Abstract
The ‘tree lobsters’ are an enigmatic group of robust, ground-dwelling stick insects (order Phasmatodea) from the subfamily Eurycanthinae, distributed in New Guinea, New Caledonia and associated islands. Its most famous member is the Lord Howe Island stick insect Dryococelus australis (Montrouzier), which was believed to have become extinct but was rediscovered in 2001 and is considered to be one of the rarest insects in the world. To resolve the evolutionary position of Dryococelus, we constructed a phylogeny from approximately 2.4 kb of mitochondrial and nuclear sequence data from representatives of all major phasmatodean lineages. Our data placed Dryococelus and the New Caledonian tree lobsters outside the New Guinean Eurycanthinae as members of an unrelated Australasian stick insect clade, the Lanceocercata. These results suggest a convergent origin of the ‘tree lobster’ body form. Our reanalysis of tree lobster characters provides additional support for our hypothesis of convergent evolution. We conclude that the phenotypic traits leading to the traditional classification are convergent adaptations to ground-living behaviour. Our molecular dating analyses indicate an ancient divergence (more than 22 Myr ago) between Dryococelus and its Australian relatives. Hence, Dryococelus represents a long-standing separate evolutionary lineage within the stick insects and must be regarded as a key taxon to protect with respect to phasmatodean diversity.
Keywords: convergent evolution, conservation genetics, Phasmatodea, Dryococelus, Eurycanthinae, Lanceocercata
1. Introduction
The rediscovery of an organism long thought extinct is a very rare and fortunate event. The discovery of a small population of ‘tree lobsters’ on a rocky offshore islet in the South Pacific Ocean in 2001 was sensational news for conservationists (Priddel et al. 2003; Pain 2006; Robertson 2006). The ‘tree lobsters’ or ‘land lobsters’ (Rentz 1996) are distinct ground-dwelling ecomorphs of stick insects (insect order Phasmatodea) that are instantly recognizable due to a unique combination of morphological and behavioural characters: tree lobsters are flightless, with a dorsoventrally flattened body, a robust, stocky habitus (body form) and square-edged thoracic segments, often exhibiting an elongated secondary ovipositor in the female and enlarged, powerfully armed hind legs in the male (figure 1a–f; Gurney 1947; Hsiung 1987). In contrast to the majority of stick insects, which are solitary canopy-dwellers that drop or flick their eggs to the ground, tree lobsters aggregate in large numbers in cavities near the ground and deposit their eggs into the soil (Lea 1916; Bedford 1976; Honan 2008; S. Bradler 2007, personal observation).
Figure 1.
Photo composition of different ‘tree lobsters’ compared with a winged, canopy-dwelling stick insect. (a) Male and (b) female of D. australis, (c) male and (d) female of Canachus alligator, (e) male and (f) female of Eurycantha horrida, and (g) male of Phasma gigas.
The most famous ‘tree lobster’ is the Lord Howe Island stick insect, Dryococelus australis (Montrouzier) (figure 1a,b). This species is a large (up to 130 mm) ground-dwelling stick insect that was formerly common throughout Lord Howe Island. The introduction of rats via a shipwreck in 1918 led to the extinction of the species on Lord Howe Island by the 1960s at the latest (Priddel et al. 2003). Long considered to be extinct (Paramonov 1963; Key 1991; Rentz 1996), a population of the species was rediscovered in 2001 on Balls Pyramid, a very small, 200 metre wide rock pyramid approximately 25 km from Lord Howe Island (Priddel et al. 2003). The population size appeared not to exceed two dozen individuals, indicating that the species is indeed one of the rarest insects in the world (Robertson 2006). Since its rediscovery, a captive population has been established at Melbourne Zoo and plans to reintroduce the species to Lord Howe Island after eradication of the rats has been prepared (Pain 2006; Honan 2008).
Traditionally, the tree lobsters pertain to the subfamily Eurycanthinae, which has its greatest diversity in New Guinea and associated islands (Günther 1953); for example, the well-known genera Eurycantha (figure 1e,f) and Thaumatobactron. These taxa are considered to be the closest relatives of Dryococelus (Gurney 1947; Hennemann & Conle 2006; Honan 2008), thus implying a dispersal of tree lobsters from New Guinea to Lord Howe Island along the former Lord Howe Rise. However, the Eurycanthinae is also recorded from New Caledonia, where it is represented by Canachus (figure 1c,d) among other less studied genera. The tree lobsters are also recorded from a few other islands in the southwest Pacific and off the northern tip of Australia. The subfamily Eurycanthinae also comprises genera that are not considered tree lobsters, such as the bush-dwelling and slender stick insects Asprenas from New Caledonia and Neopromachus from New Guinea. Other arboreal taxa such as Cnipsus from New Caledonia have been placed in the Eurycanthinae by some (Günther 1953), but not all authors (Zompro 2001).
