Abstract
Ecological immunology attempts to explain variation in immune function. Much of this work makes predictions about how potential hosts should invest in overall immunity. However, this ‘overall’ perspective under-emphasizes other critical aspects, such as the specificity, inducibility and timing of an immune response. Here, we investigate these aspects by examining gene regulation across several immune system components in both male and female Drosophila melanogaster prior to and after mating. To elucidate potentially important temporal dynamics, we also assayed several genes over time. We found that males and females emphasized different components of their immune system, however overall investment was similar. Specifically, the sexes emphasized different gene paralogues within major gene families, and males tended to invest more in gram-negative defence. By contrast, the inducibility of the immune response was both transient (lasting approx. 24 hours) and equal between the sexes. Furthermore, mating tended to induce humoral gene upregulation, while cell-mediated genes were unaffected. Within the humoral system, gram-negative bacterial defence genes exhibited a greater inducibility than those associated with fungal or gram-positive bacterial defence. Our results suggest that variation in the effectiveness of the immune response between the sexes may be driven by differences in emphasis rather than overall investment.
Keywords: mating, immunity, sexual dimorphism, specificity, inducibility, Drosophila
1. Introduction
Hosts frequently vary in their ability to defend themselves against infectious pathogens. This variation has been observed between closely related species (Relsen & Hahn 2007), among populations (Mucklow et al. 2004), across the sexes (Schwarzenbach et al. 2005) and within individuals (Lee 2006). The burgeoning field of ecological immunity attempts to describe the evolutionary forces that shape and maintain this variation (Rolff & Siva-Jothy 2003). Much of this work has taken a life-history perspective (Zuk & Stoehr 2002; McKean & Nunney 2005; Stoehr & Kokko 2006), and attempts to predict which groups of organisms should invest more in immune function. Because these studies often focus on the total allocation of resources towards an immune response, however, they tend to under-emphasize other important aspects, such as an immune response's specificity, inducibility and timing (Harvell 1990; Schmid-Hempel & Ebert 2003; Schmid-Hempel 2005). Immune function is not a simple, static process, but rather a dynamic and complex system of interrelated mechanisms that are differentially effective against specific pathogens or pathogen types (Lambrechts et al. 2005), and whose expression may vary considerably during an immune response (Fedorka et al. 2007). Thus, potential hosts may differ not only in their overall allocation to the immune system, but also in how and when that allocation is used.
Recently, the potential for sexual differences in immunological competency has become of great interest (Rolff 2002; McKean & Nunney 2005; Stoehr & Kokko 2006). These studies often hypothesize that females allocate more of their limited resources to immune function than males, assuming that investment in life-prolonging processes pays females a greater fitness return (Zuk & Stoehr 2002; but see Stoehr & Kokko 2006). To address this hypothesis, immune allocation has typically been assessed by inoculating individuals with large amounts of pathogens (Wedekind & Jakobsen 1998; Kaltz & Shykoff 2001; McKean & Nunney 2005; Fedorka et al. 2007) or pathogen mimics (Fedorka et al. 2004; Fedorka & Mousseau 2007). Although such methods are often necessary to observe significant differences between the sexes (or any other group), they do not (i) examine a broad range of immunological pathways or effectors to determine the response's specificity, (ii) provide clear baseline data necessary to determine the magnitude of the immune response (i.e. its inducibility) or (iii) examine the dynamics of the response over time. Moreover, the concentration of pathogens used, as well as the method of delivery (e.g. direct injection into the body cavity), does not usually represent biologically realistic immune challenges. Although these procedures provide important insights into the dynamics and limitations of the immune response when confronted with an acute immune challenge, a more natural challenge may be appropriate.
One alternative to these artificial approaches would be the natural immune challenge posed by mating. Mating is known to induce an immune response in a wide variety of vertebrate and invertebrate species (Denison et al. 1999; Birkhead 2000; Lawniczak & Begun 2004; Peng et al. 2005), and is presumably a defence against sexually transmitted pathogens (Nunn et al. 2000; Nunn 2002; Peng et al. 2005). This effect has been clearly demonstrated in female Drosophila melanogaster, where several immune genes are upregulated shortly after mating (Lawniczak & Begun 2004; McGraw et al. 2004; Peng et al. 2005; Fedorka et al. 2007). These observations are also consistent with some recent work in mice and crickets, where mated females exhibited an increased ability to defend themselves against an immunological challenge relative to their virgin counterparts (Johansson et al. 2004; Shoemaker et al. 2006).
However, numerous other studies have found that the effectiveness of the female immune response decreases after mating (Norris & Evans 2000; McKean & Nunney 2001; Rolff & Siva-Jothy 2002; Zuk & Stoehr 2002; Fedorka et al. 2004). This apparent discrepancy may be due to the temporal dynamics of the post-mating immune response (Fedorka et al. 2007). Components of the female's immune system may increase relative to virgin levels soon after mating, as a defence against sexually transmitted pathogens, but then decrease to levels lower than those observed in virgins several hours later to free up resources for other functions (e.g. offspring production). Under this scenario, the relative effectiveness of the female's immune response would depend on when it was assayed. As such, a detailed temporal assessment of the female's post-mating immune response may help resolve this apparent discrepancy in the literature.
Recently, invertebrates have proven to be useful models for ecological immunologists (Kimbrell & Beutler 2001; Kurtz 2004; Schwarzenbach & Ward 2006), in part, because of the simplicity and ubiquity of the innate immune system relative to acquired immunity (Kraaijeveld & Godfray 1997; Moret & Schmid-Hempel 2000; Hoffmann & Reichhart 2002). Moreover, advances in molecular biology, especially in quantitative PCR (Heid et al. 1996; Livak & Schmittgen 2001), now allow for the variation in immune function across groups of hosts to be examined in great detail. The invertebrate innate immune system can be divided roughly into the humoral and cell-mediated responses (Lemaitre & Hoffmann 2007). The humoral response is most effective against prokaryotic pathogens and generally comprises two separate enzymatic cascades known as the Imd and Toll pathways (Kimbrell & Beutler 2001). These pathways produce immunoactive peptides that primarily target gram-negative bacteria and gram-positive bacteria/fungi, respectively (Hultmark 2003). By contrast, the cell-mediated response involves the phagocytosis and haemocyte encapsulation of other potentially pathogenic invaders (Kimbrell & Beutler 2001). Encapsulation entails surrounding a foreign body (often too large to be phagocytized) with haemocytes. Once surrounded, the pro-phenoloxidase enzymatic cascade nullifies the invader through the production of melanin and other toxic compounds (Soderhall & Cerenius 1998). These compounds damage not only the invader but also the host (Nappi et al. 1995). As such, the pro-phenoloxidase cascade is tightly controlled through both positive and negative regulatory proteins (Zou et al. 2005; Lu & Jiang 2007). Because each major component of the innate immune system is most effective against a specific pathogen type, investigators can indirectly measure the specificity of an immune response by monitoring the activity of multiple immune components simultaneously. We refer to this indirect measure of specificity as the immune response's ‘emphasis’.
