Abstract
Compared to temperate and tropical relatives, some high-latitude marine species are large-bodied, a phenomenon known as polar gigantism. A leading hypothesis on the physiological basis of gigantism posits that, in polar water, high oxygen availability coupled to low metabolic rates relieves constraints on oxygen transport and allows the evolution of large body size. Here, we test the oxygen hypothesis using Antarctic pycnogonids, which have been evolving in very cold conditions (−1.8–0°C) for several million years and contain spectacular examples of gigantism. Pycnogonids from 12 species, spanning three orders of magnitude in body mass, were collected from McMurdo Sound, Antarctica. Individual sea spiders were forced into activity and their performance was measured at different experimental levels of dissolved oxygen (DO). The oxygen hypothesis predicts that, all else being equal, large pycnogonids should perform disproportionately poorly in hypoxia, an outcome that would appear as a statistically significant interaction between body size and oxygen level. In fact, although we found large effects of DO on performance, and substantial interspecific variability in oxygen sensitivity, there was no evidence for size×DO interactions. These data do not support the oxygen hypothesis of Antarctic pycnogonid gigantism and suggest that explanations must be sought in other ecological or evolutionary processes.
Keywords: sea spider, oxygen, polar gigantism, temperature, Antarctica, symmorphosis
1. Introduction
Compared to temperate and tropical relatives, some high-latitude marine species are particularly large-bodied, a phenomenon known as polar gigantism (Chapelle & Peck 1999). Latitudinal clines in body size must be driven by one or more latitude-associated factors. Postulated factors include changes in food availability, competitive environments, and developmental and growth responses to temperature and oxygen availability (Atkinson 1994; Atkinson & Sibly 1997; Barnes & Arnold 2001; Collins et al. 2005; Linse et al. 2006). The last idea—that gigantism stems from high polar oxygen availability coupled to low metabolic rates—has received substantial attention (see McClain & Rex 2001; Chapelle & Peck 2004; Woods & Moran 2008) ever since Chapelle & Peck's (1999) paper on the body sizes of benthic amphipod crustaceans. Chapelle & Peck (1999) surveyed maximum body sizes in collections of amphipods from different latitudes, finding that the maximum 5 per cent of body sizes from each collection locality correlated strongly with potential oxygen availability determined from water temperature. They concluded that ‘maximum potential size is limited by oxygen availability’.
Here, we test the oxygen hypothesis of polar gigantism using Antarctic pycnogonids, which have been evolving in very cold conditions (−1.8–0°C) for several million years (Peck et al. 2006) and contain spectacular examples of gigantism (Arnaud 1974; Clarke & Johnston 2003). There are approximately 190 described species of Antarctic pycnogonids, and these make up approximately 15 per cent of known pycnogonid species worldwide (Gusso & Gravina 2001; Clarke & Johnston 2003; Munilla & Soler-Membrives 2008). As a group, pycnogonids are ancient (more than 425 Myr old; Siveter et al. 2004). Recent studies have examined the phylogenetic affinities among pycnogonids (Arango & Wheeler 2007; Nakamura et al. 2007), but their relationships with other arthropods are under debate (see Dunlop & Arango 2005; Maxmen et al. 2005; Jager et al. 2006) and their biology is poorly known (King 1973; Arnaud & Bamber 1988).
Pycnogonids appear to possess neither respiratory organs nor pigments (Redmond & Swanson 1968; Markl 1986). Instead, oxygen diffuses directly across the chitinous exoskeleton (Douglas et al. 1969), which may act as a significant barrier to oxygen uptake (Markl 1986). Such a respiratory scheme may be tenable only when surface-area-to-volume ratios are high, as in pycnogonids. Diffusive oxygen uptake has an additional implication: it may permit the evolution of large body size only under cold conditions. Cold simultaneously depresses metabolic demand for oxygen, while not interfering with—and possibly supplementing—oxygen supply (Woods 1999; Pörtner 2001, 2002; Peck et al. 2002; Woods & Moran 2008). In general, pycnogonids are thought to employ low energy-use strategies (Clarke & Johnston 2003), and the one study directly measuring oxygen consumption found low metabolic rates compared with crustaceans of similar size (Douglas et al. 1969).
