothalamus increases food intake (25,34). The primary neurones in the lateral hypothalamus that affect feeding and energy homeostasis are the melanin-concentrating hormone (MCH) neurones and the orexin neurones (25,28,34,35). Stimulation of MCH neurones induces hyperphagia while MCH deficiency causes hypophagia and loss of body fat (36). Orexin neurones primarily control sleeping behaviour and arousal but also regulate feeding behaviour. Thus, orexin neurones are the important modulators of these two behavioural states to maintain survival and appear to control energy homeostasis by synapsing on neurones in the paraventricular nucleus of the hypothalamus (PVN) (37).
The other hypothalamic areas involved in energy homeostasis are the ventromedial hypothalamus (VMH), the dorsomedial hypothalamus (DMH) and the PVN. Neurones from the VMH are glucose-sensitive and have direct connections with other nuclei such as the PVN and the DMH (25). The DMH also has a role in energy homeostasis (38) and expresses both the orexigenic peptide, NPY (39) and the anorectic peptide, CART (35,40). The DMH is also involved in thermoregulation and, with direct connections to other hypothalamic feeding centres (PVN and LH), may function as an integrator of energy homeostasis (metabolism), stress and thermoregulation (41). The PVN is the hypothalamic nucleus where multiple signals from the lateral hypothalamus and arcuate nucleus converge to control energy homeostasis as well as the site where the effects of stress and metabolism (corticotrophin-releasing factor (CRF) and thyrotrophin-releasing hormone (TRH)) are most likely engaged in the control of energy homeostasis and feeding (25,28,42,43).
Expression of ERα and ERβ in the hypothalamus and their involvement in the control of energy homeostasis
To regulate gene expression, oestrogen uses two classical nuclear steroid receptors, ERα and ERβ. These two receptors are widely, but differentially, distributed throughout the central nervous system including the relevant hypothalamic nuclei (20–22). ERα is highly expressed the arcuate nucleus, the ventrolateral VMH, and the DMH. The lateral hypothalamus also expresses ERα while there is little expression in the PVN of the rat or the mouse but robust expression in the guinea pig and the human (20–23,44). Although ERβ is expressed in the arcuate nucleus, the DMH and the lateral hypothalamus, the highest expression of ERβ in the hypothalamus is found in PVN primarily in the magnocellular division of the PVN (20–22). The expression of ERβ in the PVN is also differentially regulated during pregnancy and postnatal development (45) indicating that sex steroids regulate ERβ expression in the PVN.
In the neurones of the hypothalamic feeding circuits, ERα is highly expressed in POMC neurones of the female guinea pig (24). The functionally opposing NPY neurones of the ARC also express ER’s and the expression of NPY may depend on the ratio of ERα to ERβ (46–48). It appears that the orexigenic MCH and orexin neurones in the lateral hypothalamus do not express ERα, whereas adjacent neurones in the lateral hypothalamus do express the receptor (49,50). The lack of ER in these neurones indicates that oestrogen probably controls the expression of MCH or orexin transcripts through an indirect mechanism from neighbouring neurones or through afferents from other ERα-containing neurones such as POMC/NPY neurones in the arcuate nucleus. Other neuronal cell types involved in energy homeostasis such as CRF and TRH neurones also express classical ER. Based on immunohistochemical studies, CRF neurones express ERα in the PVN of the human (44) but express ERβ in the rat PVN (51). TRH neurones express ERα in the PVN of the rat (51).
Over the past decade since the production of ER knockout mice strains, there has been conflicting evidence as to which ER subtype is involved in the effects of oestrogen on energy homeostasis. An early observation of ER knockout mice suggested that the nuclear oestrogen receptor involved in the effects of oestrogen on energy homeostasis was ERα. These knockouts exhibited an obesity phenotype while the ERβ knockout mice did not (52,53). In general, studies in αERKO animals have found that females gain fat deposits at the expense of muscle mass, although there are some inconsistencies depending on the KO mouse model (52,54,55). Initial data examining the role of ER subtypes in energy homeostasis found that ERα was involved in the oestrogenic decrease in white adipose accumulation (52). ERα was also deemed important for the attenuation of weight gain and food intake and potentiation of cholecystokinin (CCK) function by oestrogen (19) and is involved in the extrahypothalamic (NTS) control of food intake (56,56). Conversely, ERβ may have a central role in the control of energy homeostasis because co-administration of oestradiol with ERβ anti-sense oligodeoxynucleotides (ODN) into the third ventricle attenuated the inhibitory effects of oestradiol on food intake in ovariectomised rats while infusion of ERα anti-sense ODN did not (57). Another study suggested a role for ERβ in adipose tissue accumulation based on an increase in weight gain and fat accumulation during oestrogen treatment in ERα knockout mice (53). In specific nuclei, such as the VMH, ERα is a possible mediator of oestrogen’s actions on multiple aspects of energy homeostasis (58). Musatov et al., induced a phenotype defined by obesity, hyperphagia, glucose intolerance and reduced activity (energy expenditure) by silencing ERα in the VMH by RNAi and that this phenotype was resistant to oestrogen’s effect on activity. The loss of ERα in the VMH also reduced basal metabolic rates, total energy expenditure and physical activity suggesting that ERα may be a primary nuclear receptor for the control of energy expenditure, at least, through the VMH. Collectively, these findings indicate that the primary nuclear receptor for the central (hypothalamic and NTS) control of feeding behaviour and food intake by oestrogen is mostly likely ERα. Both ERα- and ERβ–mediated signalling may have a role in the regulation of peripheral fat deposition.