The purpose of this study was to examine male and female immune investment strategies prior to and after a natural immune challenge with regard to the emphasis, inducibility and timing of the immune response. Using D. melanogaster as our model, we were specifically interested in: (i) determining whether the sexes invest equally across several distinct immune components, or whether they differ in which immune components they emphasize, (ii) determining whether mating induces sex-specific changes in immune component investment, and (iii) providing a detailed temporal assessment of the post-mating immune response in both males and females. Considering that previous studies have suggested that males and females differ in immune component investment (Fedorka et al. 2004), we predicted that sexual differences in emphasis would exist. By contrast, we predicted that the sexes would equally induce the same immune components after copulation, given that the mating immune response is probably a defence against sexually transmitted pathogens (Nunn et al. 2000; Nunn 2002; Peng et al. 2005), and that both sexes need to defend against such pathogens. We further predicted that the cell-mediated immune components would exhibit a greater inducibility compared with the antimicrobial pathways, considering that most known sexually transmitted pathogens of invertebrates are macroscopic parasites (Knell & Webberley 2004; Webberley et al. 2006). Last, we predicted that the previously documented increases in immune function after mating would be transient, and that any heightened investment would soon return to, if not decline below, pre-mating investment levels.
2. Material and methods
(a) Fly stocks and maintenance
The experimental stock used in our study was derived from flies originally collected by Vanessa Corby (University of Georgia) from Macon County, Georgia, USA in 2005. Twenty gravid female flies were collected from the wild and maintained as isofemale lines for 15 generations on a standard cornmeal–yeast–molasses medium. Four individuals from each line (n=80) were then combined to create an outbreeding stock for the next 15 generations prior to being sent to the University of Central Florida. Once the outbred stock arrived, it was maintained as a medium size outbred stock (approx. 200 individuals per generation) on standard cornmeal medium with a 12 L : 12 D photoperiod at 24°C for approximately 12 more generations. Adult flies were separated by sex upon emergence and maintained in fresh vials at a low density (two flies per vial). These flies were then randomly assigned to either our virgin or mated treatment. All flies were 5±0.5 days old at the start of the experiment.
(b) Mating protocols
For our mated treatment, we combined one vial of adult male flies with one vial of adult females without anaesthesia (two females and two males per new vial), where they were allowed to mate for 60 min. Any pairs of flies that failed to mate were removed from the study (mating success was greater than 95%). After mating, males and females were anaesthetized with CO2, separated by sex and placed in fresh vials at medium density (seven flies per vial). At the same time, flies from the virgin treatment were also anaesthetized and placed into similar sex-specific vials. Six hours after mating was completed, the medium density vials from all treatments were again anaesthetized with CO2 and placed into Trizol (Invitrogen). Previous work (Lawniczak & Begun 2004; McGraw et al. 2004; Peng et al. 2005; Fedorka et al. 2007) and preliminary data (W. E. Winterhalter & K. M. Fedorka 2007, unpublished data) suggested that the 6 hour time point would be our best opportunity to observe differences in gene regulation between males and females. Once in Trizol, the flies were homogenized and then stored at −80°C for future RNA extraction, resulting in 5.6±1.3 (s.d.) samples for each of our four treatments (male or female and mated or virgin).
(c) Inducibility and emphasis
In order to assess immune investment, we examined immune gene regulation before and after mating. To obtain a detailed perspective, we divided immune investment into four hierarchical levels (table 1). The first level was the immune system (i.e. the humoral versus the cell-mediated immunity). The second level was the gene's functional group nested within immune system. For the humoral system, this involved genes that primarily target gram-negative bacteria (i.e. the Imd pathway) or those that primarily target gram-positive bacteria and fungi (i.e. the Toll pathway). For the cell-mediated component of the innate immune system, the functional groups were either positive regulators/activators or negative regulators of the pro-phenoloxidase cascade. Nested within these functional groups were the gene families (i.e. attacins, cecropins, etc.) and nested within the gene families were gene paralogues (i.e. attacin A, attacin B, etc.). We assayed a total of 15 genes distributed throughout this hierarchy (table 1). This sampling led to a total of 11 genes from the humoral system (representing 71% of the known humoral gene families that code directly for antimicrobial peptides) and 4 from the cell-mediated system. Because some gene families comprise only a single member (e.g. metchnikowin), our nested design was unbalanced.
Table 1.
Hierarchy of immune investment components.
| immune system | functional group | gene family | paralogue | gene ID |
|---|---|---|---|---|
| humoral | targets gram negative bacteria (Imd pathway) | attacin | attA | CG10146 |
| attB | CG18372 | |||
| attC | CG4740 | |||
| cecropin | crpA1 | CG1365 | ||
| crpB | CG1878 | |||
| crpA2 | CG1376 | |||
| targets gram positive bacteria/fungi (Toll pathway) | metchnikowin | mtk | CG8175 | |
| defensin | def | CG1385 | ||
| drosomycin | dmy1 | CG10810 | ||
| dmy5 | CG10812 | |||
| dmy2 | CG32279 | |||
| cell mediated | positive regulation | serine protease 7 | sp7 | CG3066 |
| pro-phenoloxidase AE | proAE | CG9733 | ||
| negative regulation | hemese | hem | CG31770 | |
| serpin-27A | sp27A | CG11331 |
We estimated the expression levels of these immune genes by first isolating mRNA from our samples using a standard chloroform/isopropanol extraction with a DNase treatment to remove residual genomic DNA. We then reverse transcribed the samples using the Invitrogen Superscript III kit. The resulting cDNA was maintained at −80°C, until real-time qPCR could be performed. Gene expression quantification was accomplished using a Bio-Rad MyIQ single-colour optical detection system and the SybrGreen Supermix (Bio-Rad). All primers were designed from the published sequence (available at www.Flybase.org) using Primer3 (v. 0.4.0) and NetPrimer software. Only primers that exhibited high PCR efficiency (higher than 95%) and no spurious amplification were used. To test primer specificity, we blasted each primer pair against the Drosophila genome (www.ensembl.org), as well as performed a melt curve and agarose gel separation on the PCR product.