The oxygen hypothesis of polar gigantism posits that large body size is an evolutionarily allowable outcome in cold, well-oxygenated polar waters arising from the release of constraints on oxygen delivery—i.e. cold-water species can evolve into the open ‘biophysical space’ available at the upper end of the body-size spectrum. All else being equal, therefore, experimental hypoxia should have disproportionately large effects on the performance of large-bodied animals (see Harrison & Lighton 1998). Alternatively, pycnogonids of different size may show symmorphosis (Weibel et al. 1991), in which respiratory cascades have been modified over evolutionary time to both provide adequate oxygen under most circumstances and remove excess capacity when it is not needed. Symmorphosis predicts that large and small pycnogonids should perform equally well (or poorly) in hypoxia.
We tested these predictions on a sample of pycnogonids collected from McMurdo Sound, Antarctica, that spanned three orders of magnitude in body mass. Individuals were forced into sustained activity and their performance was measured at different levels of dissolved oxygen (DO). Forced activity is a reasonable simulation of the respiratory demands experienced by pycnogonids during intense activity, e.g. during fights or precopulatory behaviour, which can last for minutes to weeks (Nakamura & Sekiguchi 1980). The specific predictions we tested were that large pycnogonids would (oxygen hypothesis) or would not (symmorphosis) perform disproportionately poorly in hypoxia. Thus, the key statistical test is whether there is a significant interaction between body size and oxygen level.
This functional approach to the oxygen hypothesis differs from most other studies of gigantism, which have focused on patterns of body size across spatial gradients in temperature or oxygen availability (Chapelle & Peck 1999, 2004; McClain & Rex 2001; Soetaert et al. 2002; Peck & Chapelle 2003; Collins et al. 2005). One exception is recent work of Peck et al. (2007), which examined how body size affects burying ability of the Antarctic clam Laternula elliptica. The largest clams in their sample were disproportionately unable to rebury themselves at higher temperatures (up to 6°C) and lower oxygen levels (down to 10.2% of air saturation). However, the effects of oxygen disappeared below 0°C. Their results provide some support for the idea that large-bodied clams are disproportionately limited by oxygen (but only at higher temperatures). Our results show, by contrast, that under environmentally realistic temperatures, large body size did not adversely affect performance under low-oxygen conditions among a sample of Antarctic pycnogonids. The oxygen hypothesis, thus, does not fully explain large body size in modern Antarctic pycnogonids.
2. Material and methods
Pycnogonids were collected by hand on SCUBA near McMurdo Station, Antarctica (77°51′ S, 166°40′ E), in October and November 2007. Individuals were returned in coolers filled with icy seawater (−1.9°C) to seawater tables (approx. −1°C) at McMurdo Station, where they were kept until used for experiments (usually within two weeks).
Experiments were carried out in a 1000 l insulated aquarium partially submerged in a seawater table, with additional cooling provided by a submersible ‘cold finger’ (model IC-6, Lauda; measured aquarium temperature −1.13±0.02°C). The aquarium was fitted with a lid so that O2 levels in the water could be easily controlled. During each run, aquarium O2 levels were monitored with an O2 electrode (YSI Model 58 m with 5700 series DO probe, with stirring). Normoxic conditions were obtained by bubbling room air into the aquarium and hypoxic conditions by bubbling N2. Hypoxia tended to be quite stable by virtue of the large aquarium volume. During individual trials, small bubble streams (air or N2) were sustained to help flush out the head space and stabilize oxygen levels.
The righting ability of each of 49 pycnogonids was tested at three oxygen levels (measured values of percentage of air saturation±s.e.m. were: low=17.6±0.4; medium=43.0±0.6; and high=92.0±0.7; n=49 for each) presented in a haphazard order. Prior to the trials, pycnogonids were given approximately 20 min to acclimatize to new surroundings and water conditions. At the beginning of each trial, the pycnogonid was grasped gently by one of its legs with a pair of forceps and turned onto its back. If it righted itself, it was turned over again. Trials lasted for 1 hour. The time to right and total number of rightings during the trial were noted; the primary statistic used was rightings per hour. No individual was tested at more than one oxygen level in a day. Righting trials involved a total of 147 pycnogonid hours of observations (49 pycnogonids×3 trials pycnogonid−1×1 hour trial−1).