Oestrogenic control of peptide gene expression and relevant nuclei involved in energy homeostasis
Oestrogen regulates the expression and activity of many of the genes involved in the control of energy homeostasis. In the arcuate nucleus, oestrogen may increase or decrease the expression of the POMC gene or one of its peptide products, β-endorphin, depending up the treatment paradigm and experimental model. In the short-term in vivo treatment (4–48 hr), oestradiol increases the expression of the POMC gene or the immunostaining of β-endorphin peptide in mice, rats, guinea pigs and sheep (33,59–62). Presumably the increase in POMC gene expression consequently means an increase the expression of α–MSH, the anorectic POMC peptide. In other long-term treatment of ovariectomised female rats, oestradiol decreases the expression of the POMC gene and its related peptides in the medial basal hypothalamus (63); however, in a recent study, long-term (30 day) systemic treatment of ovariectomised guinea pigs with oestradiol benzoate the expression of the POMC gene increase by almost 3-fold in the arcuate nucleus (64). The short-term response to oestrogen treatment may be the normal physiological response to oestrogen peak levels during the ovulatory cycle, which also corresponds to a decrease in food intake. Therefore, the chronic treatment paradigm may negate the effects of the normal oestrogen cycle and allows for other compensatory mechanisms during the oestrogenic control of energy homeostasis. Furthermore, alterations in gene expression in POMC neurones may not be the only mechanism oestrogen utilises to affect targets downstream of the melanocortin circuit (12,32).
The other arcuate nucleus neuronal cell type involved in the oestrogenic control of energy homeostasis is the NPY neurone. Similar to POMC expression, NPY has a differential response to oestrogen treatment paradigms. Oestrogen deficiency (ovariectomy) results in an increase in NPY expression in the PVN (65). In the arcuate nucleus, ovariectomy increases NPY mRNA expression with oestradiol replacement suppressing the increase in gene expression (31). In ovariectomised female rats, long-term (18 day) oestradiol treatment significantly decreases the expression of NPY protein in the PVN based on RIA of NPY (30). Oestradiol treatment also decreases the expression of NPY mRNA in the arcuate nucleus of the ovariectomised mouse within 3 hr of injection that lasted for up to 24 hr (33). Oestradiol benzoate alone decreases NPY protein expression in the arcuate nucleus and median eminence 48 hr post-treatment, but, when followed by progesterone, oestradiol treatment increases the NPY expression in the median eminence but not in the arcuate nucleus (66). Furthermore, during the pre-ovulatory/pro-oestrous phase of the ovarian cycle associated with LH surge, NPY mRNA expression is increased in the medial basal hypothalamus and, specifically, in the arcuate nucleus (67,68). In a clonal hypothalamic neuronal cell line, oestradiol differentially regulates NPY and AgRP expression via ERα and ERβ–mediated mechanisms with the direction of regulation dependent on the ratio of ERα to ERβ in the cell (48). Interestingly, systemic oestradiol replacement in ovariectomised rats attenuates the increase in food intake caused by central administration of NPY but not AgRP (69). Regardless of the conflicting evidence pertaining to the reproductive effects on NPY expression, it is apparent that some of the anorectic effects of oestrogen are mediated, in part, by decreases in NPY and/or AgRP expression in the hypothalamus.
In the lateral hypothalamus, the orexigenic signals, MCH and orexin, is regulated by oestrogen treatment although apparently through indirect mechanisms in castrated male rats (49,50). In the ovariectomised female rat, pre-proMCH mRNA is down-regulated in the lateral hypothalamus after 52 hr oestradiol benzoate + 4 h progesterone treatment but not by oestradiol treatment alone (70). Furthermore, in the intact male rat, chronic oestradiol treatment delivered via pellets significantly decreases the expression of MCH in the medial basal hypothalamus (36). In wild-type, diet-induced obese male mice, MCH expression is significantly decreased by chronic oestradiol treatment (71). Orexin peptide is potentially affected by oestrogen treatment; however, there is little data on the regulation of orexin peptide expression by oestrogen in females. In castrated males, pre-pro-orexin protein levels are restored to normal levels by oestradiol benzoate treatment (49). One of the peptide products of orexin neurones, orexin-A, is down-regulated by high doses of oestradiol benzoate (2 mg every 7 days for 3 weeks) in intact female rats in the hypothalamus although lower (5 μg daily for 22 days) doses of oestradiol in ovariectomised rats did not significantly affect orexin-A peptide content of the hypothalamus (72). The physiological irrelevance of the high dose of oestrogen in this experiments casts doubts on any significant regulatory effects of oestrogen on the expression of orexin-A in the lateral hypothalamus in females although further investigation is warranted.
The PVN expresses two other hypothalamic peptides involved in the control of energy homeostasis, TRH and CRF. While ERα and ERβ co-localise with TRH neurones in the PVN (51), there is no evidence suggesting a direct effect of oestrogen on the expression of the TRH gene. On the other hand, oestrogen is known to alter the expression of the stress-related hormone CRF in the PVN depending upon treatment paradigm and model. In ovariectomised female mice, oestradiol replacement restored the level of CRF mRNA expression to that of intact animals within 12 hr of treatment (33). In ovariectomised monkeys, chronic oestradiol treatment increases the CRF mRNA expression in the PVN compared to untreated females and females treated with both oestradiol and progesterone (73), while more recent data suggests that oestradiol and oestradiol plus progesterone treatment decreases the CRF immunostaining and mRNA expression in the PVN of ovariectomised female monkeys (74). Another new anorectic peptide associated with the control of energy homeostasis by the PVN is the CRF receptor 1 and 2 ligand, urocortin (urocortin 1) and is thought to provide a negative feedback mechanism to NPY induced-feeding (75). In the PVN, ovariectomy decreases the number of cells expressing urocortin mRNA expression, which is restored by oestradiol benzoate treatment (76).