Because a considerable investment in the survival of the species is being made, it is crucial to determine the evolutionary heritage and phylogenetic distinctiveness of Dryococelus (Vane-Wright et al. 1991; Faith 1992; Mooers 2007). It is currently unknown how phylogenetically distinct Dryococelus is with regard to the remaining tree lobsters and what its biogeographic history is. For example, because Lord Howe Island only emerged 6.4–6.9 Myr ago (McDougall et al. 1981), it is possible that Dryococelus is only very recently derived from the New Guinean or New Caledonian lineages of tree lobsters. Alternatively, it may have a much more ancient evolutionary history, which would enhance its conservation value.
To examine the phylogenetic position of Dryococelus among stick insects, to reconstruct its biogeographic history and to assess its conservation value, we obtained nuclear and mitochondrial DNA sequence data from almost all major lineages of phasmatodean diversity. In particular, we sampled all key eurycanthine genera in addition to a broad selection of other stick insect taxa from the Pacific and Australasian regions. We also applied a Bayesian relaxed clock with fossil calibrations to infer the evolutionary age of Dryococelus and provide the first divergence time estimates across the Phasmatodea.
2. Material and methods
(a) Taxon sampling
Our sampling was designed to include all major lineages of Phasmatodea and also to maximize the sampling of genera in the Australasian region. We sampled 16 of the 18 traditional euphasmatodean subfamilies, following the classification of Günther (1953) for the reasons outlined by Klug & Bradler (2006), with amendments from Zompro (2001). Generic nomenclature follows Otte & Brock (2005) and Brock & Hasenpusch (2007), except in the cases where our phylogenetic analysis suggests newly proposed genera to be non-monophyletic. We have sampled all four major genera of New Guinean Eurycanthinae, in addition to Dryococelus and most genera of New Caledonian tree lobsters. The phylogenetic tree was rooted using Timema, which has been shown to be the sister group to the Euphasmatodea in previous phylogenetic studies (Whiting et al. 2003; Bradler in press). Authorities for all taxonomic names are given in the electronic supplementary material, table 1.
(b) DNA sequence data collection
DNA extractions were performed from muscle tissue using Aqua pure genomic DNA tissue kit (Bio-Rad, USA) following the manufacturer's instructions. We used PCR to obtain sequence data from two non-contiguous mitochondrial and two nuclear genes. The mitochondrial data included 753 bp from the cytochrome oxidase subunit I (COI) gene and 689 bp from the cytochrome oxidase subunit II (COII) gene. The nuclear DNA sequence data included 350 bp from the Histone subunit 3 (H3) gene and 707 bp from the large subunit rRNA (28S) gene. The COI and COII genes were amplified using the primers C1-J-2195+TL2-N-3014 and TL2-J-3034+TK-N-3785, respectively (Simon et al. 1994). The 28S gene was amplified using the primers 28S-356+28S-1009 from Buckley et al. (2008) and the H3 gene was amplified using the primers H3F (ATGGCTCGTACCAAGCAGAC) and H3R (ATATCCTTRGGCATRATRGTGAC) from Colgan et al. (1998). PCR cycling conditions were 94°C for 1 min, followed by 35–40 cycles of 94°C for 1 min, 53–57°C for 1 min and 72°C for 1.5 min, and 1 cycle for 72°C for 10 min. DNA products were purified for sequencing using MinElute 96 UF PCR purification kit (Qiagen, USA). Purified PCR products were sequenced using BigDye Terminator v. 3.1 cycle sequencing kit (Applied Biosystems, USA). Cycle sequencing products were cleaned by 96 well plate ethanol precipitation and analysed on ABI 3100 Avant Genetic Analyzer (Applied Biosystems).
(c) Alignment, phylogenetic analysis and molecular dating
The COI and H3 genes were length-invariable and the COII gene contained only a small region of length variation. Alignment of this latter gene was achieved using ClustalX (Larkin et al. 2007). The 28S gene was more variable and contained a number of regions of length variation. The ClustalX alignment was refined using the 28S rRNA secondary structure information from the Chrysomelidae (Coleoptera) model (Gillespie et al. 2004). Helices were identified using this model and used to delimit the boundary of regions for exclusion (typically unpaired regions).
We estimated phylogenetic relationships using BEAST v. 1.4.8 (Drummond & Rambaut 2007) under an uncorrelated lognormal model (UCLD; Drummond et al. 2006). We partitioned the data into codon positions for the mitochondrial DNA and assigned the H3 and 28S sites to a different partition each to yield a five-partition model. For each partition we used the AIC to select the best fit model as implemented in PAUP* v. 4.0.b10 (Swofford 1998) and Modeltest v. 3.6 (Posada & Crandall 1998). We used a Yule prior for tree shape and exponential priors for the substitution model and relaxed clock parameters. Priors for the model parameters were μ=100.0, GTR rate parameters=100.0, transition/transversion rate ratio=100.0, partition rate multipliers=100, α shape parameter=1.0, UCLD mean=1.0, UCLD standard deviation=1.0, Yule birth rate=1.0, mean substitution rate=1.0, coefficient of variation=1.0 and covariance=1.0. We iteratively optimized the Markov chain Monte Carlo (MCMC) operators by performing short runs (10×106 cycles) and then adjusting the operators as suggested by BEAST and gradually increasing the run length. When the MCMC operators were set at optimal levels, as indicated by the BEAST output, we ran five runs at 40×106 cycles, sampling every 1000th step in the chain, and used Tracer (Drummond & Rambaut 2007) to monitor convergence, select the burn-in and calculate effective sample sizes. All runs that were consistent with convergence were concatenated and used to estimate the posterior distributions of topology and divergence time.