In order to determine whether our cDNA samples were contaminated with genomic DNA despite our DNase treatment, we performed two tests. First, we tested the efficacy of our DNase treatment by adding DNase to several cDNA samples and performed the subsequent PCR (with controls). Second, we randomly treated RNA aliquots from our original samples with RNase (after the initial DNase treatment) and performed the subsequent RT and PCR (with controls). Both PCRs failed, indicating that genomic DNA did not contaminate our cDNA samples.
(d) Time series
In order to examine the temporal dynamics of the post-mating immune response in male and female Drosophila, we needed to sample components of the innate immune system over multiple time points. However, because of time and resource limitations, we were able to examine only these dynamics across a subset of genes. As such, we randomly chose one gene from each of the three major innate immune pathways (attacinA–Imd; metchnikowin–Toll; and serine protease 7–pro-phenoloxidase) to assay over multiple time points. Flies were maintained and mated as above. At 3, 6, 12, 24, 48 and 72 hours after mating, a single medium density vial (containing seven flies) from each treatment (i.e. mated and virgins) was chosen at random, the flies were anaesthetized with CO2 and then homogenized in Trizol (Invitrogen). In addition, we collected virgin samples from both sexes just prior to mating (time point 0). Extraction of the mRNA, reverse transcription and quantification of the cDNA was performed in the same manner as above. We had a total of 159 samples distributed among the seven time points and two sexes. Each sample comprised seven flies for a total of 1113 individuals. The samples used for the 6 hour time point were the same as those used for our emphasis and inducibility analysis (see above).
(e) Data analyses
We generated gene expression estimates (dCt) by scaling the target gene's cycle threshold value (Cts) to the control gene's (actin-5) Ct value within each individual sample (i.e. dCti=Ct_controli−Ct_targeti, where i represents the sample). No differences in actin-5 expression levels were found between virgin and mated treatments or between time points.
In our first set of tests, we looked for differences between males and females in emphasis and inducibility at each level of immune investment (i.e. system, function, gene family and gene paralogue; table 1) by performing a mixed-model nested ANOVA on the expression level data collected at the 6 hour time point. sex, mating (i.e. mated or virgin individuals), immune system (i.e. humoral or cell mediated) and functional group nested within immune system were considered fixed factors in this analysis. gene family nested within functional group and gene paralogue nested with gene family were considered random factors.
We were particularly interested in the interactions of this analysis. A significant three-way interaction involving sex, mating and one of the four immune investment levels (i.e. sex×mating×(level)) would be evidence for variation in inducibility across the sexes at that level. For example, a significant sex×mating×gene paralogue (gene family) interaction would indicate that mating induced different transcriptional changes in the sexes for at least some gene paralogues within gene families.
Similarly, a significant two-way interaction between sex and one of the immune investment levels (table 1) would indicate that the level was differentially emphasized by males and females. These interactions averaged gene expression estimates across the mating and virgin treatment groups. For example, a significant sex×gene family (functional group) interaction would indicate that, overall, males and females emphasize different gene families within functional groups.
Finally, a significant two-way interaction involving mating, but not sex, would indicate that the effect of mating varied across that level of immune investment. These interactions average gene expression estimates across the sexes. For example, a significant mating×functional group (immune system) interaction would indicate that the functional groups nested within the two immune systems (i.e. the humoral and cell-mediated system) responded differently to mating.
To obtain a clearer understanding of how inducibility and emphasis differed between males and females (assuming such differences were present), we also performed a series of two-factor fixed-effect ANOVAs for each of the genes we sampled. These analyses provided insight into how the emphasis and inducibility differed between the sexes as well as across immune investment components.
To test for differences in the temporal dynamics of the post-mating immune response, we first compared the virgin expression levels across the seven time points (0, 3, 6, 12, 24, 48 and 72 hours) within each sex using a single-factor ANOVA. No significant differences were detected among these virgin samples (table 2). Therefore, we averaged the virgin target gene dCT values within a given sex prior to the subsequent analyses. We then tested for differences between the virgin (now designated as hour 0) and post-mating (i.e. hours 3–72) expression levels for each gene within each sex using a series of single-factor ANOVAs. To control for the possibility of a type I error, we employed a sequential Dunn–Šidák correction (Sokal & Rohlf 1995) across time points within each gender (k=6). Next, we tested for differences between males and females across all seven time points (hours 0–72) using another set of single-factor ANOVAs. Again, we corrected for the possibility of a type I error by using a sequential Dunn–Šidák correction (k=7). All analyses were performed using SAS v. 9.1 (SAS 2002).
Table 2.
The effect of time (age) on virgin immune gene expression. (Each gene was assayed at 0, 3, 6, 12, 24, 48 and 72 hours after the experimental group mated in order to determine whether a temporal effect on gene expression existed. F-statistics based on single-factor ANOVAs performed separate for each gene and sex. See table 1 for gene abbreviations.)
| sex | gene | F-statistics | significance |
|---|---|---|---|
| female | attA | F6,38=0.80 | p=0.5726 |
| mtk | F6,38=1.36 | p=0.2557 | |
| sp7 | F6,38=0.59 | p=0.7383 | |
| male | attA | F6,32=0.88 | p=0.5230 |
| mtk | F6,32=1.46 | p=0.2217 | |
| sp7 | F6,32=0.82 | p=0.5649 |
3. Results
(a) Emphasis
Rather than one sex consistently investing more across all aspects of immunity, we found that males and females emphasized different aspects of immunity. The expression levels of immune genes were similar (i.e. not significantly different) between males and females when averaged across the entire study (table 3, row B) as well as within immune system components, functional groups and gene families (table 3, rows G–I). However, significant differences between the sexes were detected among gene paralogues nested within gene families (table 3, row J). That is to say, the sexes emphasized different immune genes within a gene family, although no difference in gene family usage could be detected.