Lesion and implantation/cannulation studies of relevant hypothalamic nuclei have determined which hypothalamic nuclei are involved in the control of energy homeostasis and the anorectic effects of oestrogen. As early as 1975, the VMH/arcuate region of the basal hypothalamus was shown to play a role in the anorectic effects of oestrogen. Beatty et al. demonstrated that lesions of the VMH/arcuate attenuate the effects of peripheral oestradiol administration (4 μg/kg) on body weight and food intake in ovariectomised rats (77). A later study showed that lesions of the lateral hypothalamus had no significant effect on the anorectic effects of oestrogen (78). The VMH/arcuate and the PVN were identified as the major nuclei involved in these oestrogenic effects in ovariectomised guinea pigs with intracranial injections of crystalline oestradiol (13). A subsequent study in ovariectomised rats implanted with diluted and undiluted crystalline oestradiol indicated that the PVN was more important than the VMH/arcuate in the anorectic effects of oestradiol (79). However, lesions of the PVN significantly attenuated the suppression of food intake by short-term (3 day) administration of oestradiol but not body weight gain in ovariectomised rats (80). Conversely, another study found that lesions of the PVN did not significantly alter the chronic effects of oestradiol on food intake and body weight (81). These studies, while partially in conflict, have identified at least three hypothalamic nuclei; the arcuate nucleus, the VMH and the PVN, involved in either the acute and/or chronic effects of oestradiol on energy homeostasis. However, the interpretation of these data must be made with caution because the inconsistent nature of lesions or implantations, the concentrations of implanted oestradiol and the neuroendocrinology differences between the two animal models (rats and guinea pigs). These studies do suggest that oestrogenic control of energy homeostasis in the hypothalamus may originate in the basal hypothalamus (arcuate and VMH) with secondary integration or modulation via other nuclei in the hypothalamus (PVN and lateral hypothalamus).
Membrane-initiated steroid signalling and energy homeostasis
Typically, oestrogen controls gene expression via the classical ER (ERα and ERβ) binding to oestrogen-response elements or other promoter sites through protein-protein interactions such as Sp-1 and Fos-Jun (AP-1) and activating transcription of important oestrogen responsive genes (nuclear-initiated steroid signalling or NISS) (82). Oestrogen can also activate a host of rapid signalling cascades that affect cell physiology and activate gene transcription through other transcription factor (non-oestrogen response element) promoter sites (membrane-initiated steroid signalling or MISS). The MISS mechanisms of oestrogen have been extensively reviewed by many others (83–86) and will not be thoroughly described herein. Essentially, oestrogen can activate transcription via complexes with other transcription factors through protein-protein interactions including pCREB, STATs, Elk-1-SRF, ATF-2-Jun andNFκB inducing transcription via their respective promoter sites. Oestrogen through a membrane-associated ER activates multiple signalling pathways including phosphatidylinositol 3′-kinase (PI3K), phospholipase C (PLC), mitogen-activated protein kinase (MAPK) and protein kinase pathways (PKA, PKC, etc.) (83–87). The primary ER involved in oestrogenic MISS is thought to be ERα (84) although recent data suggests that in a transfected neuronal cell line, ERβ is also associated with the membrane and is translocated to the membrane by oestrogen to activate MAPK signalling (88). Oestrogen regulates gene expression for at least a few of these signalling molecules in various hypothalamic nuclei including the arcuate nucleus (24,89,90). Oestrogen receptors are activated independent of ligand binding through phosphorylation of the receptor (84). In the hypothalamus, oestrogen MISS signalling has been identified in the VMH to potentiate the effects of oestrogenic NISS signalling during particular reproductive behaviours (lordosis) (91) and may also affect oestrogen’s control of homeostasis via VMH neurones. While there is little to no direct evidence suggesting a role for the activation of membrane-associated classical ER pathways in the control of energy homeostasis, there is evidence for other rapid, MISS events that affect energy homeostasis.
Oestrogen can attenuate food intake within 6 hours of administration into the third ventricle via cannula after an overnight fast compared to saline in mice (92) and between 4 and 14 hours in fed rats (93). Besides activation of membrane-associated ER, oestrogen can also activate signalling cascades initiated through G-protein coupled receptors (GPCR) that would rapidly alter neuronal activity and initiate changes in feeding behaviour (12,32). GPCR are vital membrane mediators for a host of central and peripheral signals that control energy homeostasis in the hypothalamus (94). Since GPCR target potassium channels to control neuronal excitability, an oestrogen-responsive GPCR may account for the one of the rapid mechanism oestrogen utilises to control energy homeostasis. Indeed, acute administration of oestrogen to hypothalamic slices will alter neuronal excitability of many different types of relevant neurones through the modulation of various ion channels (95,96). Recent evidence confirms that oestrogen has at least two different GPCRs (87,95) in the brain. GPR30 was the first oestrogen-binding GPCR identified and localised in the brain. GPR30 in the hypothalamus is localised primarily to the PVN and the supraoptic nucleus but also is found in arcuate nucleus neurones (97). Whereas GRP30 is expressed in a few of the hypothalamic nuclei that control energy homeostasis, the obesity phenotype of GPR30 knockout mice is unknown but is currently being investigated (Chambers et al., 2007) (98).
The other potential GPCR is the putative membrane oestrogen receptor (Gq-mER) functionally characterised by Kelly and colleagues (12,32). While the Gq-mER has not been cloned, the putative GPCR has been functionally identified in at least three types of arcuate nucleus neurones including POMC, dopamine and γ amino butyric acid (GABA) neurones. The Gq-mER was initially characterised in female guinea pigs but has also been functionally examined in ERα and ERβ knockout both male and female and in male ERαβ double-knockout mice (12). The Gq-mER activates a Gq-PLC-PKA pathway that inhibits the activation of G-protein coupled inwardly rectifying potassium channels (GIRK) by both GABAB receptors and μ–opioid receptors. The inhibition of the GIRK channel activity will depolarise the POMC neurone and increase neuronal activity and potentially add another mechanism for oestrogen to control energy homeostasis.