Phasmatodea fossils are exceedingly rare and currently only a few fossils can be unambiguously placed on an extant lineage (Wedmann et al. 2007). We used the occurrence of fossil euphasmatodean eggs in mid-Cretaceous Burmese amber (Rasnitsyn & Ross 2000) to place a prior distribution on the age of the root. These fossils have been dated to between 95 and 110 Myr ago (Grimaldi & Engel 2005), therefore we assumed that the divergence between Timema and the Euphasmatodea occurred more than 95 Myr ago. We also used the presence of a leaf insect fossil dated at 47 Myr ago (Wedmann et al. 2007) to set the minimum age on the divergence of the leaf insects from their closest relatives. For the root and leaf insect calibration points, we used exponential distributions with a mean of 1.0 and 5.0, respectively, and offset these distributions by 95 and 47 Myr ago, respectively. Although these fossil calibrations may represent substantial underestimates of the age of the lineages they are placed on, we have no information to constrain the upper age of those lineages. Therefore, despite the tight prior distribution assumed, the resulting dates are interpreted as lower limits.
The monophyly of the Eurycanthinae was tested by constructing a maximum-likelihood tree in PAUP* v. 4.0 and comparing the likelihood of this tree to that of a tree where the Eurycanthinae were constrained to be monophyletic. The significance of the likelihood ratio was tested using the Shimodaira–Hasegawa (S–H) test (Shimodaira & Hasegawa 1999) as implemented in PAUP* v. 4.0.b10. For the bootstrap step of the S–H test, we used 10 000 replicates of the RELL approximation.
3. Results
(a) Phylogenetic relationships among the tree lobsters
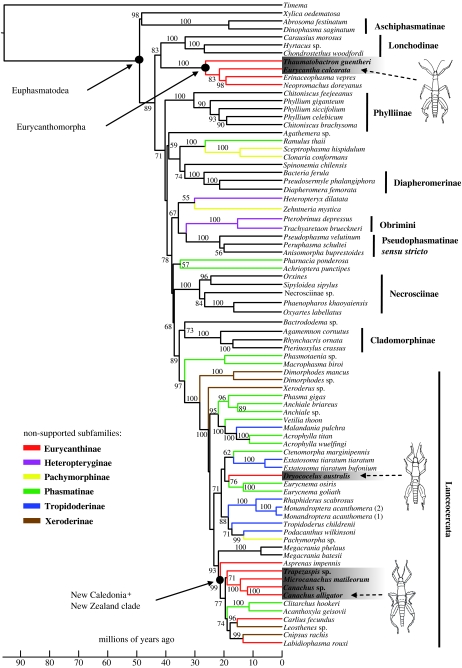
We obtained 2.4 kb of nuclear and mitochondrial sequence data from 78 euphasmatodean individuals and 1 individual of the out-group Timema. All newly obtained sequences have been submitted to GenBank under accession numbers FJ474100–FJ474403. We observed excellent support (1.0 Bayesian posterior probability) for the monophyly of Diapheromerinae, Pseudophasmatinae sensu stricto (excluding Agathemera and the Heteronemia group, e.g. Spinonemia), Phylliinae, Aschiphasmatinae, Necrosciinae, Lonchodinae, Cladomorphinae, Obrimini and Lanceocercata in agreement with the previous phylogenetic studies (figure 2; Whiting et al. 2003; Bradler in press). In general, there are very few relationships between traditional subfamilies that are well supported, the exceptions being Eurycanthomorpha+Lonchodinae (83% posterior probability), Bacillinae (Xylica)+Aschiphasmatinae (98% posterior probability) and Palophinae (Bactrododema)+Cladomorphinae (73% posterior probability), the first also recovered in a previous molecular phylogenetic study (Whiting et al. 2003). We find support for neither the monophyly of Phasmatinae, nor its subgroup Pharnaciini (the latter represented by Pharnacia and the Macrophasma+Phasmotaenia clade). The UCLD model inferred the root of the tree to lie on the branch between Timema and the Euphasmatodea with a posterior probability of 1.0, as also supported by previous molecular (Whiting et al. 2003) and morphological (Bradler in press) data.
Figure 2.
Bayesian phylogenetic tree showing relationships among euphasmatodean taxa and placement of the ‘tree lobster’ ecomorphs. Branch lengths are drawn proportional to time and values above branches are Bayesian posterior probabilities. Non-monophyletic subfamilies are indicated by coloured branches according to the inset key. The tree lobster taxa are highlighted grey. Monophyletic taxa are indicated by vertical bars.
