Abstract
During the screening of a Mycobacterium tuberculosis lambda gt-11 gene library with monoclonal antibodies, we detected a recombinant clone, lambda PH7311, which contained a mycobacterial DNA insert that hybridized specifically with DNA of M. tuberculosis complex strains. Part of this insert was sequenced and used for the development of an M. tuberculosis complex-specific polymerase chain reaction (PCR). Only strains belonging to species of the M. tuberculosis complex group contained an amplifiable fragment of 158 base pairs (bp). This fragment was absent in all strains tested belonging to 15 other mycobacterial species. After amplification by PCR and dot blot hybridization with a digoxigenin-labeled oligonucleotide, the limit of detection of purified genomic M. tuberculosis DNA amounted to a quantity corresponding to 20 bacterial cells. By this technique about 10(3) M. tuberculosis bacteria were detectable in sputum. Using PCR, we were also able to detect M. tuberculosis cells in clinical material such as pleural fluid, bronchial washings, and biopsies, and these results were comparable with those obtained by classical bacterial culture. Of 34 M. tuberculosis strains, 5 did not carry the amplifiable 158-bp fragment, which occurs usually as a single copy in the chromosome. Evidence is presented that the 158-bp fragment is located near a repeated sequence in the chromosome. We presume that strains which did not carry the 158-bp fragment have lost a chromosomal segment by a genetic rearrangement induced by the repetitive DNA element.
Full text
PDF



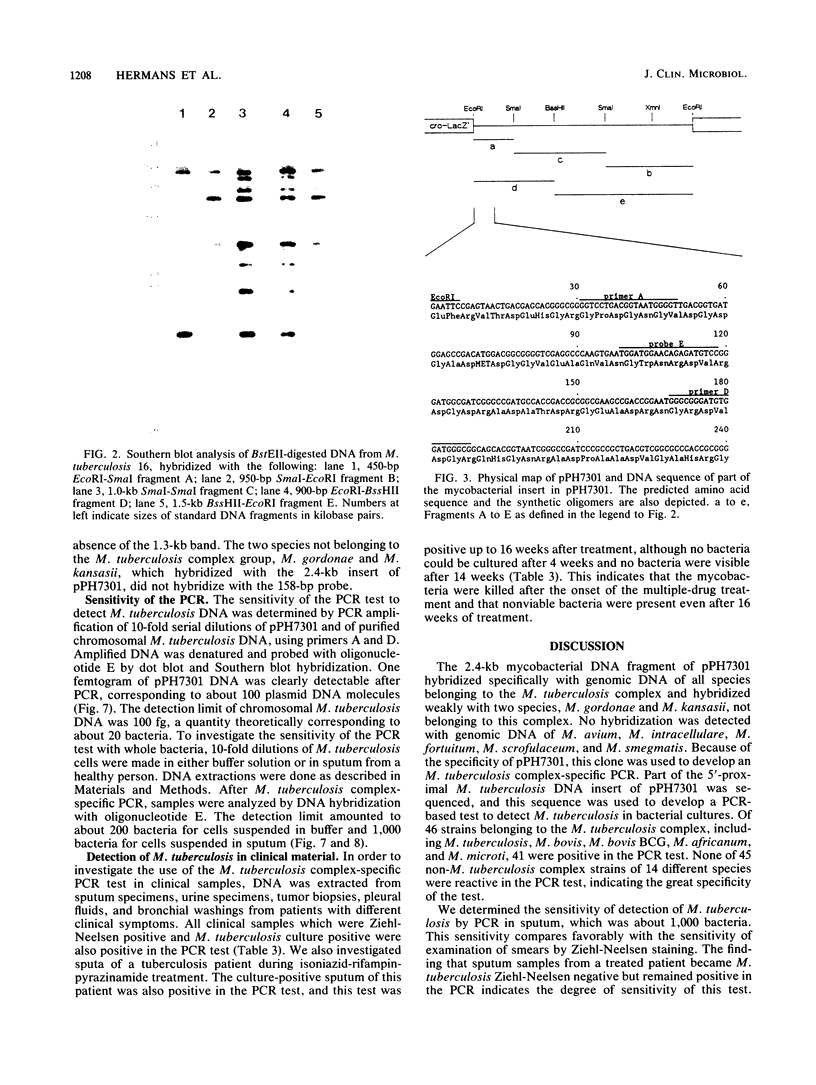


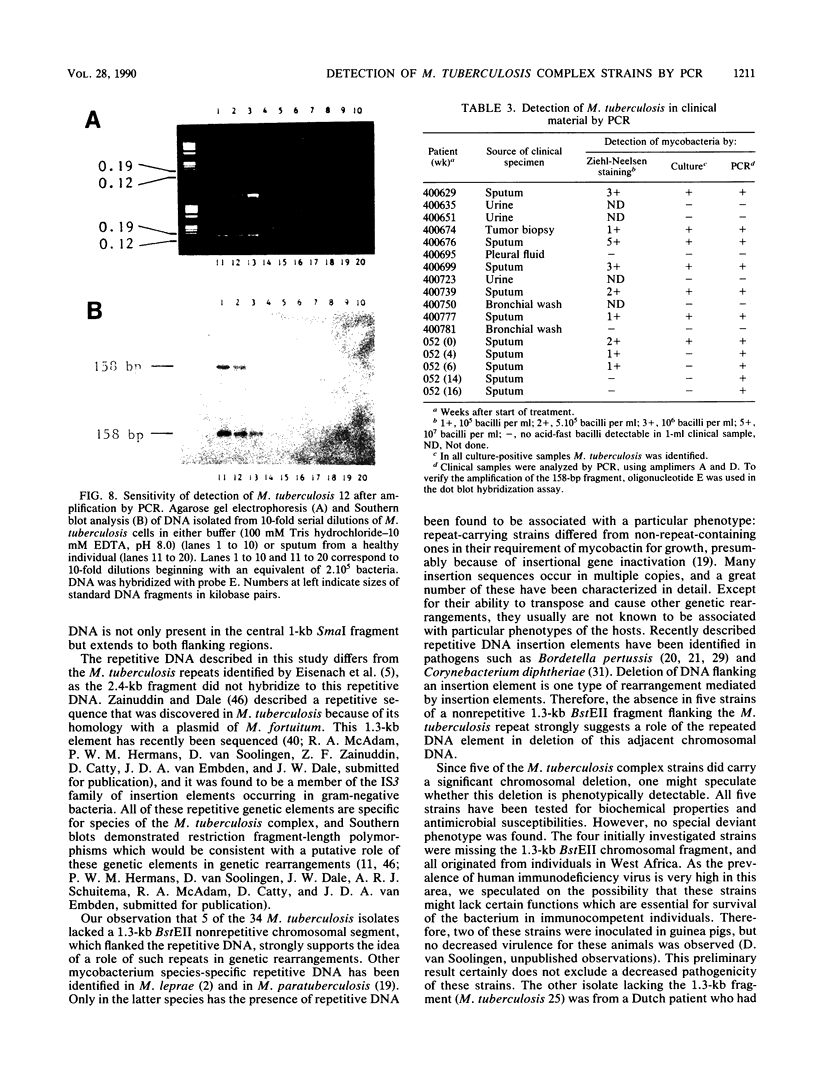


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brisson-Noël A., Gicquel B., Lecossier D., Lévy-Frébault V., Nassif X., Hance A. J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989 Nov 4;2(8671):1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Docherty M. A. A species-specific repetitive sequence in Mycobacterium leprae DNA. J Infect Dis. 1989 Jan;159(1):7–15. doi: 10.1093/infdis/159.1.7. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Debanne S. M. The serodiagnosis of tuberculosis and other mycobacterial diseases by enzyme-linked immunosorbent assay. Am Rev Respir Dis. 1987 May;135(5):1137–1151. doi: 10.1164/arrd.1987.135.5.1137. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Hindler J. A., Berlin O. G., Bruckner D. A. Rapid identification of Mycobacterium avium complex in culture using DNA probes. J Clin Microbiol. 1987 Aug;25(8):1442–1445. doi: 10.1128/jcm.25.8.1442-1445.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach K. D., Crawford J. T., Bates J. H. Repetitive DNA sequences as probes for Mycobacterium tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2240–2245. doi: 10.1128/jcm.26.11.2240-2245.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada I. C., Lamb F. I., Colston M. J., Cox R. A. Partial nucleotide sequence of 16S ribosomal RNA isolated from armadillo-grown Mycobacterium leprae. J Gen Microbiol. 1988 Jun;134(6):1449–1453. doi: 10.1099/00221287-134-6-1449. [DOI] [PubMed] [Google Scholar]