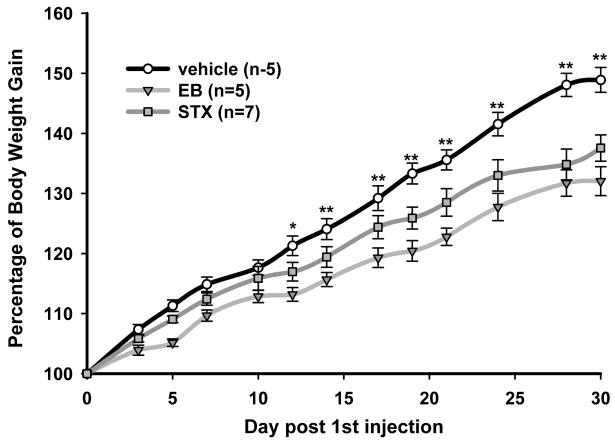
To elucidate this oestrogen pathway, a selective agonist for the Gq-mER was developed that had no binding capacity to classical ER (99). This compound, called STX, is more potent than oestrogen in attenuating the activation of GIRK channels by GABAB receptors (12,32). The ability of STX to alter POMC neuronal excitability led us to hypothesise that the putative Gq-mER has a role in energy homeostasis. Whole animal studies in which STX (2 mg/kg) was systemically injected in ovariectomised female guinea pigs over a period of four weeks supports this hypothesis (12). Recently published data indicates a dose-effect on the reduction in the body weight gain by systemic STX (6 mg/kg) treatment where the attenuation of post-ovariectomy weight gain was greater than in previous experiments (Figure 1). Both of these doses of STX, which is structurally similar to 4-OH tamoxifen, are similar to doses of tamoxifen administered to women on hormone replacement therapy. Furthermore, the administration of systemic STX generated new transcription in the arcuate nucleus of these STX-treated female guinea pigs (64). Many of the genes regulated include gene involved in the control of energy homeostasis (NPY) and neuronal activity (Cav3.1). Therefore, Gq-mER may function in the oestrogenic control of energy homeostasis presumably through activation of POMC neurones in the arcuate nucleus although other hypothalamic nuclei may be involved (12). Whole animal studies in ovariectomised rates have reported that a significant reduction in food intake by oestradiol treatment compared to oil treatment was not detected after administration of the MC3/4 antagonist SHU919. The site of action of the antagonist at the α–MSH receptors is downstream of oestradiol activation of arcuate POMC neurones which supports our hypothesis (100). It is unknown, at this time, if oestrogen has similar rapid effects in other neuronal cell types that control energy homeostasis in the hypothalamus such as NPY, MCH, and orexin. See Figure 2 for a schematic summarisation of the oestrogenic effects on the nuclei of the hypothalamus and specifically the hypothesis described above regarding the activation of arcuate POMC neurones by the Gq-mER.
Figure 1.
Oestrogen and STX significantly attenuate the body weight gain in female guinea pigs after ovariectomy. Female guinea pigs were ovariectomised and allowed to recover for 1 week (day 0) before being given bi-daily subcutaneous injections of propylene glycol (vehicle), oestradiol benzoate (EB; 8 μg/kg, triangles), or STX (6 mg/kg, squares). A two-way ANOVA (repeated measures) revealed an overall significant effect of both EB and STX (p < 0.001), and post hoc NewmañKeuls analysis revealed daily significant differences between STX and vehicle-treated groups (*p < 0.5; ** p < 0.01). Symbols represent the mean ± SEM of five, five and seven animals per group for vehicle, EB and STX treatment, respectively. Modified from Roepke et al., 2008, Endocrinology online.
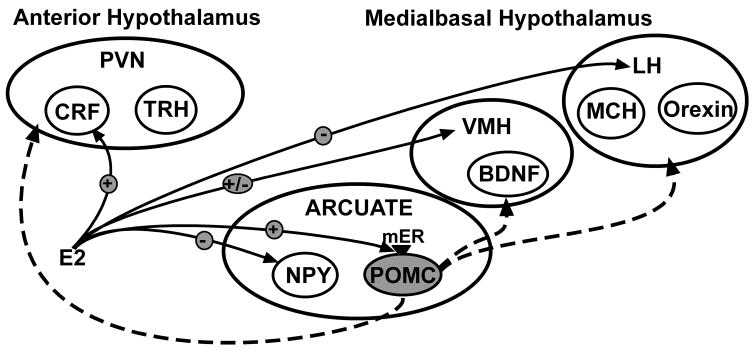
Figure 2.
Oestrogen is known to affect neurones in the arcuate nucleus, the VMH, the PVN and lateral hypothalamus (LH). Nuclear oestrogen receptors (ERα, ERβ) are expressed in all of these hypothalamic nuclei. Two membrane ER, GPR30 and Gq-mER are also expressed in the hypothalamus. GPR30 is expressed in the PVN and the arcuate nucleus while the Gq-mER is has been functionally identified in arcuate POMC neurones. The Gq-mER rapidly increases the neuronal excitability, and over the long term gene expression, of these anorectic neurones which have multiple projections to the VMH, the PVN and the lateral hypothalamus (LH). These projections (dashed line) potentially synapse on several of the neurones that control feeding behaviour, energy expenditure, etc., and affect these neurones through the α–MSH MC3/4 receptor. Kelly and colleagues have hypothesised that the oestrogenic activation of POMC neurones by the Gq-mER is important for the control of energy homeostasis as evidenced by the effects of the selective Gq-mER agonist, STX, on post-ovariectomy body weight gain. This hypothesis is supported by Polidori & Geary (100) in which the anorectic effects of oestrogen were blocked by antagonists of the MC3/4 receptor infused into the lateral ventricle near the PVN.
Oestrogen modulates of synaptic plasticity in the hypothalamus
A final mechanism for oestrogen to control energy homeostasis is the remodelling of excitatory and inhibitory synapses on hypothalamic neurones. Synaptic connections in the hypothalamus are not hardwired and synaptic plasticity has been reported in multiple hypothalamic neuronal systems including PVN magnocellular neurones and GnRH neurones of the arcuate nucleus and preoptic area (35). Recent data indicates that oestrogen-induced synaptic remodelling in the arcuate nucleus also affects the excitatory input on POMC neurones and has a potential role in the control of feeding and energy expenditure (93). The rewiring of synaptic inputs into POMC neurones occurs within 4–6 hours of oestrogen treatment, similar to the inhibition of food intake in fasted mice (92), and significantly increases the frequency of EPSCs on POMC neurones. Gao et al. (94) suggest that this rapid effect is mediated by ERα; however, the role of the Gq-mER receptor present in POMC neurones was not examined. Oestrogen also increases the growth of dendritic spines and alters synaptic plasticity in the ventrolateral VMH and in other parts of the brain (101,102).