The Lanceocercata contain a wide array of Australasian phasmids conventionally thought to be unrelated to one another, comprising species of Tropidoderinae, Xeroderinae, Platycraninae, Phasmatini, Acanthoxylini and Pachymorphini (Bradler 2001, in press). Within the Lanceocercata, Dimorphodes (subfamily Xeroderinae) constitutes the sister group to all other members (100% posterior probability), which include the remaining Xeroderinae and the polyphyletic Tropidoderinae, and Phasmatini as previously suggested (Whiting et al. 2003). Perhaps the most surprising result is that the subfamily Eurycanthinae, containing the tree lobsters, are also polyphyletic, forming five separate lineages. Members of the virtual tree lobsters are found in three unrelated regions of the tree (grey boxes in figure 2), two of them (Trapezaspis+Canachus+Microcanachus and Dryococelus) nested within Lanceocercata. Using an S–H test, we were able to reject monophyly of the Eurycanthinae with a P-value of 0.00001. The members of the Eurycanthinae outside the Lanceocercata clade comprise Eurycantha and related genera from New Guinea, the Eurycanthomorpha sensu Bradler (2002, in press). Within Lanceocercata, the New Caledonian eurycanthines form a clade with various other taxa from New Caledonia and New Zealand (100% posterior probability), including the enigmatic Cnipsus, which has been assigned to different subfamilies in the past (Redtenbacher 1908; Günther 1953; Zompro 2001). The Lord Howe Island tree lobster Dryococelus appears to be unrelated to the New Guinean and New Caledonian Eurycanthinae, forming a rather isolated lineage, but related to the widespread Australian genus Eurycnema (76% posterior probability).
Using the two calibration points discussed above, we obtained a lower limit on the age of the extant Euphasmatodea radiation of 51.9 Myr ago (95% posterior intervals 47.0–58.7). Most of the subfamilies diverged over a period of ca 20 Myr ago, indicating that the Euphasmatodea underwent a rapid radiation.
(b) Analysis of morphological characters
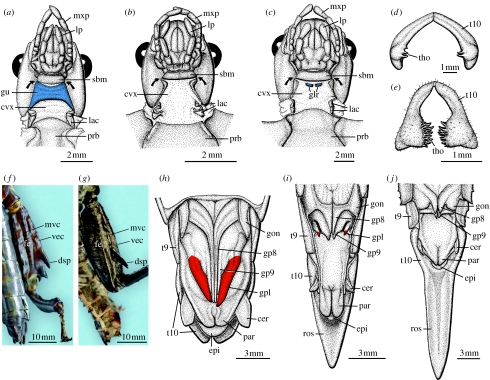
Our findings contradict the view that Dryococelus is closely related to the New Guinean or New Caledonian tree lobsters as has been suggested for almost 150 years by all previous authors (Westwood 1859; Redtenbacher 1908; Gurney 1947; Günther 1953; Key 1991; Zompro 2001; Hennemann & Conle 2006; Brock & Hasenpusch 2007; Honan 2008). The results of our phylogenetic analysis demand a reassessment of the morphological evidence. One putative synapomorphic character of Dryococelus and the New Guinean Eurycantha and Thaumatobactron are the conspicuously enlarged and strongly armed hind legs of the males. Both Dryococelus and Eurycantha, for example, have a large defensive spine on ventral surface of the hind femora (dsp in figure 3f,g). However, closer inspection of this armature shows that it appears to be non-homologous between these genera. In Dryococelus, the prominent ventral spine is formed by the ventroexternal carina (vec in figure 3f), whereas in Eurycantha and Thaumatobactron a similarly prominent spine is produced by the midventral carina (mvc in figure 3g; Gurney 1947) and in Canachus this spine is absent.
Figure 3.
Morphological details of D. australis compared with key taxa of the remaining ‘tree lobster’ clades. Ventral view of the male head region of (a) Thaumatobactron guentheri, (b) C. alligator and (c) D. australis. Male clasper (tergum 10) in posterior view of (d) D. australis and (e) C. alligator. Ventral view of left hind leg of (f) male D. australis and (g) male Eurycantha calcarata. Ventral view of female genitalia (sternum 8 removed) of (h) D. australis, (i) C. alligator and (j) Eurycantha insularis. cer, cercus; cvx, cervix; dsp, defensive spine; epi, epiproct; fe, femur; glr, gularia; gon, gonangulum; gp8–9, gonapophysis 8–9; gpl, gonoplac; gu, gula; lac, laterocervicalia; lp, labial palpus; mvc, midventral carina; mxp, maxillar palpus; par, paraproct; prb, probasisternite; ros, rostrum; sbm, submentum; t9–10, tergum 9–10; tho, thorn pad; vec, ventroexternal carina. The arrows in (a–c) indicate the position of the posterior tentorial pits. Gular sclerotization shaded blue, gonoplacs shaded red.
In the neck region of all Eurycanthomorpha, e.g. Thaumatobactron, the cervix is protected by a prominent sclerite, the gula (shaded blue in figure 3a), whereas Canachus exhibits no gular sclerotization whatsoever (figure 3b), and Dryococelus specimens possess small gular sclerites in the otherwise membranous cervical region (figure 3c).