Table 3.
Mixed-model nested ANOVA for the relative expression levels (dCts). (Sex (male versus female), mated (virgin versus mated) and immune component (humoral versus cell mediated) were the main effects and function within immune system, gene family within function and paralogue within gene family were the nested effects. Whether a particular effect was fixed (F) or random (R) appears in brackets.)
| source | d.f. | MS | F-ratio | F | p-value |
|---|---|---|---|---|---|
| A. mating- (F) | 1 | 67.3 | A/M | 96.14 | 0.0002 |
| B. sex- (F) | 1 | 17.2 | B/I | 1.30 | 0.3059 |
| C. immune component- (F) | 1 | 7.4 | C/E | 0.04 | 0.8494 |
| D. functional group (imm)- (F) | 2 | 288.3 | D/E | 1.70 | 0.2734 |
| E. gene family (fun)- (R) | 5 | 170.0 | E/F | 0.53 | 0.7486 |
| F. paralogue (fam)- (R) | 6 | 321.0 | F/T | 91.29 | <0.0001 |
| G. sex×imm | 1 | 2.2 | G/I | 0.17 | 0.6972 |
| H. sex×fun (imm) | 2 | 24.3 | H/I | 1.84 | 0.2518 |
| I. sex×fam (fun) | 5 | 13.2 | I/J | 0.52 | 0.7551 |
| J. sex×par (fam) | 6 | 25.2 | J/T | 7.20 | <0.0001 |
| K. mat×imm | 1 | 35.2 | K/M | 50.29 | 0.0009 |
| L. mat×fun (imm) | 2 | 4.4 | L/M | 6.29 | 0.0431 |
| M. mat×fam (fun) | 5 | 0.7 | M/N | 0.58 | 0.7167 |
| N. mat×par (fam) | 6 | 1.2 | N/T | 0.34 | 0.9187 |
| O. sex×mat | 1 | 8.3 | O/R | 4.37 | 0.0908 |
| P. sex×mat×imm | 1 | 0.6 | P/R | 0.32 | 0.5960 |
| Q. sex×mat×fun (imm) | 2 | 1.9 | Q/R | 1.00 | 0.4226 |
| R. sex×mat×fam (fun) | 5 | 1.9 | R/S | 0.90 | 0.5362 |
| S. sex×mat×par (fam) | 6 | 2.1 | S/T | 0.60 | 0.7303 |
| T. error | 281 | 3.5 |
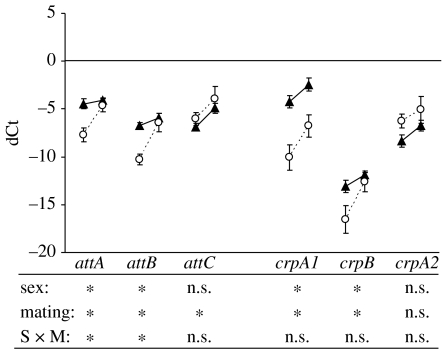
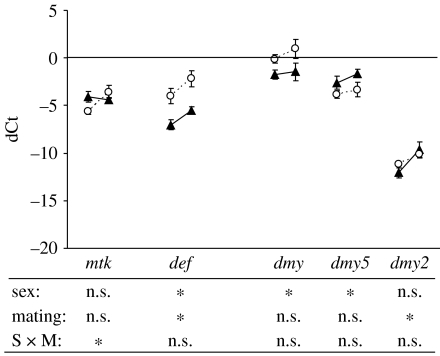
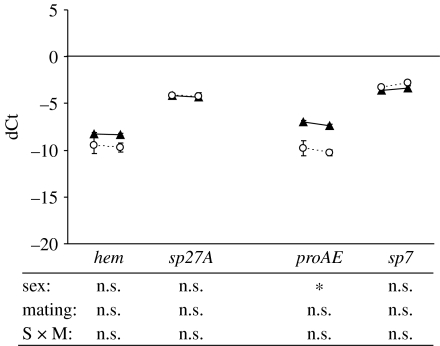
In general, males emphasized genes that were part of the Imd pathway (figure 1). Based on our gene-specific two-way ANOVAs, male expression levels were significantly greater than female levels in four of the six genes examined within this pathway (attA, attB, crpA1 and crpB). The Toll pathway was more variable in terms of immune gene emphasis (figure 2). Here, females had significantly greater expression levels than males for two of the genes examined (def and dmy1), males had greater expression levels for one of the genes (dmy5) and the expression levels of the remaining two genes were not significantly different when averaged across treatments (figure 2). Fewer differences in the emphasis of the immune response were detected within the pro-phenoloxidase cascade (figure 3). The only significant difference detected was for proAE, which was greater for males than females (figure 3).
Figure 1.
Expression patterns (relative to actin-5)±s.e. of six humoral genes from the Imd pathway for males (triangles) and females (circles) as virgins (left data point) and 6 hours after mating (right data point). Below are the significant tests of a two-way model I ANOVA with sex (male versus female) and mating treatment (virgin versus mated) as fixed effects. The asterisks indicate a significant difference at α=0.05. See table 1 for gene abbreviations and table S1 for statistical information.
Figure 2.
Expression patterns (relative to actin-5)±s.e. of five humoral genes from the Toll pathway for males (triangles) and females (circles) as virgins (left data point) and 6 hours after mating (right data point). Below are the significant tests of a two-way model I ANOVA with sex (male versus female) and mating treatment (virgin versus mated) as fixed effects. The asterisks indicate a significant difference at α=0.05. See table 1 for gene abbreviations and table S2 for statistical information.
Figure 3.
Expression patterns (relative to actin-5)±s.e. of four genes from the cell-mediated immune system for males (triangles) and females (circles) as virgins (left data point) and 6 hours after mating (right data point). Below are the significant tests of a two-way model I ANOVA with sex (male versus female) and mating treatment (virgin versus mated) as fixed effects. The asterisks indicate a significant difference at α=0.05. See table 1 for gene abbreviations and table S3 for statistical information.