- Gonzalez R., Hanna B. A. Evaluation of Gen-Probe DNA hybridization systems for the identification of Mycobacterium tuberculosis and Mycobacterium avium-intracellulare. Diagn Microbiol Infect Dis. 1987 Oct;8(2):69–77. doi: 10.1016/0732-8893(87)90152-0. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Grandchamp B., Lévy-Frébault V., Lecossier D., Rauzier J., Bocart D., Gicquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989 Jul;3(7):843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Hart C., Schochetman G., Spira T., Lifson A., Moore J., Galphin J., Sninsky J., Ou C. Y. Direct detection of HIV RNA expression in seropositive subjects. Lancet. 1988 Sep 10;2(8611):596–599. doi: 10.1016/s0140-6736(88)90639-3. [DOI] [PubMed] [Google Scholar]
- Hartskeerl R. A., de Wit M. Y., Klatser P. R. Polymerase chain reaction for the detection of Mycobacterium leprae. J Gen Microbiol. 1989 Sep;135(9):2357–2364. doi: 10.1099/00221287-135-9-2357. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F., Barnes W. M., Clement J. M., Hofnung M. A novel intercistronic regulatory element of prokaryotic operons. Nature. 1982 Aug 19;298(5876):760–762. doi: 10.1038/298760a0. [DOI] [PubMed] [Google Scholar]
- Hoffner S. E. Improved detection of Mycobacterium avium complex with the Bactec radiometric system. Diagn Microbiol Infect Dis. 1988 May;10(1):1–6. doi: 10.1016/0732-8893(88)90121-6. [DOI] [PubMed] [Google Scholar]
- Kadival G. V., Mazarelo T. B., Chaparas S. D. Sensitivity and specificity of enzyme-linked immunosorbent assay in the detection of antigen in tuberculous meningitis cerebrospinal fluids. J Clin Microbiol. 1986 May;23(5):901–904. doi: 10.1128/jcm.23.5.901-904.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn T. E., Edwards F. F. Rapid identification using a specific DNA probe of Mycobacterium avium complex from patients with acquired immunodeficiency syndrome. J Clin Microbiol. 1987 Aug;25(8):1551–1552. doi: 10.1128/jcm.25.8.1551-1552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krambovitis E., McIllmurray M. B., Lock P. E., Hendrickse W., Holzel H. Rapid diagnosis of tuberculous meningitis by latex particle agglutination. Lancet. 1984 Dec 1;2(8414):1229–1231. doi: 10.1016/s0140-6736(84)92792-2. [DOI] [PubMed] [Google Scholar]