In the female ovipositor of Dryococelus, all three pairs of valves are well developed, with the gonoplac forming the largest sheath (shaded red in figure 3h), which appears to represent the primitive condition among neopteran insects (Kristensen 1975) as well as in Phasmatodea (Tilgner et al. 1999). The ovipositor of Canachus exhibits only remnants of the gonoplac (figure 3i) and in Eurycantha the gonoplac is completely reduced (figure 3j). In females of Eurycantha and Canachus, the abdominal tergum 10 is elongated forming a rostrum as part of a secondary ovipositor for depositing eggs in soil and other substrates (figure 3i,j). A secondary ovipositor is absent in Dryococelus, traditionally interpreted as the result of a secondary reduction (Zompro 2001). Our reconstruction, however, suggests that a secondary ovipositor was not present in the ancestral lineage of Dryococelus, suggesting its absence in Dryococelus to constitute the primary condition. Thus, the female genitalia are quite different among each of the three tree lobster lineages, and there appear to be no obvious synapomorphies to link Dryococelus and the New Guinean and New Caledonian tree lobsters. This variation in female terminalia is reflected in the differences in the genital armature of the male claspers, where Dryococelus and Canachus resemble typical Lanceocercata (tho in figure 3d,e; cf. Bradler in press). Finally, the New Caledonian eurycanthines (Canachus and the related Asprenas) have small wing rudiments, whereas the Eurycanthomorpha and Dryococelus are fully apterous.
In summary, Dryococelus lacks all of the apomorphic characters that define the New Guinean Eurycanthomorpha, including the presence of a gula, reduced gonoplacs in the female ovipositor and the presence of a secondary ovipositor for egg deposition (Bradler 2002, in press). These relatively inconspicuous but significant anatomical differences between the different tree lobster lineages mirror their separate phylogenetic placement. Nevertheless, the overall strong similarity in general body form of the different tree lobsters led to their improper and hitherto unquestioned classification.
4. Discussion
(a) Diversification and convergent evolution in stick insects
Similarities between species can arise in two fundamentally different ways. Either each species has retained a comparable trait from their common ancestor, or each has acquired it independently (Hall 1994). Although the first possibility might seem far more likely, convergence is in fact a common phenomenon (Morris 2003), often found as a consequence of adaptive radiations in separate evolutionary lineages (Givnish 1997). Our data strongly suggest that the tree lobster body form evolved independently on the three different landmasses of New Guinea, New Caledonia and Lord Howe Island. Each lineage of these ground-dwelling ecomorphs have probably descended from arboreal ancestors as they are all either nested within arboreal clades (Dryococelus and Canachus) or sister-group to them (New Guinean tree lobsters). Their overall uniformity in body form and behaviour is probably the product of similar selective pressure associated with adaptations to ground-dwelling life. Individuals of Dryococelus and Eurycantha congregate in large numbers and close spatial proximity in tree hollows and cavities during the day (Lea 1916; Gurney 1947; Bedford 1976). Females of all tree lobster genera deposit their eggs into the soil (Hsiung 1987; Pain 2006; Honan 2008; personal observations), with females of Canachus and Eurycantha even using a similarly developed secondary ovipositor (Bradler 2002; figure 3i,j). Individuals of all three clades exhibit a robust habitus with dorsoventrally flattened body and sturdy legs. The greatly enlarged and armed hind legs of some males probably evolved as a response to ground-hunting predators and might also be used against other males (Lea 1916; Gurney 1947; Bedford 1976; Hsiung 1987; Honan 2008), although if this is the case this defence mechanism did not save Dryococelus from rats (the rats were apparently eating the nymphs, not adults).
Among Phasmatodea the Lanceocercata exhibit morphological and ecological parallelisms comparable with those found between placental mammals and marsupials (Springer et al. 1997) and between afrotherian and laurasiatherian mammals (Madsen et al. 2001). Examples of extensive and multiple convergences have also been demonstrated between lineages of African cichlid fishes (Meyer et al. 1990; Brakefield 2006), lizards on Caribbean islands (Losos et al. 1998) and between several bird families (Fain & Houde 2004). Such phenotypic similarity between unrelated species is probably generated by extrinsic selective pressure, as well as by intrinsic factors such as shared trajectories in the underlying developmental architecture (Brakefield 2006), which might provide constraints on the direction of stick insect evolution. Our results indicate that the Australasian Lanceocercata and the remaining Euphasmatodea underwent parallel adaptive radiations that resulted, in addition to the tree lobsters, in further astounding examples of convergence. The Lanceocercata also comprise giant-winged stick insects of the canopy, such as the Australian Acrophylla, exceeding 26 cm in body length, which is paralleled by the equally large, morphologically and ecologically similar African Bactrododema (Palophinae). The leaf-imitating forms include Malandania and Tropidoderus in Lanceocercata and true leaf insects in the Phylliinae. Gracile flyers are also found within Lanceocercata (e.g. Carlius), which are strikingly similar to the forms from the Necrosciinae (e.g. Sipyloidea). In addition, the small wingless Australian Lanceocercata with exceedingly short antennae (e.g. Pachymorpha) are highly reminiscent of the Afro-Oriental Gratidiini (e.g. Sceptrophasma, Clonaria, Gratidia). Examples among diminutive spiny trunk-dwellers include Cnipsus in Lanceocercata and Neopromachus in Eurycanthomorpha.