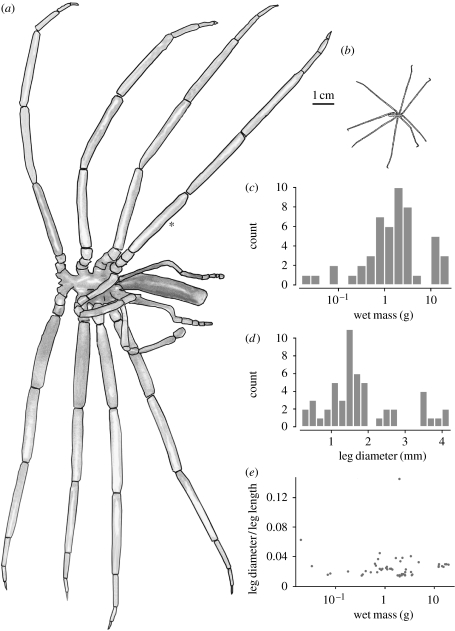
We assessed pycnogonid body size using both weight and linear dimensions. After each pycnogonid had completed trials at all three oxygen concentrations, it was blotted dry and weighed on a balance (Model AL54, Mettler Toledo). We then used calipers or rulers to measure five morphological variables (figure 1): dorsoventral and lateral diameters of the first leg; length of the first leg; proboscis length; and the body length excluding proboscis. Pycnogonids were subsequently frozen for shipment to the United States (University of Montana) and preserved in ethanol prior to shipment to the Queensland Museum (CPA) for identification.
Figure 1.
Morphological summary of the 49 pycnogonids used in the righting experiment. Line drawings from scans of (a) the largest individual (Colossendeidae: Colossendeis australis, 19.5 g) and (b) one of the smallest individuals in our sample (Endeidae: Endeis australis, 0.02 g). Histograms of (c) wet masses, (d) leg diameters and (e) leg aspect ratios (diameter/length) as a function of wet mass. Leg diameters were measured at the location indicated by the asterisk in (a).
The data were analysed using linear mixed-effects models (Pinheiro & Bates 2000) implemented in S-Plus v. 6.1 (Insightful Corporation Seattle, WA, USA), with individual pycnogonids incorporated as random effects. Body size was modelled as both body mass and leg diameter. A case can be made for both variables. Body mass provides a measure of the total mass of respiring tissues. However, because pycnogonids are almost entirely legs, leg diameter may be a better functional description of the distance traversed by oxygen from the environment to sites of consumption.
Some species in our analysis were represented by only one individual. These were excluded from the statistical analysis to avoid assigning both species and random effects to the same individual.
3. Results
The sample of 49 pycnogonids (table 1) was distributed across 12 species in five families, with the majority of specimens (36) identified to the genus Colossendeis (Colossendeidae). Body masses spanned three orders of magnitude (figure 1), from 0.02 to 19.5 g, and leg diameters varied from 0.2 to 4.2 mm.
Table 1.
Summary of sex and morphology of individuals used in the righting experiment. (Asterisk indicates sex undetermined for one or more individuals.)