(b) Inducibility
We found little evidence for differences between males and females in the inducibility of immune investment 6 hours after mating. None of the interactions involving both sex and mating were significant in our overall ANOVA (table 3, rows O–S), although the sex×mating interaction was low (F1,5=4.37, p=0.0908). Despite this lack of significance in our overall model (table 3), our gene-specific two-way ANOVAs did detect a significant sex×mating interaction for 3 of the 15 genes (attA, attB and mtk) we sampled (figures 1 and 2). However, in each of these cases, the p-values did not withstand a correction for multiple tests (attA: F1,21=4.45, p=0.0470; attB: F1,11=5.30, p=0.0418; mtk: F1,21=5.72, p=0.0262).
Although we were unable to detect any significant differences in the inducibility of immune genes between males and females, significant differences in inducibility were detected across the different components of the immune system independent of sex. The mating×immune system and mating×functional group (immune system) interactions of our overall ANOVA were significant (table 3, rows K and L). Nearly all of the genes from the humoral immune system exhibited an upregulation 6 hours after mating (figures 1 and 2). Based on our gene-specific two-way ANOVAs, this upregulation was significant for 7 of the 11 genes sampled (figures 1 and 2). By contrast, none of the genes from the cell-mediated immune system responded significantly to mating (figure 3).
The patterns associated with the significant mating×functional group (immune system) were more complex. Because none of the cell-mediated genes responded significantly to mating at the 6 hour time point (figure 3), the differences between functional groups appeared to be limited to the humoral immune system. Here, the genes that target gram-negative bacteria (i.e. the Imd pathway) generally exhibited a stronger response to mating, then the genes that target gram-positive bacteria and fungi (i.e. the Toll pathway). Based on least-square means, the upregulation of the Imd pathway genes was on average approximately twice that of the genes from the Toll pathway (ddCtimd=2.08±0.06 se; ddCttoll=1.20±0.19), and this difference was significant (t3=3.6; p=0.0376; Both the estimates and statistics were performed using a log2 scale).
(c) Time series
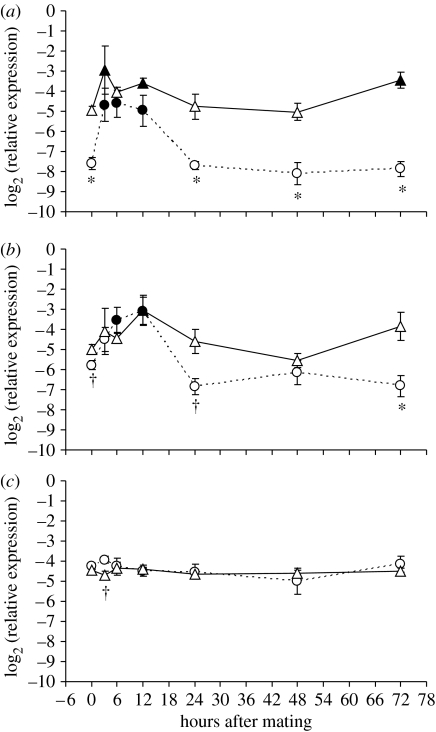
In female D. melanogaster, the expression levels of attA and mtk increased dramatically during the first few hours after mating, but then returned to pre-mating levels 24 hours later (figure 4). By 3 hours after mating, female attA levels were 11.4 times greater than virgin levels, which was significant (F1,51=33.13, p<0.0001). These expression levels decreased steadily after reaching this maximum until 24 hours after mating, at which point they could no longer be differentiated from pre-mating levels statistically (figure 2). These pre-mating levels were then maintained throughout the rest of the time series.
Figure 4.
Expression patterns (relative to actin-5)±s.e. of (a) attacinA (attA), (b) metchnikowin (mtk) and (c) serine protease 7 (sp7) for males (triangles) and females (circles) at several time points after mating. Closed symbols indicate that the expression level was significantly different from virgin levels (i.e. hour 0) within each sex even after a sequential Dunn–Šidák correction. ‘Asterisks’ indicate significant differences as detected across the sexes. ‘Daggers’ indicate that a significant difference was detected prior to a sequential Dunn–Šidák correction, but not after the correction was applied.
Mtk followed a slightly different pattern. Here, post-mating expression levels were only 6.3 times greater than pre-mating levels at their maximum, and this maximum was not reached until the 6 hour time point (F1,54=25.13, p<0.0001). In addition, rather than a steady decrease in expression after the maximum was reached, female mtk levels were maintained at similar levels through the 12 hour time point (figure 4). As with attA, mtk returned to approximately pre-mating levels from 24 to 72 hours after mating (figure 4). However, the expression levels of these later time points were consistently lower than pre-mating levels, although these differences were not significant (figure 4).
As with females, male attA and mtk levels increased in the first few hours after mating and then returned to roughly pre-mating levels by 24 hours (figure 4). However, unlike females, the expression levels of both of these genes did not reach their maximum until the 12 hour time point, and the extent of the upregulation was less dramatic (figure 4). At their maximum, male attA and mtk levels were only 2.9 and 4.9 times greater than pre-mating levels, respectively. In addition, while the expression of both of these genes returned to approximately pre-mating levels at the 24 and 48 hour time points, another significant upregulation was observed 72 hours after mating (attA: F1,42=20.19, p<0.0001; mtk: F1,41=7.66, p=0.0085; figure 2). Here, the expression levels for attA were 3.2 times greater than pre-mating levels, while mtk levels were 2.7 times greater.
Despite the fact that females upregulated both attA and mtk to a greater extent than males in the first few hours after mating, no significant differences were detected between males and females during these time points (figure 4). This interesting observation resulted from the fact that males exhibited significantly higher pre-mating levels for both genes (attA: F1,82=144.09, p<0.0001; mtk: F1,83=7.17, p=0.0089). In other words, male and female expression levels for these genes appeared to be similar in the hours just after mating, despite the significant difference in expression prior to mating.
Unlike attA and mtk, we found almost no differences in the expression levels of sp7 (figure 4). The only exception to this pattern was at the 3 hour time point, in which females exhibited slightly higher, but significantly different expression levels than males (F1,10=5.15, p=0.0466).