Our data show that the Lanceocercata radiated not only in Australia and New Guinea but also from as far west as the Mascarene Archipelago in the Indian Ocean and as far east as New Zealand and New Caledonia. Given the low dispersal abilities of phasmatodeans overseas (Nakata 1961), tectonic movements might have been of major importance in shaping their historical distribution. Because we have no information from which to derive the upper limits on any of the divergences times within the Phasmatodea, all our reported dates must be interpreted as minimum estimates, and future discoveries of fossil phasmatodeans pertaining to extant crown groups could result in significantly older age estimates of their most recent common ancestor. Our divergence time estimates using a Bayesian relaxed clock (Drummond et al. 2006) suggest that the Lanceocercata began to diversify at least 32 Myr ago (29.4–37.5 Myr ago), in the Oligocene. The radiation of the subfamilies and other major clades occurred over a period of 20 million years (and much less if the Diapheromerinae and Phylliinae are excluded) and may represent a classic case of rapid ancient radiation.
Our divergence time estimates suggest that Dryococelus shared a most recent common ancestor with its closest Australian relative, the genus Eurycnema, at least 22 Myr ago (15.9–26.2 Myr ago), which contrasts with the emergence of Lord Howe Island 6.4–6.9 Myr ago as the result of volcanic activity along the Lord Howe Rise (McDougall et al. 1981). Unless future sampling of other Lanceocercata taxa reveals more closely related extant relatives then Dryococelus must have existed elsewhere prior to the formation of Lord Howe Island, possibly to the north on the now-submerged seamounts known as the Lord Howe seamount chain, or to the northwest along the submerged Tasmantid Guyots (McDougall et al. 1981). Interestingly, the oldest of the submerged islands in the Lord Howe seamount chain, Nova Bank, is roughly estimated to be 23 Myr old (McDougall et al. 1981), which accords well with our minimum divergence estimate of Dryococelus from its mainland Australian relatives. The Lord Howe Island tree lobster may have evolved on now-drowned islands far to the north of Lord Howe and progressively dispersed down the island chain, leaving its ancestral populations to become extinct as their islands eroded away.
(b) Conservation genetics of D. australis
Numerous authors have suggested that the conservation of phylogenetic diversity is preferable over the protection of pure species richness, taking into account that different species can vary drastically in their evolutionary isolation and heritage (Vane-Wright et al. 1991; Faith 1992; Mooers 2007). The isolated phylogenetic position and great age of Dryococelus among the Lanceocercata highlights the importance of its conservation with respect to phasmatodean diversity. Considerable effort has already been undertaken to conserve the small remaining population of Dryococelus (Honan 2008). The results of this study indicate that this investment is decidedly warranted.
Acknowledgments
Specimens and assistance with fieldwork were provided by H. Blafard, M. Brinkert, P. Brock, S. Brown, J.-J. Cassan, S. Cazeres, D. Clark, J. Colville, P. Cranston, N. Evenhuis, K. Hill, R. Hoare, M. Humphrey, T. Jewell, B. Kneubühler, R. Leschen, D. Marshall, J. Midgley, C. Millé, P. Miller, G. Monteith, M. Moulds, D. Otte, D. Paulaud, K. Rabaey, R. Simoens, K. Vandervennet and K. Will. Thanks to T. Reischig and P. Schwendinger for taking the photos. U. Aspöck, S. Randolf and P. Schwendinger loaned specimens. Permits for New Caledonia were issued by D. Paulaud and J.-J. Cassan with assistance from C. Millé. R. Leschen, A. Stumpner and two anonymous reviewers provided comments on the manuscript. Funding was provided by the National Geographic Society (7906-05), the Royal Society of New Zealand Marsden Fund (LCR302), the Foundation for Research, Science and Technology through the Defining New Zealand's Land Bioti OBI and an Ernst Mayr Travel Grant in Animal Systematics.
Supplementary Material
Taxonomic and geographic sampling of stick insect species
References
- Bedford G.O. Defensive behaviour of the New Guinea stick insect Eurycantha (Phasmatodea, Phasmatidae, Eurycanthinae) Proc. Linn. Soc. NSW. 1976;100:218–222. [Google Scholar]
- Bradler S. The Australian stick insects, a monophyletic group within the Phasmatodea? Zoology. 2001;104(Suppl. IV (DZG 94.1)):69. [Google Scholar]
- Bradler S. Evolution of secondary ovipositors in stick insects (Insecta: Phasmatodea) Zoology. 2002;105(Suppl. V (DZG 95.1)):60. [Google Scholar]
- Bradler, S. In press. Phylogeny of the stick and leaf insects (Insecta: Phasmatodea). Species Phylogenet. Evol.
