Abstract
Stinging mechanisms generally deliver venomous compounds to external targets. However, nematocysts, the microscopic stinging organelles that are common to all members of the phylum Cnidaria, occur and act in both external and internal tissue structures. This is the first report of such an internal piercing mechanism. This mechanism identifies prey items within the body cavity of the sea anemone and actively injects them with cytolytic venom compounds. Internal tissues isolated from sea anemones caused the degradation of live Artemia salina nauplii in vitro. When examined, the nauplii were found to be pierced by discharged nematocysts. This phenomenon is suggested to aid digestive phagocytic processes in a predator otherwise lacking the means to masticate its prey.
Keywords: nematocyst, sea anemone, internal stinging mechanism, venom, digestion
1. Introduction
Species of Cnidaria (approx. 10 000) all bear nematocysts, microscopic allomone-delivery systems (Hyman 1940). The nematocyst is a highly specialized secretory structure, loaded with the venom it delivers (Lotan et al. 1995). In cnidarians, as in all other venomous animals, the stinging apparatus actively translocates venom compounds away from the originator and into an external target.
Cnidarian predators use this exquisite mechanical device to capture and subdue prey. For the initial stage of prey capture, they generally rely on tentacles replete with nematocysts to interact with their prey. Tentacular nematocysts discharge in response to appropriate mechanical and chemical stimulation. Penetrable prey are primarily secured to the tentacle by discharged nematocysts and by the inherent stickiness of the tentacle. The nematocyst response results in prey capture by envenomating prey and attaching it to the tentacle. Following prey capture, the feeding response involves the movement of the tentacles towards the mouth and ingestion of prey (Thorington & Hessinger 1996, 1998; Yanagihara et al. 2002). Having caught their prey, cnidarians face the difficulty of digestion, particularly since many marine prey organisms are protected by a hard exterior, e.g. a cuticle, scales, etc. Other predators solve this by using mechanical means for the preliminary disintegration or mastication of prey, e.g. teeth, gizzards or other hard components of their digestive tract. Masticated food is then extracellularly digested into small molecules such as amino acids and simple sugars within the gut lumen; and it is only these small molecules that are absorbed by the cells lining the intestine (Ruppert & Barnes 1996). Cnidarians, however, do not possess such morphological features (Hyman 1940), and both prey digestion and absorption occur in the same general gastrovascular cavity (GVC; van Praet 1985). This same cavity also serves as a circulatory system, takes part in gas exchange and excretion, and forms the hydrostatic skeleton (Fautin & Mariscal 1991). These multiple roles necessitate a periodical exchange of water with the environment, most probably limiting the ability to control the gut chemical–enzymatic properties. This type of gut could, in essence, resemble a direct branch of the outside seawater (SW) environment. Thus, it is reasonable to assume that chemical–enzymatic digestion alone may not be effective in the open environment of the GVC.
Anthozoans, which comprise approximately 60 per cent of cnidarian species (Hyman 1940), possess a venom apparatus within their body in internal, gastrodermal tissue structures (Fautin & Mariscal 1991). These internal structures actively mobilize stinging cells to function externally, in extracoelenteric digestion and competitive interactions (Yonge 1930, 1973; Abe 1938; Goreau et al. 1971). Several authors have hypothesized that anthozoans may also employ internal nematocysts in digestive processes (van Praet 1985; Fautin & Mariscal 1991; Shick 1991). In this study, we provide the first direct evidence to support this hypothesis.
2. Material and methods
(a) Anemone culture
Sea anemones of the species Aiptasia diaphana were cultured in a flow-through SW system. Conditions during the experimental period were set to match natural ambient conditions. The anemones in the culture apparatus were attached to a removable nylon mesh, facilitating their handling. They produced numerous asexual clones, which were subsequently cultured to adult dimensions, enabling rapid and efficient nematocyst harvesting procedures. In order to test the toxicity of these internal nematocysts (microbasic p-mastigophores), we isolated the organelles and assayed their soluble content.