4. Discussion
(a) Emphasis
We found strong evidence that males and females emphasized different immune components rather than one sex investing more across all immune components (figures 1–3). Interestingly, the sexes did not emphasize different immune system components (i.e. humoral versus cell-mediated immunity), functional groups or gene families (table 3, rows G–I), but rather different gene paralogues within major gene families. For instance, within the drosomycin gene family, females emphasized dmy1, males emphasized dmy5 and no difference was detected for dmy2 (figure 2). A similar pattern was seen for the cecropins (figure 1). It is unclear why one sex would favour a particular gene paralogue over another, particularly if all members of a given gene family are similarly effective against specific pathogens. Nevertheless, this is an intriguing result that suggests that the sexes might differentially use duplicated genes within a genome.
If members of a single gene family do vary in their effectiveness against specific pathogens, then several possibilities exist that could explain our observations. First, males and females may have different probabilities of being infected by different sexually transmitted pathogens. If the risk of becoming infected varies across the sexes, then we might expect differences in the emphasis of their immune response. Another possibility is that the fitness consequence of infection varies across the sexes. If true, then we might expect differences in the emphasis of their immune response, even if the probability of becoming infected was identical. A third possibility is that damage in the female or male reproductive tract caused by the act of copulation could result in different immune challenges. Thus, the differences observed in our study may simply reflect differences in the cost of copulation between the sexes. Regardless of the mechanism(s), our data suggest that while the immune system defends males and females from similar pathogenic threats, the mechanisms through which these defences are obtained may be different.
An alternative interpretation of our results is that males and females do differentially emphasize other immune components in addition to the gene paralogue component, but we lacked sufficient statistical power to detect such differences. For example, in all of the genes sampled from the Imd pathway, male expression levels were significantly higher or not significantly different from female levels (figure 1). This suggests that males may emphasize defence against gram-negative bacteria more than females, despite the fact that we were unable to demonstrate this observation in the overall statistical model (table 3, row H). Emphasis within the Toll pathway exhibited greater variation, with males and females emphasizing different genes that are effective against gram-positive bacteria and fungi (figure 2). Clearly, the emphasis of the immune system differs between males and females, but the variation among specific genes within each aspect of immune defence makes generalizing those differences difficult. Regardless, our data suggest that attempts to compare ‘overall immune investment’ between the sexes may obscure important differences in immune investment variation, particularly if only a single component of immunity is examined.
(b) Inducibility
While males and females differed in the emphasis of their immune components, we found that the inducibility of the immune system in response to mating was generally similar across the sexes. None of the interactions involving both sex and mating were significant in our overall ANOVA (table 3, rows O–S). These results suggest that the variation in immune response effectiveness frequently observed between the sexes (Zuk & McKean 1996) is more likely due to variation in the emphasis of immune investment rather than its inducibility. By contrast, our gene-specific two-way ANOVAs did find significant mating×sex interactions for 3 of the 15 genes assayed (figure 1: attA and attB; figure 2: mtk). In each of these cases, male expression levels were higher than female levels prior to mating, but females upregulated those levels to a greater degree 6 hours after the mating had occurred. Thus, it appears that males and females respond to mating in a similar overall manner, but that the inducibility of some genes may vary across the sexes.
When inducibility was examined independent of sex, we found that the immune components responded differently to mating. Both immune system (i.e. humoral versus cell mediated) and functional group nested within immune system had a significant interaction with mating (table 3, rows K and L). Most of the genes within the humoral immune system were upregulated 6 hours after mating (figures 1 and 2), while genes within the cell-mediated system (figure 3) did not respond to this immune challenge. This pattern directly contradicts our hypothesis that the cell-mediated immune system would be more responsive to mating because most known sexually transmitted pathogens of arthropods are macroparasites (Knell & Webberley 2004; Webberley et al. 2006). Several possibilities could explain this observation. First, the cell-mediated immune system may be regulated post-transcriptionally. Because our data were based on the relative amounts of mRNA in our samples, any regulatory mechanisms that had acted after transcription would not be reflected in our results. Second, the upregulation of the cell-mediated immune system may occur later than our experimental design could detect. Because macroparasites generally grow more slowly than microparasites, the cell-mediated immune response does not have to be activated as quickly in order to be as effective (Anderson & May 1981). Third, genes other than the four we sampled may be responsible for the post-mating regulation of the cell-mediated immune response. Last, microparasites may be more important as sexually transmitted pathogens of Drosophila than that is currently appreciated.
In addition to finding significant differences between the inducibility of the humoral and cell-mediated systems, we also found significant differences between the two functional groups within the humoral system. In general, genes from the Imd pathway were upregulated to a greater degree than genes from the Toll pathway. If the upregulation of these humoral genes is a defence against sexually transmitted pathogens, our results suggest that gram-negative bacteria may be transferred during mating more frequently than gram-positive bacteria and fungi.
(c) Time series
Generally, our time-series data verified the patterns we observed in the emphasis and inducibility portion of our study. The two humoral genes (attA and mtk) were upregulated during the first few hours after mating in both sexes and then returned to roughly pre-mating levels by the 24 hour time point (figure 4). By contrast, the cell-mediated gene (sp7) had relatively similar levels of expression in both sexes throughout the post-mating response, although a significant difference was detected between males and females at the 3 hour time point (figure 4).
Although most of the post-mating immune response occurred prior to the 24 hour time point, two interesting trends were observed during the later time points. First, mated female levels of mtk (but not attA) were consistently lower than virgin levels at 24, 48 and 72 hours after mating, although these differences were not significant (figure 4). If the expression levels of other immune genes are also reduced 24 hours after mating, then this trend could contribute to the lower effectiveness of the female's immune response that is frequently observed after mating in females (Norris & Evans 2000; Rolff 2002; Zuk & Stoehr 2002; Fedorka et al. 2004), despite the upregulation of these same genes during the first few hours of the response. Second, males exhibited an additional upregulation of attA and mtk during the 72 hour time point (figure 4). These results suggest that males may increase the baseline (i.e. pre-immune challenge) expression levels of at least some of their humoral genes as they age and/or experience additional matings. The effect of age and multiple matings on the expression levels of immune components may provide additional insights into immune allocation strategies of males.