The neurotrophin brain-derived neurotrophic factor (BDNF) controls synaptic plasticity in many parts of the brain (103) and has a role in the control of energy homeostasis (104) primarily through the VMH and the PVN (105,106). In the VMH, BDNF expressing neurones also express ERα (107) although oestrogen apparently does not regulate the mRNA expression of BDNF in the VMH using in situ hybridisation (108). While oestrogen does not directly regulate the expression of BDNF, it may interact with the BDNF signalling pathway through at least two mechanisms. BDNF is up-regulated by the melanocortin pathway (MC4 receptor) in the VMH (109,110) and melanocortin signalling is a target for the rapid and classical signalling of oestrogen in the arcuate nucleus (32,60). Furthermore, BDNF signals through its tyrosine kinase receptor (TrKB) to activate PI3K signalling pathways along with MAPK and PLC pathways (103). Interactions between BDNF and oestrogen in the hypothalamus may occur through the activation of similar pathways. Recent evidence suggests that oestrogen also rapidly activates PI3K signalling in the hypothalamic arcuate nucleus as well as increases the expression of a PI3K regulatory subunit in a number of hypothalamic nuclei including the arcuate and the VMH (90).
Concluding Remarks
In the hypothalamus, oestrogen controls energy homeostasis, which includes metabolic processes, fat deposition and feeding behaviour, by interacting with specific neurones in the hypothalamus. The dominant paradigm is that oestrogen regulates these neurones through classical oestrogen signalling - i.e., regulation of gene transcription. Based on findings from transgenic knockout mice, ERα appears necessary for the anorectic effects of oestrogen (19). ERα is localised in many of the relevant hypothalamic nuclei, although there is little to no expression found in the PVN of both rats and mice (20–22). A few authors have suggested that the PVN is the primary site for oestrogen’s actions on energy homoeostasis particularly feeding behaviour (111). However, since the PVN does not express significant levels of ERα, these two hypotheses are incongruent and need further examination. Therefore, the hypothesis that other receptors are involved in the control of energy homeostasis by oestrogen should be investigated.
A candidate for another oestrogenic mechanism recently identified is the control of neuronal excitability through a membrane GPCR receptor (12,32). The Gq-mER affects not only neuronal activity but alters metabolic processes such as body weight gain. The molecular characterisation of this Gq-mER (cloning, localisation and regulation) is crucial for fully understanding the role of the rapid, membrane-mediated effects of oestrogen on energy homeostasis. STX, the selective agonist for the Gq-mER, is an excellent tool for the elucidation of the role of the Gq-mER in energy homeostasis and gene regulation by MISS (64). Experiments are underway to determine if the Gq-mER, utilising STX to target the receptor, actually contributes to the regulation of energy homeostasis in αERKO mice. Furthermore, oestrogen activation of rapid signalling pathways via the classical ER needs to be thoroughly investigated. A recent advance in ERα transgenic technology is the development of a mouse strain (NERKI, nonclassical ER knock-in) that express a selective mutation in the DNA binding domain of ERα eliminating the capacity to activate gene transcription via oestrogen response elements but retaining the ability to activate multiple signalling pathways via ERα (112). These two developments are excellent tools to investigate the involvement of non-classical oestrogen signalling in the control of energy homeostasis.
In summary, oestrogen has multiple neuronal and transcriptional targets in the hypothalamus to control numerous hypothalamic functions including energy homeostasis but also reproduction, sexual behaviour, body temperature and stress responses. The control of energy homeostasis through all these receptor-mediated mechanisms must be integrated with the control of all these hypothalamic functions as well as the regulation of extrahypothalamic nuclei (NTS) and peripheral hormonal signals (gut peptides) that impact feeding behaviour and adiposity. Oestrogen can indirectly control the processes of hypothalamic control of energy homeostasis by altering gene expression of signalling pathways important for other hormonal signals including PI3K, PLC, PKC and calmodulin (64,24). Oestrogen can directly activate and/or inhibit relevant neuronal cell types either by altering neuronal excitability or altering gene transcription through MISS mechanisms. Oestrogen has at least two receptor types with multiple membrane and nuclear signalling pathways that are activated during the immediate and long-term effects on energy homeostasis. Furthermore, oestrogen can also alter the synaptic morphology of and pre-synaptic inputs into important hypothalamic neurones. Ultimately, it is the interactions of all the receptor-mediated processes in the diverse, pertinent hypothalamic nuclei and neuronal cell types that will determine the effects of oestrogen on feeding behaviour, fat metabolism and energy homeostasis.
Table 1.
List of anorectic and orexigenic peptides and the effects of oestrogen
| Nucleus | Peptide | Effect on energy homeostasis | Regulation by Oestrogen |
|---|---|---|---|
| Arcuate Nucleus | POMC/α-MSH | − | + |
| CART | − | ND | |
| NPY | + | − | |
| AgRP | + | − | |
| Ventromedial Hypothalamus | BDNF | − | NC |
| NPY | + | − | |
| Dorsomedial hypothalamus | NPY | + | − |
| CART | − | ND | |
| Lateral Hypothalamus | MCH | + | − |
| Orexin | + | ? | |
| CART | − | ND | |
| Paraventricular Nucleus | CRF | − | +/− |
| TRH | − | ND | |
| Urocortin | − | + | |
| NPY | − | − | |
| CART | − | ND | |
| BDNF | − | ND |
(+) denotes a positive effect or regulation.
(−) denotes negative effect or regulation. NC means no regulation. ND means not determined.
(?) denotes mixed results.
Acknowledgments
T. A. Roepke was supported by PHS training grants 5T32 DA07262 and NIH NRSA 1F32 DK079508. The author would like to thank Drs. Martin J. Kelly and Oline K. Ronnekleiv for their support, guidance and review of this manuscript.