(b) Determination of internal nematocyst function
In order to test the hypothesis of internal nematocyst discharge, three anemone specimens were fed with fry of the fish Sparus auratus. One hour after prey ingestion, the animals were relaxed by adding 10 per cent MgCl2 SW to their medium at ambient temperature, and then cooled to 4°C for 30 min, yielding unresponsive anemones, which fully recuperated if returned to the aquaria. The relaxed anemones were fixed in 4 per cent formaldehyde SW. After 24 hours, the formaldehyde was removed and specimens were rinsed three times in distilled water and placed in 70 per cent ethanol. The fixed specimens were embedded in paraffin and histological sections (5 μm thick) of anemones containing fry within their coelenterons were prepared. The sections were dyed using the Alcian blue–nuclear fast red stain.
(c) Nematocyst isolation and crude venom preparation
Nematocyst isolation was modified from Blanquet & Lenhoff (1966). This method yielded more than 99 per cent microbasic p-mastigophores. Aiptasia diaphana, reared as described above, were induced to eject acontial filaments. In order to isolate internal microbasic p-mastigophore nematocysts, acontia were harvested from several anemone specimens for each analysis. The ejected acontia were cut with stainless steel dissection scissors and siphoned with a Pasteur pipette into a small Petri dish on ice. The accumulated filaments were then transferred to a 50 ml test tube on ice, in a minimal volume of SW. Intact nematocysts were freed from the acontial filaments by transferring 5 ml of the solution to a 25 ml Erlenmeyer flask containing 5 ml of 0.5 M Na3 citrate SW at room temperature. At 0.5 M Na3 citrate SW, the release of nematocysts from the filaments did not appear to cause tissue damage, which could potentially contaminate the final solution of crude venom (CV) with extra-nematocyst active substances. The flask was shaken for 10 min after which the solution was gathered with a syringe and gently injected through a 100 μm nylon mesh held in a 25 mm polypropylene filter holder (Advantec MFS). The filter was changed frequently to avoid the build-up of pressure and to increase the yield of nematocysts, which decreases as tissue build-up on the mesh reduces its filtering capacity. The filtrate was then gathered into 50 ml tubes and kept on ice. The preparation was rinsed twice in 0.5 M Na3 citrate SW by centrifuging at 4°C at 1000g for 60 min. The supernatant was collected for protein content detection and haemolysis assays in order to control the source of assayed activity. The pellet was placed in a 1.5 ml micro test tube on ice; it contained microbasic p-mastigophores and a small fraction (less than 1 per cent) of basitrichous isorhiza nematocysts.
Isolated nematocysts were induced to discharge by adding 3 mM CaCl2 ddH2O to the pellet. Nematocyst discharge was confirmed under a light microscope. The discharged nematocysts were separated from the soluble phase by centrifuging at 10 000g at 4°C for 15 min. Protein concentration was determined in duplicate by the Bradford dye-binding procedure (Bradford 1976). Bovine serum albumin (Promega) was used as a protein standard. A well containing 200 μl Bradford reagent and 10 μl ddH2O served as the blank reference, 3 mM CaCl2 ddH2O used for nematocyst release and the 0.5 M Na3 citrate SW supernatant served as distinct negative controls. Spectrophotometric analysis was carried out on a Spectra Max 190 (Molecular Devices), which was set to read absorbance at 595 nm. The sample concentration was calculated against the standard curve.
(d) SDS-PAGE analysis of nematocyst soluble protein content
Gels were run according to the method of Laemmli (1970) using an Xcell II mini-gel apparatus (Novex). Nematocyst venom extracts were prepared at a concentration of 1 mg ml−1. The venom extracts were separated under non-reducing conditions using a 4 per cent stacking gel and a 12 per cent resolving gel.
The samples were mixed with a sample buffer (1 : 5) containing 0.0625 M Tris, pH 6.8, 10 per cent glycerol, 2 per cent SDS, 0.02 per cent bromophenol blue and loaded onto the gel. The sample volumes were identical. Pre-stained molecular weight markers (Bio-Rad, Hercules, CA) were used as molecular mass standards for each gel. The gels were run at 15 mA per gel at 4°C. Following electrophoresis, the gel was stained with Coomassie blue.