In summary, we found no evidence for the overinvestment in immunity by one sex. Instead, male and female D. melanogaster appear to emphasize different immune components, such as the differential emphasis on paralogous genes within a gene family, or a male-biased investment in gram-negative bacterial defence. Thus, comparisons of ‘overall’ investment between the sexes may obscure evolutionarily important patterns, and future work should focus on which immune components are emphasized by each sex and why. We also found that induction of the post-mating immune response is both transient (lasts approx. 24 hours) and equal between the sexes. Furthermore, we found that the humoral response is more sensitive to mating than the cell-mediated response at the level of gene transcription. Whether or not these patterns are consistent across other systems, as well as other immunological challenges is currently unknown. However, we feel that evaluating the emphasis, specificity and timing of the immune response under a range of alternative contexts will provide important new insights into the field of ecological immunity.
Acknowledgments
We thank Brian Ware for assistance in generating the gene expression data and Daniel Promislow for providing the fly stocks. This work was supported by a National Science Foundation grant (IOS-0922123) to K.M.F.
Supplementary Material
Table S1. Two-way ANOVA of Imd pathway immune genes. Table S2. Two-way ANOVA of Toll pathway immune genes. Table S3. Two-way ANOVA of prophenoloxidase pathway immune genes. Table S4. Descriptive statistics of temporal immune gene expression.
References
- Anderson R.M., May R.M. The population-dynamics of micro-parasites and their invertebrate hosts. Phil. Trans. R. Soc. B. 1981;291:451–524. doi:10.1098/rstb.1981.0005 [Google Scholar]
- Birkhead T.R. Harvard University Press; Cambridge, MA: 2000. Promiscuity: an evolutionary history of sperm competition. [Google Scholar]
- Denison F.C., Grant V.E., Calder A.A., Kelly R.W. Seminal plasma components stimulate interleukin-8 and interleukin-10 release. Mol. Hum. Reprod. 1999;5:220–226. doi: 10.1093/molehr/5.3.220. doi:10.1093/molehr/5.3.220 [DOI] [PubMed] [Google Scholar]
- Fedorka K.M., Mousseau T.A. Immune system activation affects male sexual signal and reproductive potential in crickets. Behav. Ecol. 2007;18:231–235. doi:10.1093/beheco/arl067 [Google Scholar]
- Fedorka K.M., Zuk M., Mousseau T.A. Immune suppression and the cost of reproduction in the ground cricket, Allonemobius socius. Evolution. 2004;58:2478–2485. doi: 10.1111/j.0014-3820.2004.tb00877.x. doi:10.1554/04-399 [DOI] [PubMed] [Google Scholar]
- Fedorka K.M., Linder J.E., Winterhalter W., Promislow D. Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proc. R. Soc. B. 2007;274:1211–1217. doi: 10.1098/rspb.2006.0394. doi:10.1098/rspb.2006.0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvell C.D. The ecology and evolution of inducible defenses. Q. Rev. Biol. 1990;65:323–340. doi: 10.1086/416841. doi:10.1086/416841 [DOI] [PubMed] [Google Scholar]
- Heid C.A., Stevens J., Livak K.J., Williams P.M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. doi:10.1101/gr.6.10.986 [DOI] [PubMed] [Google Scholar]
- Hoffmann J.A., Reichhart J.M. Drosophila innate immunity: an evolutionary perspective. Nat. Immunol. 2002;3:121–126. doi: 10.1038/ni0202-121. doi:10.1038/ni0202-121 [DOI] [PubMed] [Google Scholar]
- Hultmark D. Drosophila immunity: paths and patterns. Curr. Opin. Immunol. 2003;15:12–19. doi: 10.1016/s0952-7915(02)00005-5. doi:10.1016/S0952-7915(02)00005-5 [DOI] [PubMed] [Google Scholar]
- Johansson M., Bromfield J.J., Jasper M.J., Robertson S.A. Semen activates the female immune response during early pregnancy in mice. Immunology. 2004;112:290–300. doi: 10.1111/j.1365-2567.2004.01876.x. doi:10.1111/j.1365-2567.2004.01876.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltz O., Shykoff J.A. Male and female Silene latifolia plants differ in per-contact risk of infection by a sexually transmitted disease. J. Ecol. 2001;89:99–109. doi:10.1046/j.1365-2745.2001.00527.x [Google Scholar]
- Kimbrell D.A., Beutler B. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2001;2:256–267. doi: 10.1038/35066006. doi:10.1038/35066006 [DOI] [PubMed] [Google Scholar]
- Knell R.J., Webberley K.M. Sexually transmitted diseases of insects: distribution, evolution, ecology and host behaviour. Biol. Rev. 2004;79:557–581. doi: 10.1017/s1464793103006365. doi:10.1017/S1464793103006365 [DOI] [PubMed] [Google Scholar]
- Kraaijeveld A.R., Godfray H.C.J. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. doi:10.1038/38483 [DOI] [PubMed] [Google Scholar]
- Kurtz J. Memory in the innate and adaptive immune systems. Microbes Infect. 2004;6:1410–1417. doi: 10.1016/j.micinf.2004.10.002. doi:10.1016/j.micinf.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Lambrechts L., Halbert J., Durand P., Gouagna L.C., Koella J.C. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum. Malar. J. 2005;4:3. doi: 10.1186/1475-2875-4-3. doi:10.1186/1475-2875-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawniczak M.K.N., Begun D.J. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome. 2004;47:900–910. doi: 10.1139/g04-050. doi:10.1139/g04-050 [DOI] [PubMed] [Google Scholar]
- Lee K.A. Linking immune defenses and life history at the levels of the individual and the species. Integr. Comp. Biol. 2006;46:1000–1015. doi: 10.1093/icb/icl049. doi:10.1093/icb/icl049 [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Hoffmann J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. doi:10.1146/annurev.immunol.25.022106.141615 [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu Z.Q., Jiang H.B. Regulation of phenoloxidase activity by high- and low-molecular-weight inhibitors from the larval hemolymph of Manduca sexta. Insect Biochem. Mol. Biol. 2007;37:478–485. doi: 10.1016/j.ibmb.2007.02.004. doi:10.1016/j.ibmb.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw L.A., Gibson G., Clark A.G., Wolfner M.F. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr. Biol. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. doi:10.1016/j.cub.2004.08.028 [DOI] [PubMed] [Google Scholar]