References
- 1.Milewicz A, Bidzinska B, Mikulski E, Demissie M, Tworowska U. Influence of obesity and menopausal status on serum leptin, cholecystokinin, galanin and neuropeptide Y levels. Gynecol Endocrinol. 2000;14:196–203. doi: 10.3109/09513590009167682. [DOI] [PubMed] [Google Scholar]
- 2.Geary N. Estradiol, CCK and satiation. Peptides. 2001;22:1251–1263. doi: 10.1016/s0196-9781(01)00449-1. [DOI] [PubMed] [Google Scholar]
- 3.Poehlman ET. Menopause, energy expenditure, and body composition. Acta Obstet Gynecol Scand. 2002;81:603–611. doi: 10.1034/j.1600-0412.2002.810705.x. [DOI] [PubMed] [Google Scholar]
- 4.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Phil Trans R Soc B. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 6.Augoulea A, Mastorakos G, Lambrinoudaki I, Christodoulakos G, Creatsas G. Role of postmenopausal hormone replacement therapy on body fat gain and leptin levels. Gynecol Endocrinol. 2005;20:227–235. doi: 10.1080/09513590400027372. [DOI] [PubMed] [Google Scholar]
- 7.Jasienska G, Ziomkiewicz A, Gorkiewicz M, Pajak A. Body mass, depressive symptoms and menopausal status: an examination of the “jolly fat” hypothesis. Women’s Health Issues. 2005;15:145–151. doi: 10.1016/j.whi.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Ahdieh HB, Wade GN. Effects of hysterectomy on sexual receptivity, food intake, running wheel activity, and hypothalamic estrogen and progestin receptors in rats. J Comp Physiol Psychol. 1982;96:886–892. [PubMed] [Google Scholar]
- 9.Colvin GB, Sawyer CH. Induction of running activity by intracerebral implants of estrogen in ovariectomized rats. Neuroendocrinology. 1969;4:309–320. doi: 10.1159/000121762. [DOI] [PubMed] [Google Scholar]
- 10.Shimomura Y, Shimizu H, Takahashi M, Sato N, Uehara Y, Fukatsu A, Negishi M, Kobayashi I, Kobayashi S. The significance of decreased ambulatory activity during the generation by long-term observation of obesity in ovariectomized rats. Physiol Behav. 1990;47:155–159. doi: 10.1016/0031-9384(90)90055-9. [DOI] [PubMed] [Google Scholar]
- 11.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 12.Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy S, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- 14.Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Exp Biol Med. 2007;228:1175–1180. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- 15.Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Parker GC, McKee ME, Bishop C, Coscina DV. Whole-body metabolism varies across the estrous cycle in Sprague-Dawley rats. Physiol Behav. 2001;74:399–403. doi: 10.1016/s0031-9384(01)00599-6. [DOI] [PubMed] [Google Scholar]
- 17.Varma M, Chai J-K, Meguid MM, Laviano A, Gleason JR, Yang Z-J, Blaha V. Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats. Physiol Behav. 1999;68:99–107. doi: 10.1016/s0031-9384(99)00152-3. [DOI] [PubMed] [Google Scholar]
- 18.Jones MEE, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao SG, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 20.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptorα and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 22.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor α and β in the mouse central nervous system in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 23.Warembourg M, Leroy D. Comparative distribution of estrogen receptor alpha and beta immunoreactivities in the forebrain and the midbrain of the female guinea pig. Brain Res. 2004;1002:55–66. doi: 10.1016/j.brainres.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Roepke TA, Malyala A, Bosch MA, Kelly MJ, Ronnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- 25.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: Different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 26.Cowley MA. Hypothalamic melanocortin neurons integrate signals of energy state. Eur J Pharmacol. 2003;480:3–11. doi: 10.1016/j.ejphar.2003.08.087. [DOI] [PubMed] [Google Scholar]
- 27.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 28.Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity - a review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- 30.Bonavera JJ, Dube MG, Kalra PS, Kalra SP. Anorectic effects of estrogen may be mediated by decreased neuropeptide-Y release in the hypothalamic paraventricular nucleus. Endocrinology. 1994;134:2367–2370. doi: 10.1210/endo.134.6.8194462. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu H, Ohtani K, Kato Y, Tanaka Y, Mori M. Estrogen increases hypothalamic neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat. Neurosci Lett. 1996;204:81–84. doi: 10.1016/0304-3940(96)12322-3. [DOI] [PubMed] [Google Scholar]
- 32.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinology. 2007;19:426–431. doi: 10.1111/j.1365-2826.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 34.Nahon J-L. The melanocortins and melanin-concentrating hormone in the central regulation of feeding behavior and energy homeostasis. C R Biologies. 2006;329:623–638. doi: 10.1016/j.crvi.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Horvath TL. Synaptic plasticity in energy balance regulation. Obesity. 2006;14 (Suppl):229S–233S. doi: 10.1038/oby.2006.314. [DOI] [PubMed] [Google Scholar]
- 36.Mystkowski P, Seeley RJ, Hahn TM, Baskin DG, Havel PJ, Matsumoto AM, Wilkinson CW, Peacock-Kinzig K, Blake KA, Schwartz MW. Hypothalamic melanin-concentrating hormone and estrogen-induced weight loss. J Neurosci. 2000;20:8637–8642. doi: 10.1523/JNEUROSCI.20-22-08637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakurai T. Roles of orexins and orexin receptors in central regulation of feeding behavior and energy homeostasis. CNS Neurol Disord Drug Targets. 2006;5:313–325. doi: 10.2174/187152706777452218. [DOI] [PubMed] [Google Scholar]
- 38.Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- 39.Bi S. Role of dorsomedial hypothalamic neuropeptide Y in energy homeostasis. Peptides. 2007;28:352–356. doi: 10.1016/j.peptides.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 40.Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB, Elmquist JK. Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol. 2001;432:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- 41.DiMicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Intergr Comp Physiol. 2006;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- 42.Mastorakos G, Zapanti E. The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: the role of corticoptropin-releasing hormone. Nutr Neurosci. 2004;7:271–280. doi: 10.1080/10284150400020516. [DOI] [PubMed] [Google Scholar]