(e) Haemolysis assay system
In order to assay the soluble content of internal nematocysts, a qualitative haemolysis assay was used. The assay was modified from Primor & Zlotkin (1975). Briefly, fresh human blood was obtained from the blood bank at the Hillel Yaffe Medical Centre. Erythrocytes were rinsed by adding 6 ml of phosphate-buffered saline (PBS; 120 mM NaCl +10 mM phosphate buffer, pH 7.4) to 3 ml of blood, mixing well and centrifuging at 2000g for 5 min. The supernatant was removed and the process repeated until a clear supernatant was attained. The pellet of erythrocytes was resuspended in 3 mM CaCl2 PBS (PBSCa) to a final concentration of 20 per cent (v/v). Each assay was performed in a final volume of 500 μl containing 400 μl of the assayed sample in PBSCa (unless otherwise stated) and 100 μl erythrocytes at a final concentration of 4 per cent. All samples were well mixed and incubated for 1 hour at 37°C. Following incubation, 1 ml of PBSCa was added to all samples and they were centrifuged at 3000g for 3 min. Haemolysis was determined by comparing the colour of the supernatant in each assay with that resulting from the incubation of erythrocytes in 400 μl of 3 mM CaCl2 ddH2O. A null level of haemolysis was defined as the colour of the supernatant resulting from the incubation of erythrocytes in 400 μl PBSCa. In order to control the haemolytic effect of the nematocyst CV medium (3 mM CaCl2 ddH2O), erythrocytes were incubated with 380 μl PBSCa +20 μl 3 mM CaCl2 ddH2O. The haemolytic effect of nematocyst CV was tested on erythrocytes incubated in 380 μl PBSCa+20 μl 3 mM CaCl2 ddH2O+1.6 μg CV ml−1. In order to test the effect of CV in a calcium-free medium, 1.6 μg CV ml−1 were incubated and resuspended as above, in calcium-free PBS (table 1).
Table 1.
Haemolysis assay. (Haemolysis (+) was determined by comparing the colour of the supernatant in each assay with the colour of the supernatant resulting from the incubation erythrocytes in 3 mM CaCl2 double distilled water (ddH2O; assay no. 1). A null level of haemolysis (−) was defined as the colour of the supernatant resulting from the incubation of erythrocytes in 3 mM CaCl2 phosphate-buffered saline (PBSCa; assay no. 2). Assay no. 3 tested the nematocyst crude venom (CV) medium. Assay no. 4 tested the CV itself. Assay no. 5 tested the effect of CV in a calcium-free medium PBS.)
| assay | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| ddH2O | PBSCa | PBSCa+CV medium | PBSCa+CV | PBS+CV | |
| haemolysis | (+) | (−) | (−) | (+) | (−) |
(f) Testing the effect of acontial filaments on prey
Acontial filaments were collected and soaked in toluidine blue SW solution in order to load their nematocysts with dye. They were then rinsed several times with SW until the surrounding medium was clear. The stained acontia were transferred to a glass Petri dish containing 10 ml SW. Artemia salina nauplii were incubated with the filaments at 24°C for 12 hours. A control derived from the same batch of hatched nauplii was placed in a 10 ml (SW) Petri dish. In order to test the effect of toluidine blue on the nauplii, they were incubated in a blue toluidine SW solution. The treated nauplii and controls were sampled and photographed with a Nikon DS-5M digital camera mounted on a Reichert microscope.