- McKean K.A., Nunney L. Increased sexual activity reduces male immune function in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 2001;98:7904–7909. doi: 10.1073/pnas.131216398. doi:10.1073/pnas.131216398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean K.A., Nunney L. Bateman's principle and immunity: phenotypically plastic reproductive strategies predict changes in immunological sex differences. Evolution. 2005;59:1510–1517. doi:10.1111/j.0014-3820.2005.tb01800.x [PubMed] [Google Scholar]
- Moret Y., Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. doi:10.1126/science.290.5494.1166 [DOI] [PubMed] [Google Scholar]
- Mucklow P.T., Vizoso D.B., Jensen K.H., Refardt D., Ebert D. Variation in phenoloxidase activity and its relation to parasite resistance within and between populations of Daphnia magna. Proc. R. Soc. B. 2004;271:1175–1183. doi: 10.1098/rspb.2004.2707. doi:10.1098/rspb.2004.2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi A.J., Vass E., Frey F., Carton Y. Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Eur. J. Cell Biol. 1995;68:450–456. [PubMed] [Google Scholar]
- Norris K., Evans M.R. Ecological immunology: life history trade-offs and immune defense in birds. Behav. Ecol. 2000;11:19–26. doi:10.1093/beheco/11.1.19 [Google Scholar]
- Nunn C.L. A comparative study of leukocyte counts and disease risk in primates. Evolution. 2002;56:177–190. doi: 10.1111/j.0014-3820.2002.tb00859.x. doi:10.1554/0014-3820(2002)056[0177:ACSOLC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nunn C.L., Gittleman J.L., Antonovics J. Promiscuity and the primate immune system. Science. 2000;290:1168–1170. doi: 10.1126/science.290.5494.1168. doi:10.1126/science.290.5494.1168 [DOI] [PubMed] [Google Scholar]
- Peng J., Zipperlen P., Kubli E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr. Biol. 2005;15:1690–1694. doi: 10.1016/j.cub.2005.08.048. doi:10.1016/j.cub.2005.08.048 [DOI] [PubMed] [Google Scholar]
- Relsen W.K., Hahn D.C. Comparison of immune responses of brown-headed cowbird and related blackbirds to West Nile and other mosquito-borne encephalitis viruses. J. Wildl. Dis. 2007;43:439–449. doi: 10.7589/0090-3558-43.3.439. [DOI] [PubMed] [Google Scholar]
- Rolff J. Bateman's principle and immunity. Proc. R. Soc. B. 2002;269:867–872. doi: 10.1098/rspb.2002.1959. doi:10.1098/rspb.2002.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolff J., Siva-Jothy M.T. Copulation corrupts immunity: a mechanism for a cost of mating in insects. Proc. Natl Acad. Sci. USA. 2002;99:9916–9918. doi: 10.1073/pnas.152271999. doi:10.1073/pnas.152271999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolff J., Siva-Jothy M.T. Invertebrate ecological immunology. Science. 2003;301:472–475. doi: 10.1126/science.1080623. doi:10.1126/science.1080623 [DOI] [PubMed] [Google Scholar]
- SAS. SAS Institute Inc; Cary, NC: 2002. SAS 9.1. [Google Scholar]
- Schmid-Hempel P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005;50:529–551. doi: 10.1146/annurev.ento.50.071803.130420. doi:10.1146/annurev.ento.50.071803.130420 [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P., Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 2003;18:27–32. doi:10.1016/S0169-5347(02)00013-7 [Google Scholar]
- Schwarzenbach G.A., Ward P.I. Responses to selection on phenoloxidase activity in yellow dung flies. Evolution. 2006;60:1612–1621. doi:10.1111/j.0014-3820.2006.tb00506.x [PubMed] [Google Scholar]
- Schwarzenbach G.A., Hosken D.J., Ward P.I. Sex and immunity in the yellow dung fly Scathophaga stercoraria. J. Evol. Biol. 2005;18:455–463. doi: 10.1111/j.1420-9101.2004.00820.x. doi:10.1111/j.1420-9101.2004.00820.x [DOI] [PubMed] [Google Scholar]
- Shoemaker K.L., Parsons N.M., Adamo S.A. Mating enhances parasite resistance in the cricket Gryllus texensis. Anim. Behav. 2006;71:371–380. doi:10.1016/j.anbehav.2005.05.007 [Google Scholar]
- Soderhall K., Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. doi:10.1016/S0952-7915(98)80026-5 [DOI] [PubMed] [Google Scholar]
- Sokal R.R., Rohlf F.J. W.H. Freeman and Co; New York, NY: 1995. Biometry. [Google Scholar]
- Stoehr A.M., Kokko H. Sexual dimorphism in immunocompetence: what does life-history theory predict? Behav. Ecol. 2006;17:751–756. doi:10.1093/beheco/ark018 [Google Scholar]
- Webberley K.M., Buszko J., Isham V., Hurst G.D.D. Sexually transmitted disease epidemics in a natural insect population. J. Anim. Ecol. 2006;75:33–43. doi: 10.1111/j.1365-2656.2005.01020.x. doi:10.1111/j.1365-2656.2005.01020.x [DOI] [PubMed] [Google Scholar]
- Wedekind C., Jakobsen P.J. Male-biased susceptibility to helminth infection: an experimental test with a copepod. Oikos. 1998;81:458–462. doi:10.2307/3546767 [Google Scholar]
- Zou Z., Wang Y., Jiang H.B. Manduca sexta prophenoloxidase activating proteinase-1 (PAP-1) gene: organization, expression, and regulation by immune and hormonal signals. Insect Biochem. Mol. Biol. 2005;35:627–636. doi: 10.1016/j.ibmb.2005.02.004. doi:10.1016/j.ibmb.2005.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M., McKean K.A. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 1996;26:1009–1023. doi:10.1016/S0020-7519(96)00086-0 [PubMed] [Google Scholar]
- Zuk M., Stoehr A.M. Immune defense and host life history. Am. Nat. 2002;160:S9–S22. doi: 10.1086/342131. doi:10.1086/342131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Two-way ANOVA of Imd pathway immune genes. Table S2. Two-way ANOVA of Toll pathway immune genes. Table S3. Two-way ANOVA of prophenoloxidase pathway immune genes. Table S4. Descriptive statistics of temporal immune gene expression.