| family | species | N (♂,♀) | mass range (g) | leg diameter range (mm) |
|---|---|---|---|---|
| Colossendeidae | Colossendeis australis | 4 (0, 2)* | 14.4–19.5 | 3.6–4.2 |
| C. robusta | 1 (1, 0) | 12.3 | 3.6 | |
| C. scotti | 3 (1, 1)* | 12.3–16.6 | 3.5–3.9 | |
| C. megalonyx | 17 (8, 6)* | 0.34–3.61 | 0.9–2.0 | |
| C. hoeki | 9 (4, 5) | 0.79–1.42 | 1.2–1.7 | |
| C. notialis-like | 2 (1, 0)* | 0.18–0.41 | 0.7–1.1 | |
| Pallenopsidae | Pallenopsis patagonica | 4 (1, 3) | 0.63–0.79 | 1.2–1.6 |
| Ammotheidae | Ammothea striata | 1 (1, 0) | 4.8 | 2.5 |
| A. sexarticulata | 1 (0, 1) | 3.4 | 2.7 | |
| A. carolinensis | 3 (1, 2) | 1.67–2.27 | 2.0–2.3 | |
| Endeidae | Endeis australis | 1 (1, 0) | 0.03 | 0.6 |
| Nymphonidae | Pentanymphon antarcticum | 3 (0, 1)* | 0.02–0.08 | 0.2–0.6 |
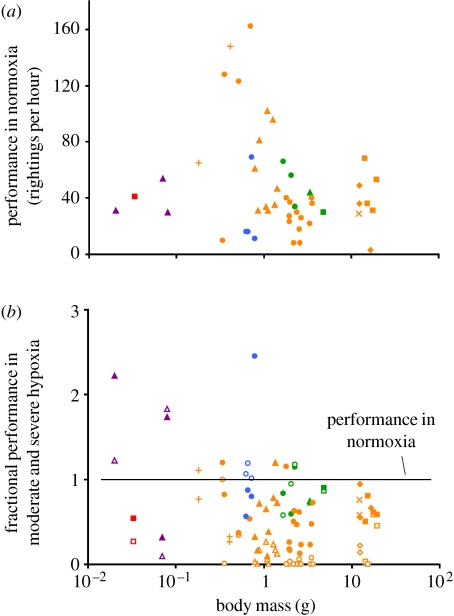
The data were analysed using linear mixed-effects models (Pinheiro & Bates 2000) with pycnogonid identity structured as a random effect. To meet assumptions of normality and give reasonable distributions of residuals, we used log-transformed body masses and square-root-transformed righting frequencies. The relevant statistical test of the oxygen-gigantism hypothesis is whether the sample shows a significant interaction between pycnogonid body size and response to hypoxia—support for the hypothesis would appear as large-bodied pycnogonids performing disproportionately poorly in hypoxia. This outcome was not apparent in plots of pycnogonid righting performance (figure 2). Righting frequency in normoxia (figure 2a) ranged from 3 to 162 per hour, and there was some indication that mid-sized pycnogonids (approx. 1 g) performed relatively better than small or large ones. Performance in moderate (43% of air saturation) and severe (17.6% of air saturation) hypoxia declined precipitously for all size classes (figure 2b); however, it appeared that large-bodied individuals performed only slightly worse in hypoxia than did small-bodied individuals.
Figure 2.
Righting performance of Antarctic pycnogonids in (a) normoxia and (b) two levels of hypoxia. Taxonomic groups are coded by colour (orange, Colossendeidae; blue, Pallenopsidae; red, Endeidae; green, Ammotheidae; purple, Nymphonidae). Species are coded within colours by symbols (orange squares, Colossendeis australis; orange diamonds, C. scotti; orange crosses, C. robusta; orange circles, C. megalonyx; orange triangles, C. hoeki; orange pluses, C. notialis-like; blue circles, Pallenopsis patagonica; green squares, Ammothea striata; green circles, A. carolinensis; purple triangles, Pentanymphon antarcticum; red squares, Endeis australis). Performance in (a) normoxia is presented as the number of rightings in 1 hour. Performance in (b) hypoxia is presented as fractional performance (compared with performance in normoxia) in either moderate hypoxia (filled symbols) or severe hypoxia (open symbols).
Linear mixed-effects modelling confirmed these patterns. Regardless of whether body size was estimated as log body mass or leg diameter, there were significant effects of all three primary predictors (species, body size and DO) on righting frequencies (table 2). In addition, some species performed relatively better in hypoxia than others; this was apparent as a significant interaction between species identity and DO. For example, figure 2 indicates that members of the family Ammotheidae (green) and the genus Pallenopsis (blue) performed substantially better in hypoxia than did members of the genus Colossendeis (orange).
Table 2.
Summary of linear mixed-effects models of pycnogonid righting performance (square-root transformed). (Size was incorporated in two different ways—as log body mass or as leg diameter.)
| log body mass | leg diameter | |||||
|---|---|---|---|---|---|---|
| source | numDF | denDF | F | p-value | F | p-value |
| intercept | 1 | 59 | 290 | <0.0001 | 286 | <0.0001 |
| species | 7 | 59 | 8.9 | <0.0001 | 8.4 | <0.0001 |
| sizea | 1 | 44 | 13.7 | 0.0006 | 7.0 | 0.012 |
| DOb | 1 | 59 | 114 | <0.0001 | 118 | <0.0001 |
| species×size | 7 | 59 | 1.6 | 0.16 | 2.4 | 0.03 |
| species×DO | 7 | 59 | 4.7 | 0.0003 | 4.9 | 0.0002 |
| size×DO | 1 | 59 | 0.5 | 0.49 | 2.9 | 0.10 |
| species×size×DO | 7 | 59 | 1.5 | 0.20 | 1.3 | 0.28 |
Size incorporated either as log body mass or as leg diameter.