- Brakefield P.M. Evo–devo and constraints on selection. Trends Ecol. Evol. 2006;21:362–368. doi: 10.1016/j.tree.2006.05.001. doi:10.1016/j.tree.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Brock P.D., Hasenpusch J. Studies on the Australian stick insects (Phasmida), including a checklist of species and bibliography. Zootaxa. 2007;1570:1–81. [Google Scholar]
- Buckley T.R., Attanayake D., Park D.-C., Ravindran S., Jewell T.R., Normark B.B. Investigating hybridization in the parthenogenetic New Zealand stick insect Acanthoxyla (Phasmatodea) using single-copy nuclear loci. Mol. Phylogenet. Evol. 2008;46:335–349. doi: 10.1016/j.ympev.2008.02.016. doi:10.1016/j.ympev.2008.02.016 [DOI] [PubMed] [Google Scholar]
- Colgan D.J., McLauchlan A., Wilson G.D.F., Livingston S.P., Edgecombe G.D., Macaranas J., Cassis G., Gray M.R. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust. J. Zool. 1998;46:419–437. doi:10.1071/ZO98048 [Google Scholar]
- Drummond A.J., Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. doi:10.1186/1471-2148-7-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A.J., Ho S.Y.W., Phillips M.J., Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS. Biol. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. doi:10.1371/journal.pbio.0040088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain M.G., Houde P. Parallel radiations in the primary clades of birds. Evolution. 2004;58:2558–2573. doi: 10.1111/j.0014-3820.2004.tb00884.x. doi:10.1554/04-235 [DOI] [PubMed] [Google Scholar]
- Faith D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992;61:1–10. doi:10.1016/0006-3207(92)91201-3 [Google Scholar]
- Gillespie J., Cannone J., Gutell R., Cognato A. A secondary structure model of the 28S rRNA expansion segments D2 and D3 from rootworms and related leaf beetles (Coleoptera: Chrysomelidae; Galerucinae) Insect Mol. Biol. 2004;13:495–518. doi: 10.1111/j.0962-1075.2004.00509.x. doi:10.1111/j.0962-1075.2004.00509.x [DOI] [PubMed] [Google Scholar]
- Givnish T.J. Adaptive radiation and molecular systematics: issues and approaches. In: Givnish T.J., Sytsma K.J., editors. Molecular evolution and adaptive radiation. Cambridge University Press; Cambridge, UK: 1997. pp. 1–54. [Google Scholar]
- Grimaldi D.A., Engel M.S. Cambridge University Press; Cambridge, UK: 2005. Evolution of the insects. [Google Scholar]
- Günther K. Über die taxonomische Gliederung und geographische Verbreitung der Insektenordnung der Phasmatodea. Beitr. Entomol. 1953;3:541–563. [Google Scholar]
- Gurney A.B. Notes on some remarkable Australasian walkingsticks, including a synopsis of the genus Extatosoma (Orthoptera: Phasmatidea) Ann. Entomol. Soc. Am. 1947;40:373–396. [Google Scholar]
- Hall B.K. Academic Press; San Diego, CA: 1994. Homology: the hierarchical basis of comparative biology. [Google Scholar]
- Hennemann F., Conle O.V. Papuacocelus papuanus n. gen., n. sp.—a new Eurycanthinae from Papua New Guinea, with notes on the genus Dryococelus Gurney, 1947 and description of the egg (Phasmatodea: Phasmatidae: Eurycanthinae) Zootaxa. 2006;1375:31–49. [Google Scholar]
- Honan P. Notes on the biology, captive management and conservation status of the Lord Howe Island Stick Insect (Dryococelus australis) (Phasmatodea) J. Insect Conserv. 2008;12:399–413. doi:10.1007/s10841-008-9162-5 [Google Scholar]
- Hsiung C. Aspects of the biology of the of the Melanesian stick-insect Eurycantha calcarata Lucas (Cheleutoptera: Phasmatidae) J. Nat. Hist. 1987;21:1241–1258. doi:10.1080/00222938700770761 [Google Scholar]
- Key, K. H. L. 1991 Phasmatodea (stick-insects). In The insects of Australia, vol. 1 (ed. CSIRO), pp. 394–404. Ithaca, NY: Cornell University Press.