3. Results
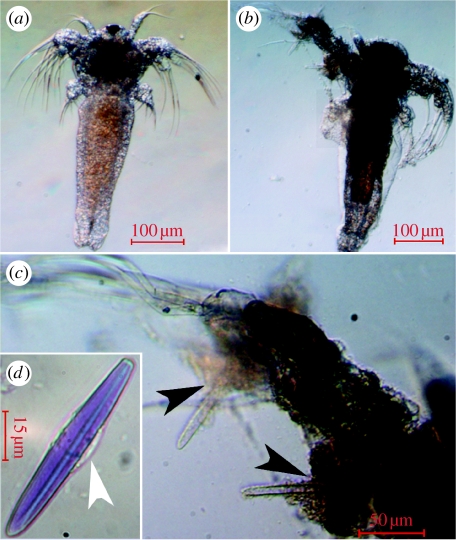
Following ingestion (figure 1a), ciliated acontial filaments appeared to wrap around the ingested prey in the sea anemone's GVC (figure 1b). Histological examination of this interaction revealed that internal nematocysts (acontial microbasic p-mastigophores, identified by a capsule length of 60 μm) had pierced and penetrated the ingested prey (figure 1c). The solutes released from these nematocysts through discharge contained at least five different protein components, ranging in size between approximately 10 and approximately 70 kDa (figure 1d). A double band is clearly depicted approximately 45 kDa (arrow). This result corresponds with the α (45 000 MW) and β (43 000 MW) phospholipase A2 (PA2) isoforms found in the acontial microbasic p-mastigophores of the congener Aiptasia pallida (Grotendorst & Hessinger 1999). The nematocyst solutes were haemolytic, and similarly to PA2 found in the acontial microbasic p-mastigophores of A. pallida (Hessinger et al. 1973), haemolytic activity was calcium dependent, i.e. the venom showed no haemolytic activity in a calcium-free medium (table 1).
Figure 1.
The sea anemone Aiptasia diaphana feeding upon a fry of the fish Lates calcarifer. (a) The fish's head (H) is facing the anemone's pedal disc, its tail (T) is located between the anemone pharynx and the body wall. (b) Acontial filaments wrap around the ingested prey and the arrows depict distinct filaments. (c) Nematocyst–prey interaction in the coelenteron. Acontial microbasic p-mastigophore capsules (CA) aligned adjacent to the prey's scale (SC). The discharged tubules (TU) are embedded within prey muscle tissue (MU). The black arrows depict points of tubule penetration into the prey (scale bar, 10 μm). (d) SDS-PAGE analysis of acontial microbasic p-mastigophore nematocyst soluble protein content. (i) Molecular weight markers and (ii) nematocyst venom soluble protein components.
In vitro, the stained acontia stained the nauplii and had a lethal, degrading effect on them (figure 2). The nauplii in both control treatments were viable and intact following the 12 hours incubation, and the control nauplii incubated in toluidine blue stained blue.
Figure 2.
Acontial filaments disintegrate Artemia salina tissue. (a) Control treatment nauplii after 12 hours incubation in SW (scale bar, 100 μm). (b) After 12 hours incubation with stained acontial filaments, A. salina nauplius is partially disintegrated and its coloration is darker than the control (scale bar, 100 μm). (c) Tissue disintegration is evident adjacent to clear (discharged) nematocyst capsules (black arrows; scale bar, 50 μm). (d) Toluidine binds to and stains the acontial microbasic p-mastigophore capsule matrix and tubule but not to the cytoplasm-containing girdle (white arrow; scale bar, 15 μm). The dark coloration in (b) is probably due to nematocyst injection of toluidine blue into the prey.
4. Discussion
Envenoming of prey involves the delivery of complex mixtures of bioactive compounds, ultimately enabling digestion of the prey by gastric enzymes. Nematocysts consist of a capsule containing a highly folded eversible tubule. The capsule is filled with a matrix of charged γ-glutamate polymers and cations, generating a high internal pressure (15 MPa) that drives discharge at accelerations reaching 5.41×106 g (g=9.81 m s−2). Thus, the estimated pressure at the point of impact is more than 7 GPa, which is similar to that exerted by technical bullets (Nuchter et al. 2006). Our results provide the first direct evidence that envenoming of prey by this mechanism occurs within the body cavity (figure 1).
Microbasic mastigophore nematocysts are prevalent elements of internal tissue structures (i.e. acontia and mesenterial filaments) in all hexacorallian orders (Hexacorallia=Zoantharia; a subclass of Anthozoa; currently containing six orders; Pires & Castro 1997; Sebens 1998; Daly et al. 2003). To date, the function of acontial microbasic mastigophore nematocysts has not been definitively established, although the fact that acontia are often extruded in reaction to disturbance has supported a role in defence (Shick 1991). While acontial filaments are found in Acontiaria, a clade of actinians (sea anemones), and in Ceriantharia (Fautin & Mariscal 1991), the most basal order within Hexacorallia (Daly et al. 2003), mesenterial filaments are ubiquitous in Hexacorallia. Similarly to acontia, these internal structures can be extruded. They may be used externally for agonistic or feeding purposes (Rinkevich & Loya 1983; Goldberg 2002), yet they are presumed to be primarily digestive in function (van Praet 1985; Fautin & Mariscal 1991).