- Laure F., Courgnaud V., Rouzioux C., Blanche S., Veber F., Burgard M., Jacomet C., Griscelli C., Brechot C. Detection of HIV1 DNA in infants and children by means of the polymerase chain reaction. Lancet. 1988 Sep 3;2(8610):538–541. doi: 10.1016/s0140-6736(88)92659-1. [DOI] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Thompson J., Chiodini R., Hermon-Taylor J. The use of DNA probes identifying restriction-fragment-length polymorphisms to examine the Mycobacterium avium complex. Mol Microbiol. 1987 Nov;1(3):283–291. doi: 10.1111/j.1365-2958.1987.tb01934.x. [DOI] [PubMed] [Google Scholar]
- McLafferty M. A., Harcus D. R., Hewlett E. L. Nucleotide sequence and characterization of a repetitive DNA element from the genome of Bordetella pertussis with characteristics of an insertion sequence. J Gen Microbiol. 1988 Aug;134(8):2297–2306. doi: 10.1099/00221287-134-8-2297. [DOI] [PubMed] [Google Scholar]
- McPheat W. L., Hanson J. H., Livey I., Robertson J. S. Analysis of separate isolates of Bordetella pertussis repeated DNA sequences. J Gen Microbiol. 1989 Jun;135(6):1515–1520. doi: 10.1099/00221287-135-6-1515. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. A., Horstmeier C. D., DeYoung D. R., Roberts G. D. Comparison of a radiometric method (BACTEC) and conventional culture media for recovery of mycobacteria from smear-negative specimens. J Clin Microbiol. 1983 Aug;18(2):384–388. doi: 10.1128/jcm.18.2.384-388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassau E., Parsons E. R., Johnson G. D. The detection of antibodies to Mycobacterium tuberculosis by microplate enzyme-linked immunosorbent assay (ELISA). Tubercle. 1976 Mar;57(1):67–70. doi: 10.1016/0041-3879(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Noordhoek G. T., Hermans P. W., Paul A. N., Schouls L. M., van der Sluis J. J., van Embden J. D. Treponema pallidum subspecies pallidum (Nichols) and Treponema pallidum subspecies pertenue (CDC 2575) differ in at least one nucleotide: comparison of two homologous antigens. Microb Pathog. 1989 Jan;6(1):29–42. doi: 10.1016/0882-4010(89)90005-3. [DOI] [PubMed] [Google Scholar]
- Olszewska E., Jones K. Vacuum blotting enhances nucleic acid transfer. Trends Genet. 1988 Apr;4(4):92–94. doi: 10.1016/0168-9525(88)90095-9. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Lin S. S., Wu S. Y., Juang W. M., Chang C. H., Lin J. Y. The detection of mycobacterial DNA sequences in uncultured clinical specimens with cloned Mycobacterium tuberculosis DNA as probes. Tubercle. 1988 Mar;69(1):27–36. doi: 10.1016/0041-3879(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Lu R., Floyd C., Nakasone A., Friedly G., de la Maza L. M. Direct identification of Mycobacterium tuberculosis, Mycobacterium avium, and Mycobacterium intracellulare from amplified primary cultures in BACTEC media using DNA probes. J Clin Microbiol. 