Dissolved oxygen (percentage of air saturation).
The main interaction of interest—size×DO—was non-significant regardless of the size measure used and so was the three-way interaction, species×size×DO. The remaining interaction—species×size—was non-significant for one measure of body size (log body mass) and marginally significant for the other (leg diameter; p=0.03), suggesting that species containing larger-bodied individuals may have had somewhat lower righting frequencies overall (figure 2a), although this effect was unrelated to DO. We examined two additional statistical models targeting subsets of the data for which we had the best replication (all members of the genus Colossendeis combined, 36 individuals; and all members of the species C. megalonyx, 17 individuals). In both subsets, oxygen level had the largest single effect followed by body size (both factors p<0.001). However, in neither case was the size×DO interaction significant (p=0.38 within Colossendeis and p=0.42 within C. megalonyx).
4. Discussion
If the oxygen hypothesis of polar gigantism is correct, larger extant pycnogonids should have more difficulty obtaining sufficient oxygen—they should have smaller safety margins for oxygen delivery (see Harrison & Lighton 1998)—than do small pycnogonids. We performed a functional test of this prediction by imposing different levels of oxygen on individuals whose body masses spanned three orders of magnitude. The data, which show a strong effect of hypoxia across size classes, provide no evidence of an interaction between body size and DO and thus are inconsistent with predictions from the oxygen hypothesis. This experimental outcome is instead more consistent with predictions from symmorphosis—that small- and large-bodied species should show similar decrements in performance in hypoxia stemming from evolutionary matching of their oxygen cascades to local temperature and oxygen conditions. Note that symmorphosis alone cannot explain why, as a group, Antarctic pycnogonids contain larger-bodied species than do temperate and tropical assemblages; explanations for gigantism must be sought in other ecological or evolutionary processes.
Our data do not suggest that pycnogonids are insensitive to hypoxia—indeed, of all factors tested, DO had by far the largest effect (table 2 and figure 2b). Moreover, the result was not driven by performance in severe hypoxia; many species showed steep declines in the performance in moderate hypoxia too. These results suggest that, as a group, Antarctic pycnogonids do not have large safety margins (sensu Harrison & Lighton 1998) for oxygen delivery. Similar tests have not been performed on temperate or tropical pycnogonids, so we do not know whether these data reflect a phylogenetic constraint on pycnogonids in general, or an increased sensitivity in Antarctic species, due to millions of years of evolution in the well-oxygenated Southern Ocean.
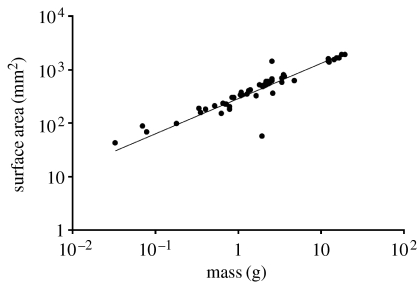
An additional question that we can address with our data is whether Antarctic pycnogonids show size-dependent increases in relative surface area (SA) for gas exchange (a potential component of symmorphosis-like alterations to the respiratory cascade). The null expectation is that SA scales to body mass with an exponent of 2/3. For each pycnogonid in our sample, we measured the radius (r, on the first main segment) and total length (L) on the most anterior leg (left or right, chosen at random). Leg SA was calculated as SA=2πrL. A log–log plot (figure 3) revealed that the relationship between SA of one leg and body mass had a scaling exponent of almost precisely 2/3 (0.66±0.6, fitted by reduced major axis regression in R v. 2.2.0), indicating that shape scaled isometrically with mass; large pycnogonids did not have disproportionately large SAs, and therefore size-dependent increases in relative SA cannot explain their relative insensitivity to hypoxia. In fact, owing to the decreased surface area to volume ratios of larger pycnogonids, these data provide an additional a priori reason to expect larger pycnogonids to be proportionally more affected by hypoxia than small ones.
Figure 3.