The tissue-disrupting effect of acontial filaments on A. salina (figure 2) was probably mediated by the cytolytic effect of nematocyst venom (table 1), as seen by the uptake of the dye toluidine blue (figure 2a,b) and tissue disintegration in the region of discharged nematocyst capsules (figure 2c). Prey disintegration into small particles in the GVC is advantageous, as sea anemone digestion occurs within the phagocytic endodermal digestive cells along the mesenteries (van Praet 1985). The beating of the mesentery cilia creates a circulating flow that manoeuvres food particles towards these cells (Ruppert & Barnes 1996).
Our results support the hypothesis (van Praet 1985; Fautin & Mariscal 1991; Shick 1991) that nematocysts in the acontia and perhaps in mesenterial filaments, in general, have a role in digestion in the Anthozoa. We suggest that cnidarians employ cytolytic venoms such as PA2, a widespread cytolytic cnidarian venom (Nevalainen et al. 2004), within the GVC in order to induce disintegration of the prey, ultimately aiding digestive processes. One problem faced by all organisms is how to protect themselves from the activity of their own digestive enzymes. While all cnidarians bear nematocytes, hydrozoans lack internal nematocysts and complex internal anatomy (Hyman 1940; Ruppert & Barnes 1996). Concomitantly, hydrozoan species show a higher degree of functional specialization than anthozoans, functionally and spatially separating digestive and reproductive processes (Beklemishev 1969; Dunn & Wagner 2006). Indeed, Sher et al. (2008) have recently shown that the hydrozoan Chlorohydra viridissima employs an extra-nematocyst pore-forming protein within its GVC to aid in digestive processes. Based on expressed sequence tag analyses and the targeted study of specific gene families within Cnidaria, the depth of the Anthozoa–Hydrozoa split is comparable to the protostome–deuterostome divergence, emphasizing the distant relationship between them (Putnam et al. 2007). Thus, it appears that the two classes may differ in their digestive physiology as well. Localization of venom activity within the body cavity in the Anthozoa, by means of a piercing delivery system, may therefore serve in protecting other vital functions that occur in the GVC, such as reproductive processes (Fautin, 1990), i.e. gametogenesis and brooding of propagules.
Acknowledgements
We are grateful to D. Pargament for her initiative and support at the Marine Laboratory facilities. We thank I. Brickner for his help with the histological work. The critical reading and constructive suggestions of three anonymous reviewers are gratefully acknowledged. This research was supported by the Israel Science Foundation (ISF) and the Raynor Chair for Environmental Conservation Research to Y.L.