1989 Jul;27(7):1543–1547. doi: 10.1128/jcm.27.7.1543-1547.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R., Perugini M., Ratti G. DNA element of Corynebacterium diphtheriae with properties of an insertion sequence and usefulness for epidemiological studies. J Bacteriol. 1987 Jan;169(1):308–312. doi: 10.1128/jb.169.1.308-312.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. D., Goodman N. L., Heifets L., Larsh H. W., Lindner T. H., McClatchy J. K., McGinnis M. R., Siddiqi S. H., Wright P. Evaluation of the BACTEC radiometric method for recovery of mycobacteria and drug susceptibility testing of Mycobacterium tuberculosis from acid-fast smear-positive specimens. J Clin Microbiol. 1983 Sep;18(3):689–696. doi: 10.1128/jcm.18.3.689-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., McMillan C., Coyle M. B. Whole chromosomal DNA probes for rapid identification of Mycobacterium tuberculosis and Mycobacterium avium complex. J Clin Microbiol. 1987 Jul;25(7):1239–1243. doi: 10.1128/jcm.25.7.1239-1243.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sherman I., Harrington N., Rothrock A., George H. Use of a cutoff range in identifying mycobacteria by the Gen-Probe Rapid Diagnostic System. J Clin Microbiol. 1989 Feb;27(2):241–244. doi: 10.1128/jcm.27.2.241-244.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Sweetser D., Thole J., van Embden J., Young R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987 Aug;55(8):1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C. Diagnostic deoxyribonucleic acid probes for infectious diseases. Clin Microbiol Rev. 1988 Jan;1(1):82–101. doi: 10.1128/cmr.1.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Cave M. D., Eisenach K. D., Crawford J. T., Bates J. H., Gicquel B., Guesdon J. L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990 Jan 11;18(1):188–188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Stabel L. F., Suykerbuyk M. E., De Wit M. Y., Klatser P. R., Kolk A. H., Hartskeerl R. A. A major immunogenic 36,000-molecular-weight antigen from Mycobacterium leprae contains an immunoreactive region of proline-rich repeats. Infect Immun. 1990 Jan;58(1):80–87. doi: 10.1128/iai.58.1.80-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáez M. A., Coppola M. P., Russo D. A., Delaha E., Chaparas S. D., Yeager H., Jr Determination of mycobacterial antigens in sputum by enzyme immunoassay. J Clin Microbiol. 1986 May;23(5):822–825. doi: 10.1128/jcm.23.5.822-825.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainuddin Z. F., Dale J. W. Polymorphic repetitive DNA sequences in Mycobacterium tuberculosis detected with a gene probe from a Mycobacterium fortuitum plasmid. J Gen Microbiol. 1989 Sep;135(9):2347–2355. doi: 10.1099/00221287-135-9-2347. [DOI] [PubMed] [Google Scholar]