Scaling of leg SA to body mass in Antarctic pycnogonids. Leg SA was calculated as SA=2πrL, where r is the radius (of the most anterior leg, on the first main segment) and L is the total leg length. The regression line was fitted by reduced major axis regression, using code for R provided by George Gilchrist (y=288×0.66). The fitted equation (with 95% CI) had a slope of 0.66 (0.60–0.72) and an intercept of 2.46 (2.42–2.51).
This analysis of course does not exclude the possibility of size-dependent changes in cuticle structure and permeability, which would be predicted if lineages undergo evolutionary adjustments of oxygen delivery cascades in response to evolutionary change in body size (i.e. symmorphosis; Weibel et al. 1991). For example, the evolution of larger body size might be accompanied by increases in cuticular permeability or altered tissue biochemistry. Likewise, in smaller animals, higher ratios of oxygen supply to demand in the Antarctic might favour thicker or less permeable cuticles if these provided biomechanical or defensive benefits. This hypothesis could, in the future, be addressed by examining cuticular gas permeability, morphology and ultrastructure in pycnogonids from the Antarctic and temperate zones.
Our results contrast in several ways with the work of Peck, Pörtner and colleagues on the Antarctic bivalve L. elliptica (Peck et al. 2002, 2007; Pörtner et al. 2006). Their studies focused on how temperature (from < 0 to +9°C) affected tissue biochemistry, internal oxygen levels and performance, and found that rising temperature led to falling internal oxygen levels and impaired performance. Experimental hyperoxia rescued some of these effects (Pörtner et al. 2006). Peck et al. (2007) recently extended these analyses to examine interactive effects of temperature and body size, finding that the performance of large clams was disproportionately compromised at high temperatures. Moreover, simultaneous manipulation of oxygen levels had disproportionately large effects at high temperatures.
Their results indicate that Laternula, especially large-bodied individuals, operate near the limits of their capacity to obtain sufficient oxygen. Our results on pycnogonids, by contrast, suggest little difference between large- and small-bodied individuals in their response to environmental oxygen. The resolution to this incongruity lies at least partly in temperature. Peck et al.'s (2007) study found interactive effects of oxygen and body size in Laternula only at temperatures greater than 0°C; at or below 0°C (the temperature range at which all of our experiments were carried out), the interaction disappeared. This suggests that, in both studies, cold-induced depression of metabolic rate entirely ameliorated the detrimental effects of size on oxygen supply to internal tissues.
Our data on pycnogonids (figure 2) suggest one additional possibility related to phylogeography: species of Colossendeis were more sensitive to DO than were species of Ammothea and Pallenopsis. Colossendeis appears as an early branch in the pycnogonid phylogeny (Arango & Wheeler 2007), has diversified in cold, deep waters, and is bipolar, suggesting that it has a long evolutionary history in cold, well-oxygenated water. By contrast, Ammothea and Pallenopsis appear to be younger taxa (see Arango & Wheeler 2007), but it remains to be determined whether they have non-Antarctic origins. The relative hypoxia insensitivity of Ammothea and Pallenopsis could thus reflect physiological vestiges of a recent evolutionary history in warmer temperate or tropical waters. Additional phylogeographic work will be necessary to evaluate this possibility rigorously.
Acknowledgements
We thank the director and staff of McMurdo Station for excellent logistical and technical support. In addition, Pema Kitaeff and Bruce Miller provided extensive experimental support. George Gilchrist provided R code for fitting RMA regressions. We also thank John Pearse and two anonymous reviewers for their comments on the manuscript. This work was supported by the US National Science Foundation (ANT-0440577 to H.A.W. and ANT-0551969 to A.L.M.) and the Australian Biological Resources Study Program (grant no. 204-61 to C.P.A.)