- 43.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. In: Kalsbeek A, Fliers E, Hofman MA, Swaab DF, Van Someren EJW, Buijs RM, editors. Hypothalamic integration of energy metabolism, Proceedings of the 24th International Summer School of Brain Research. Amsterdam, The Netherlands: Elsevier; 2006. pp. 209–234. [Google Scholar]
- 44.Bao A-M, Hestiantoro A, Van Someren EJW, Swaab DF, Zhou J-N. Colocalization of corticotropin-releasing hormone and oestrogen receptor-α in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128:1301–1313. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J-Q, Su B, Cai W-Q. Immunolocatization of estrogen receptor beta in the hypothalamic paraventricular nucleus of female mice during pregnancy, lactation and postnatal development. Brain Res. 2003;997:89–96. doi: 10.1016/j.brainres.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 46.Sar M, Sahu A, Crowley WR, Kalra SP. Localization of neuropeptide-Y immunoreactivity in estradiol-concentrating cells in the hypothalamus. Endocrinology. 1990;127:2752–2756. doi: 10.1210/endo-127-6-2752. [DOI] [PubMed] [Google Scholar]
- 47.Skinner DC, Herbison AE. Effects of photoperiod on estrogen receptor, tyrosine hydroxylase, neuropeptide Y and β-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology. 1997;138:2585–2595. doi: 10.1210/endo.138.6.5208. [DOI] [PubMed] [Google Scholar]
- 48.Titolo D, Cal F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER)α to ERβ in clonal hypothalamic neurons. Mol Endocrinol. 2006;20:2080–2092. doi: 10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- 49.Muschamp JW, Dominguez JM, Sato SM, Shen R-Y, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muschamp JW, Hull EM. Melanin concentrating hormone and estrogen receptor-α are co-exstensive but not co-expressed in cells of male rat hypothalamus. Neurosci Lett. 2007;427:123–126. doi: 10.1016/j.neulet.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki S, Handa RJ. Estrogen receptor-β but not estrogen receptor-α, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- 52.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naaz A, Zakroczymski M, Heine PA, Taylor J, Saunders PT, Lubahn DB, Cooke PS. Effect of Ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERα): a potential role for estrogen receptor beta (ERβ) Horm Metab Res. 2002;34:758–763. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- 54.Vidal O, Lindberg M, Savendahl L, Lubahn DB, Ritzen EM, Gustafsson JA, Ohlsson C. Disproportional body growth in female estrogen receptor-alpha-inactivated mice. Biochem Biophys Res Comm. 1999;265:569–571. doi: 10.1006/bbrc.1999.1711. [DOI] [PubMed] [Google Scholar]
- 55.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 56.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–1617. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor β is involved in the anorectic action of estrogen. Int J Obesity. 2002;26:1103–1109. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- 58.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broad KD, Kendrick KM, Sirinathsinghji DJ, Keverne EB. Changes in pro-opiomelanocortin and pre-proenkephalin mRNA levels in the ovine brain during pregnancy, parturition and lactation and in response to oestrogen and progesterone. J Neuroendocrinology. 1993;5:711–719. doi: 10.1111/j.1365-2826.1993.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 60.Thornton JE, Loose MD, Kelly MJ, Rønnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- 61.Priest CA, Roberts JL. Estrogen and tamoxifen differentially regulate beta-endorphin and cFos expression and neuronal colocalization in the arcuate nucleus of the rat. Neuroendocrinology. 2000;72:293–305. doi: 10.1159/000054598. [DOI] [PubMed] [Google Scholar]
- 62.Taylor JA, Goubillon M-L, Broad KD, Robinson JE. Steroid control of gonadotropin-releasing hormone secretion: associated changes in pro-opiomelanocortin and preproenkephalin messenger RNA expression in the ovine hypothalamus. Biol Repro. 2007;76:524–531. doi: 10.1095/biolreprod.106.055533. [DOI] [PubMed] [Google Scholar]
- 63.Treiser SL, Wardlaw SL. Estradiol regulation of proopiomelanocortin gene expression and peptide content in the hypothalamus. Neuroendocrinology. 1992;55:167–173. doi: 10.1159/000126111. [DOI] [PubMed] [Google Scholar]
- 64.Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008 doi: 10.1210/en.2008-0769. online: http://endo.endojournals.org/cgi/rapidpdf/en.2008-0769v1. [DOI] [PMC free article] [PubMed]
- 65.Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obesity. 2001;25:1680–1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- 66.Crowley WR, Tessel RE, O’Donohue TL, Adler BA, Kalra SP. Effects of ovarian hormones on the concentrations of immunoreactive neuropeptide Y in discrete brain regions of the female rat: correlation with serum luteinizing hormone (LH) and median eminence LH-releasing hormone. Endocrinology. 1985;117:1151–1155. doi: 10.1210/endo-117-3-1151. [DOI] [PubMed] [Google Scholar]
- 67.Bauer-Dantoin AC, Urban JH, Levine JE. Neuropeptide Y gene expression in the arcuate nucleus is increased during preovulatory luteinizing hormone surges. Endocrinology. 1992;131:2953–2958. doi: 10.1210/endo.131.6.1446633. [DOI] [PubMed] [Google Scholar]
- 68.Sahu A, Crowley WR, Kalra SP. Evidence that hypothalamic neuropeptide Y gene expression increases before the onset of the preovulatory LH surge. J Neuroendocrinol. 1995;7:291–296. doi: 10.1111/j.1365-2826.1995.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 69.Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav Brain Res. 2008;191:173–177. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray JF, Baker BI, Levy A, Wilson CA. The influence of gonadal steroids on pre-pro melanin-concentrating hormone mRNA in female rats. J Neuroendocrinol. 2000;12:53–59. doi: 10.1046/j.1365-2826.2000.00425.x. [DOI] [PubMed] [Google Scholar]
- 71.Morton GJ, Mystkowski P, Matsumoto AM, Schwartz MW. Increased hypothalamic melanin concentrating hormone gene expression during energy restriction involves a melanocortin-independent, estrogen-sensitive mechanism. Peptides. 2004;25:667–674. doi: 10.1016/j.peptides.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Russell SH, Small CJ, Kennedy AR, Stanley SA, Seth A, Murphy KG, Taheri S, Ghatei MA, Bloom SR. Orexin A interactions in the hypothalamo-pituitary gonadal axis. Endocrinology. 2001;142:5294–5302. doi: 10.1210/endo.142.12.8558. [DOI] [PubMed] [Google Scholar]