- Klug R., Bradler S. The pregenital abdominal musculature in phasmids and its implications for the basal phylogeny of Phasmatodea (Insecta: Polyneoptera) Org. Divers. Evol. 2006;6:171–184. doi:10.1016/j.ode.2005.08.004 [Google Scholar]
- Kristensen N.P. The phylogeny of hexapod ‘orders’. A critical review of recent accounts. Zool. Syst. Evol. Forsch. 1975;13:1–44. [Google Scholar]
- Larkin M.A., et al. ClustalW and ClustalX v. 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. doi:10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lea A.M. Notes on the Lord Howe phasma, and on an associated longicorn beetle. Proc. R. Soc. S. Aust. 1916;40:145–147. [Google Scholar]
- Losos J.B., Jackman T.R., Larson A., de Queiroz K., Rodríguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. doi:10.1126/science.279.5359.2115 [DOI] [PubMed] [Google Scholar]
- Madsen O., et al. Parallel adaptive radiations in two major clades of placental mammals. Nature. 2001;409:610–614. doi: 10.1038/35054544. doi:10.1038/35054544 [DOI] [PubMed] [Google Scholar]
- McDougall I., Embleton B.J.J., Stone D.B. Origin and evolution of Lord Howe Island, southwest Pacific Ocean. J. Geol. Soc. Aust. 1981;28:155–176. doi:10.1080/00167618108729154 [Google Scholar]
- Meyer A., Kocher T.D., Basasibwaki P., Wilson A.C. Monophyletic origin of Lake Victoria cichlid fishes suggested by mitochondrial DNA sequences. Nature. 1990;347:550–553. doi: 10.1038/347550a0. doi:10.1038/347550a0 [DOI] [PubMed] [Google Scholar]
- Mooers A.Ø. The diversity of biodiversity. Nature. 2007;445:717–718. doi: 10.1038/445717a. doi:10.1038/445717a [DOI] [PubMed] [Google Scholar]
- Morris S.C. Cambridge University Press; Cambridge, UK: 2003. Life's solution: inevitable humans in a lonely universe. [Google Scholar]
- Nakata S. Some notes on the occurrence of Phasmatodea in Oceania. Pacific Insects Monogr. 1961;2:107–121. [Google Scholar]
- Otte D., Brock P.D. The Orthopterists Society; Philadelphia, PA: 2005. Phasmida species file: a catalog to the stick insects of the world. [Google Scholar]
- Pain S. Return of the giants. New Sci. 2006;191:42–45. doi:10.1016/S0262-4079(06)60113-9 [Google Scholar]
- Paramonov S.J. Lord Howe Island, a riddle of the Pacific, part III. Pac. Sci. 1963;17:361–373. [Google Scholar]
- Posada D., Crandall K.A. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. doi:10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Priddel D., Carlile N., Humphrey M., Fellenberg S., Hiscox D. Rediscovery of the ‘extinct’ Lord Howe Island stick-insect (Dryococelus australis (Montrouzier)) (Phasmatodea) and recommendations for its conservation. Biodivers. Conserv. 2003;12:1391–1403. doi:10.1023/A:1023625710011 [Google Scholar]
- Rasnitsyn A.P., Ross A.J. A preliminary list of arthropod families present in the Burmese amber collection at The Natural History Museum, London. Bull. Nat. Hist. Mus. Geol. Ser. 2000;56:21–24. [Google Scholar]
- Redtenbacher J. Wilhelm Engelmann; Leipzig, Germany: 1908. Die Insektenfamilie der Phasmiden III. Phasmidae Anareolatae. [Google Scholar]
- Rentz D.C.F. University of NSW Press; Sydney, Australia: 1996. Grasshopper country. [Google Scholar]
- Robertson H. Sticking in there. Curr. Biol. 2006;16:R781–R782. doi: 10.1016/j.cub.2006.08.062. doi:10.1016/j.cub.2006.08.062 [DOI] [PubMed] [Google Scholar]
- Shimodaira H., Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 1999;16:1114–1116. [Google Scholar]
- Simon C., Frati F., Crespi B., Liu H., Flook P.K. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved PCR primers. Ann. Entomol. Soc. Am. 1994;87:651–701. [Google Scholar]
- Springer M.S., Kirsch J.A.W., Case J.A. The chronicle of marsupial evolution. In: Givnish T.J., Sytsma K.J., editors. Molecular evolution and adaptive radiation. Cambridge University Press; Cambridge, UK: 1997. pp. 129–161. [Google Scholar]
- Swofford, D. L. 1998 PAUP*: phylogenetic analysis using parsimony (*and other methods), v. 4. Sunderland, MA: Sinauer Associates.
- Tilgner E.H., Kiselyova T.G., McHugh J.V. A morphological study of Timema cristinae Vickery with implications for the phylogenetics of Phasmida. Deut. Entomol. Z. 1999;46:149–162. [Google Scholar]
- Vane-Wright R.I., Humphries C.J., Williams P.H. What to protect?—Systematics and the agony of choice. Biol. Conserv. 1991;55:235–254. doi:10.1016/0006-3207(91)90030-D [Google Scholar]
- Wedmann S., Bradler S., Rust J. The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior. Proc. Natl Acad. Sci. USA. 2007;104:565–569. doi: 10.1073/pnas.0606937104. doi:10.1073/pnas.0606937104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood J.O. Taylor & Francis; London, UK: 1859. Catalogue of the Orthopterous insects in the collection of the British Museum. Part I: Phasmidae. [Google Scholar]
- Whiting M.F., Bradler S., Maxwell T. Loss and recovery of wings in stick insects. Nature. 2003;421:264–267. doi: 10.1038/nature01313. doi:10.1038/nature01313 [DOI] [PubMed] [Google Scholar]
- Zompro O. A review of Eurycanthinae: Eurycanthini, with a key to genera, notes on the subfamily and designation of type species. Phasmid Stud. 2001;10:19–23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Taxonomic and geographic sampling of stick insect species