References
- Arango C.P., Wheeler W.C. Phylogeny of the sea spiders (Arthropoda, Pycnogonida) based on direct optimization of six loci and morphology. Cladistics. 2007;23:255–293. doi: 10.1111/j.1096-0031.2007.00143.x. doi:10.1111/j.1096-0031.2007.00143.x [DOI] [PubMed] [Google Scholar]
- Arnaud P.M. Contribution à la bionomie marine benthique des regions antarctiques et subantarctiques. Téthys. 1974;6:465–656. [Google Scholar]
- Arnaud F., Bamber R.N. The biology of Pycnogonida. Adv. Mar. Biol. 1988;24:1–96. doi:10.1016/S0065-2881(08)60073-5 [Google Scholar]
- Atkinson D. Temperature and organism size—a biological law for ectotherms? Adv. Ecol. Res. 1994;25:1–58. doi:10.1016/S0065-2504(08)60212-3 [Google Scholar]
- Atkinson D., Sibly R.M. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 1997;12:235–239. doi: 10.1016/s0169-5347(97)01058-6. doi:10.1016/S0169-5347(97)01058-6 [DOI] [PubMed] [Google Scholar]
- Barnes D.K.A., Arnold R. Competition, sub-lethal mortality and diversity on Southern Ocean coastal rock communities. Pol. Biol. 2001;24:447–454. doi:10.1007/s003000100240 [Google Scholar]
- Chapelle G., Peck L.S. Polar gigantism dictated by oxygen availability. Nature. 1999;399:114–115. doi:10.1038/20099 [Google Scholar]
- Chapelle G., Peck L.S. Amphipod crustacean size spectra: new insights in the relationship between size and oxygen. Oikos. 2004;106:167–175. doi:10.1111/j.0030-1299.2004.12934.x [Google Scholar]
- Clarke A., Johnston N. Antarctic marine benthic diversity. Oceanogr. Mar. Biol. Annu. Rev. 2003;41:47–114. [Google Scholar]
- Collins M.A., Bailey D.M., Ruxton G.D., Priede I.G. Trends in body size across an environmental gradient: a differential response in scavenging and non-scavenging demersal deep-sea fish. Proc. R. Soc. B. 2005;272:2051–2057. doi: 10.1098/rspb.2005.3189. doi:10.1098/rspb.2005.3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas E.L., Hedgpeth J.W., Hemmingsen E.A. Oxygen consumption of some Antarctic pycnogonids. Ant. J. US. 1969;4:109. [Google Scholar]
- Dunlop J., Arango C.P. Pycnogonid affinities: a review. J. Zool. Syst. Evol. Res. 2005;43:8–21. doi:10.1111/j.1439-0469.2004.00284.x [Google Scholar]
- Gusso C.C., Gravina M.F. Faunistic and biological traits of some Antarctic Pycnogonida. Ital. J. Zool. 2001;68:335–344. [Google Scholar]
- Harrison J.F., Lighton J.R.B. Oxygen-sensitive flight metabolism in the dragonfly Erythemis simplicicollis. J. Exp. Biol. 1998;201:1739–1744. doi: 10.1242/jeb.201.11.1739. [DOI] [PubMed] [Google Scholar]
- Jager M., Murienne J., Clabaut C., Deutsch J., Le Guyader H., Manuel M. Homology of arthropod anterior appendages revealed by Hox gene expression in a sea spider. Nature. 2006;441:506–508. doi: 10.1038/nature04591. doi:10.1038/nature04591 [DOI] [PubMed] [Google Scholar]
- King P.E. St Martin's Press; New York, NY: 1973. Pycnogonids. [Google Scholar]
- Linse K., Barnes D.K.A., Enderlein P. Body size and growth of benthic invertebrates along an Antarctic latitudinal gradient. Deep-Sea Res. II. 2006;53:921–931. doi:10.1016/j.dsr2.2006.03.006 [Google Scholar]
- Markl J. Evolution and function of structurally diverse subunits in the respiratory protein hemocyanin from arthropods. Biol. Bull. 1986;171:90–115. doi:10.2307/1541909 [Google Scholar]
- Maxmen A., Browne W.E., Martindale M.Q., Giribet G. Neuroanatomy of sea spiders implies an appendicular origin of the protocerebral segment. Nature. 2005;437:1144–1148. doi: 10.1038/nature03984. doi:10.1038/nature03984 [DOI] [PubMed] [Google Scholar]