- 73.Roy BN, Reid RL, Van Vugt DA. The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopression messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology. 1999;140:2191–2198. doi: 10.1210/endo.140.5.6684. [DOI] [PubMed] [Google Scholar]
- 74.Bethea CL, Centeno ML. Ovarian steroid treatment decreases corticotropin-releasing hormone (CRH) mRNA and protein in the hypothalamic paraventricular nucleus of ovariectomized monkeys. Neuropsychopharmacology. 2007;207:546–556. doi: 10.1038/sj.npp.1301442. [DOI] [PubMed] [Google Scholar]
- 75.Pan W, Kastin AJ. Urocortin and the brain. Prog Neurobiol. 2007;84:148. doi: 10.1016/j.pneurobio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haeger P, Andrés ME, Forray MI, Daza C, Araneda S, Gysling K. Estrogen receptors α and β differentially regulate the transcriptional activity of the urocortin gene. J Neurosci. 2006;26:4908–4916. doi: 10.1523/JNEUROSCI.0476-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beatty WW, O’Briant DA, Vilberg TR. Effects of ovariectomy and estradiol injections on food intake and body weight in rats with ventromedial hypothalamic lesions. Pharmacol Biochem Behav. 1975;3:539–544. doi: 10.1016/0091-3057(75)90169-0. [DOI] [PubMed] [Google Scholar]
- 78.Nance DM, Gorski RA. Similar effects of estrogen and lateral hypothalamic lesions on feeding behavior of female rats. Brain Res Bull. 1978;3:549–553. doi: 10.1016/0361-9230(78)90085-0. [DOI] [PubMed] [Google Scholar]
- 79.Butera PC, Beikirch RJ. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- 80.Butera PC, Willard DM, Raymond SA. Effects of PVN lesions on the responsiveness of female rats to estradiol. Brain Res. 1992;576:304–310. doi: 10.1016/0006-8993(92)90694-5. [DOI] [PubMed] [Google Scholar]
- 81.Dagnault A, Richard D. Lesions of hypothalamic paraventricular nuclei do not prevent the effect of estradiol on energy and fat balance. Am J Physiol Endocrinol Metab. 1994;267:E32–E38. doi: 10.1152/ajpendo.1994.267.1.E32. [DOI] [PubMed] [Google Scholar]
- 82.Hammes SR, Levin ER. Extra-nuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 83.Björnström L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 84.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 86.Ronnekleiv OK, Malyala A, Kelly MJ. Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med. 2007;25:165–176. doi: 10.1055/s-2007-973429. [DOI] [PubMed] [Google Scholar]
- 87.Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 88.Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induces rapid translocation of estrogen receptor β, but not estrogen receptor α, to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malyala A, Kelly MJ, Rønnekleiv OK. Estrogen modulation of hypothalamic neurons: Activation of multiple signaling pathways and gene expression changes. Steroids. 2005;70:397–406. doi: 10.1016/j.steroids.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 90.Malyala A, Zhang C, Bryant D, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- 91.Vasudevan N, Kow LM, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–396. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 92.Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. Serotonin 5HT2c receptor signaling in hypothalamic POMC neurons: role in energy homeostasis in females. Mol Pharmacol. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- 93.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao X-B, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature Med. 2006;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 94.Schiöth HB. G protein-coupled receptors in regulation of body weight. CNS Neurol Disord Drug Targets. 2006;5:241–248. doi: 10.2174/187152706777452263. [DOI] [PubMed] [Google Scholar]
- 95.Kelly MJ, Qiu J, Wagner EJ, Rønnekleiv OK. Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS) J Steroid Biochem Mol Biol. 2003;83:187–193. doi: 10.1016/s0960-0760(02)00249-2. [DOI] [PubMed] [Google Scholar]
- 96.Kow LM, Devidze N, Pataky S, Shibuya I, Pfaff DW. Acute estradiol application increases inward and decreases outward whole-cell currents of neurons in rat hypothalamic ventromedial nucleus. Brain Res. 2006;1116:1–11. doi: 10.1016/j.brainres.2006.07.104. [DOI] [PubMed] [Google Scholar]
- 97.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 98.Chambers JB, Schurdak JD, Benoit SC, Clegg DJ. Behavioral and metabolic phenotyping of GPR30 and neuronal estrogen receptor-α knockout mice. Appetite. 2007;49:272–341. [Google Scholar]
- 99.Tobias SC, Qiu J, Kelly MJ, Scanlan TS. Synthesis and biological evaluation of SERMs with potent nongenomic estrogenic activity. ChemMedChem. 2006;1:565–571. doi: 10.1002/cmdc.200500098. [DOI] [PubMed] [Google Scholar]
- 100.Polidori C, Geary N. Estradiol treatment fails to affect the feeding responses to melanocortin-3/4 receptor agonism or antagonism in ovariectomized rats. Peptides. 2002;23:1697–1700. doi: 10.1016/s0196-9781(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 101.Calizo LH, Flanagan-Cato LM. Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20:1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 103.Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25:77–107. doi: 10.1016/j.yfrne.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 104.Lebrun B, Bariohay B, Moyse E, Jean A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: a mini review. Auton Neurosci. 2006:126–127. doi: 10.1016/j.autneu.2006.02.027. 30–38. [DOI] [PubMed] [Google Scholar]
- 105.Wang CF, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1003–R1012. doi: 10.1152/ajpregu.00011.2007. [DOI] [PubMed] [Google Scholar]
- 106.Wang CF, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1037–R1045. doi: 10.1152/ajpregu.00125.2007. [DOI] [PubMed] [Google Scholar]
- 107.Blurton-Jones M, Kuan PN, Tuszynski MH. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J Comp Neurol. 2004;468:347–360. doi: 10.1002/cne.10989. [DOI] [PubMed] [Google Scholar]
- 108.Liu Y, Fowler CD, Young LJ, Yan Q, Insel TR, Wang Z. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J Comp Neurol. 2001;433:499–514. doi: 10.1002/cne.1156. [DOI] [PubMed] [Google Scholar]
- 109.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nicholson JR, Peter J-C, Lecourt A-C, Barde Y-A, Hofbauer KG. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol. 2007;19:974–992. doi: 10.1111/j.1365-2826.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 111.Geary N. The estrogenic inhibition of eating. In: Stricker EM, Woods SC, editors. Handbook of Behavioral Neurobiology. New York, NY: Kluwer Academic/Plenum; 2007. pp. 307–345. [Google Scholar]
- 112.O’Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]