- McClain C.R., Rex M.A. The relationship between dissolved oxygen concentration and maximum size in deep-sea turrid gastropods: an application of quantile regression. Mar. Biol. 2001;139:681–685. doi:10.1007/s002270100617 [Google Scholar]
- Munilla, T. & Soler-Membrives, A. 2008 Check-list of the pycnogonids from Antarctic and sub-Antarctic waters. Zoogeographic implications. Ant. Sci. (doi:10.1017/S095410200800151X)
- Nakamura K., Sekiguchi K. Mating behavior and oviposition in the pycnogonid Propallene longiceps. Mar. Ecol. Prog. Ser. 1980;2:163–168. doi:10.3354/meps002163 [Google Scholar]
- Nakamura K., Kano Y., Suzuki N., Namatame T., Kosaku A. 18S rRNA phylogeny of sea spiders with emphasis on the position of Rhynchothoracidae. Mar. Biol. 2007;153:213–223. doi:10.1007/s00227-007-0803-0 [Google Scholar]
- Peck L.S., Chapelle G. Reduced oxygen at high altitude limits maximum size. Proc. R. Soc. B. 2003;270(Suppl. 2):S166–S167. doi: 10.1098/rsbl.2003.0054. doi:10.1098/rsbl.2003.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L.S., Pörtner H.O., Hardewig I. Metabolic demand, oxygen supply and critical temperatures in the Antarctic bivalve Laternula elliptica. Physiol. Biochem. Zool. 2002;75:123–133. doi: 10.1086/340990. doi:10.1086/340990 [DOI] [PubMed] [Google Scholar]
- Peck L.S., Convey P., Barnes D.K.A. Environmental constraints on life histories in Antarctic ecosystems: tempos, timings and predictability. Biol. Rev. 2006;81:75–109. doi: 10.1017/S1464793105006871. doi:10.1017/S1464793105006871 [DOI] [PubMed] [Google Scholar]
- Peck L.S., Morley S.A., Pörtner H.-O., Clark M.S. Thermal limits of burrowing capacity are linked to oxygen availability and size in the Antarctic clam Laternula elliptica. Oecologia. 2007;154:479–484. doi: 10.1007/s00442-007-0858-0. doi:10.1007/s00442-007-0858-0 [DOI] [PubMed] [Google Scholar]
- Pinheiro J.C., Bates D.M. Springer; New York, NY: 2000. Mixed-effects models in S and S-Plus. [Google Scholar]
- Pörtner H.O. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88:137–146. doi: 10.1007/s001140100216. doi:10.1007/s001140100216 [DOI] [PubMed] [Google Scholar]
- Pörtner H.O. Climate variation and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. 2002;132A:739–761. doi: 10.1016/s1095-6433(02)00045-4. doi:10.1016/s1095-6433(02)00045-4 [DOI] [PubMed] [Google Scholar]
- Pörtner H.O., Peck L.S., Hirse T. Hyperoxia alleviates thermal stress in the Antarctic bivalve, Laternula elliptica: evidence for oxygen limited thermal tolerance. Pol. Biol. 2006;29:688–693. doi:10.1007/s00300-005-0106-1 [Google Scholar]
- Redmond J.R., Swanson C.D. Preliminary studies of the physiology of the Pycnogonida. Ant. J. US. 1968;3:130–131. [Google Scholar]
- Siveter D.J., Sutton M.D., Briggs D.E.G., Siveter D.J. A Silurian sea spider. Nature. 2004;431:978–980. doi: 10.1038/nature02928. doi:10.1038/nature02928 [DOI] [PubMed] [Google Scholar]
- Soetaert K., Muthumbi A., Heip C. Size and shape of ocean margin nematodes: morphological diversity and depth-related patterns. Mar. Ecol. Prog. Ser. 2002;242:179–193. doi:10.3354/meps242179 [Google Scholar]
- Weibel E.R., Taylor C.R., Hoppeler H. The concept of symmorphosis: a testable hypothesis of structure-function relationship. Proc. Natl Acad. Sci. USA. 1991;88:10 357–10 361. doi: 10.1073/pnas.88.22.10357. doi:10.1073/pnas.88.22.10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods H.A. Egg-mass size and cell size: effects of temperature on oxygen distribution. Am. Zool. 1999;39:244–252. doi:10.1093/icb/39.2.244 [Google Scholar]
- Woods H.A., Moran A.L. Temperature–oxygen interactions in Antarctic nudibranch egg masses. J. Exp. Biol. 2008;211:798–804. doi: 10.1242/jeb.014621. doi:10.1242/jeb.014621 [DOI] [PubMed] [Google Scholar]