References
- Abe N. Feeding behaviour and the nematocyst of Fungia and 15 other species of corals. Stud. Palao Trop. Biol. Stn. 1938;1:469–521. [Google Scholar]
- Beklemishev W.N. Oliver and Boyd; Edinburgh, UK: 1969. Principles of comparative anatomy. [Google Scholar]
- Blanquet R., Lenhoff H. A disulphide linked collagenous protein of nematocysts capsules. Science. 1966;154:152–153. doi: 10.1126/science.154.3745.152. doi:10.1126/science.154.3745.152 [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. doi:10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Daly M., Fautin D.G., Cappola V.A. Systematics of the hexacorallia (Cnidaria: Anthozoa) Zool. J. Linn. Soc. 2003;139:419–437. doi:10.1046/j.1096-3642.2003.00084.x [Google Scholar]
- Dunn C., Wagner G.n. The evolution of colony-level development in the Siphonophora (Cnidaria: Hydrozoa) Dev. Genes Evol. 2006;216:743–754. doi: 10.1007/s00427-006-0101-8. doi:10.1007/s00427-006-0101-8 [DOI] [PubMed] [Google Scholar]
- Fautin D.G. Cnidaria. In: Adiyodi K.G., Adiyodi R.G., editors. Reproductive biology of invertebrates. vol. 5. Oxford and I.B.H; New Delhi, India: 1990. pp. 31–55. [Google Scholar]
- Fautin D.G., Mariscal R.N. Cnidaria: Anthozoa. In: Harrison F.W., Westfall J.A., editors. Microscopic anatomy of invertebrates. vol. 2. Wiley; New York, NY: 1991. pp. 267–358. [Google Scholar]
- Goldberg W.M. Gastrodermal structure and feeding responses in the scleractinian coral Mycetophyllia reesi, a coral with novel digestive filaments. Tissue & Cell. 2002;34:246–261. doi: 10.1016/s0040-8166(02)00008-3. doi:10.1016/S0040-8166(02)00008-3 [DOI] [PubMed] [Google Scholar]
- Goreau T.F., Goreau N.I., Yonge C.M. Reef corals: autotrophs or heterotrophs? Biol. Bull. 1971;141:247–260. doi:10.2307/1540115 [Google Scholar]
- Grotendorst G.R., Hessinger D.A. Purification and partial characterization of the phospholipase A2 and co-lytic factor from sea anemone (Aiptasia pallida) nematocyst venom. Toxicon. 1999;37:1779–1796. doi: 10.1016/s0041-0101(99)00120-8. doi:10.1016/S0041-0101(99)00120-8 [DOI] [PubMed] [Google Scholar]
- Hessinger D.A., Lenhoff H.M., Kahan L.B. Haemolytic, phospholypase A and nerve affecting activities of sea anemone nematocyst venom. Nat. (Lond.) New Biol. 1973;241:125–127. doi: 10.1038/newbio241125b0. [DOI] [PubMed] [Google Scholar]
- Hyman, L. H. 1940 Protozoa through Ctenophora. In The invertebrates, vol. 1. New York, NY: McGraw-Hill.
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. doi:10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Lotan A., Fishman L., Loya Y., Zlotkin E. Delivery of a nematocyst toxin. Nature. 1995;375:456. doi: 10.1038/375456a0. doi:10.1038/375456a0 [DOI] [PubMed] [Google Scholar]
- Nevalainen T.J., Peuravuori H.J., Quinn R.J., Llewellyn L.E., Benzie J.A.H., Fenner P.J., Winkel K.D. Phospholipase A2 in Cnidaria. Comp. Biochem. Physiol. Biochem. Mol. Biol. 2004;139:731–735. doi: 10.1016/j.cbpc.2004.09.006. doi:10.1016/j.cbpc.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Nuchter T., Benoit M., Engel U., Ozbek S., Holstein T.W. Nanosecond-scale kinetics of nematocyst discharge. Curr. Biol. 2006;16:R316–R318. doi: 10.1016/j.cub.2006.03.089. doi:10.1016/j.cub.2006.03.089 [DOI] [PubMed] [Google Scholar]
- Pires D.O., Castro C.B. Scleractinia and corallimorpharia: an analysis of cnidae affinity. In: Lessios H.A., Macintyre I.G., editors. Proc. Eighth Int. Coral Reef Symposium. Smithsonian Tropical Research Institute; Republic of Panama: 1997. pp. 1581–1586. [Google Scholar]
- Primor N., Zlotkin E. On the ichthyotoxic and hemolytic action of the skin secretion of the flatfish Pardachirus marmoratus (Soleidae) Toxicon. 1975;13:227–231. doi: 10.1016/0041-0101(75)90128-2. doi:10.1016/0041-0101(75)90128-2 [DOI] [PubMed] [Google Scholar]
- Putnam N.H., et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. doi:10.1126/science.1139158 [DOI] [PubMed] [Google Scholar]
- Rinkevich B., Loya Y. Intraspesific competitive networks in the Red Sea coral Stylophora pistillata. Coral Reefs. 1983;1:161–172. doi:10.1007/BF00571193 [Google Scholar]
- Ruppert E.E, Barnes D.R. Harcourt College Publishers; Orlando, FL: 1996. Invertebrate zoology. [Google Scholar]
- Sebens, K. 1998. Marine flora and fauna of the eastern United States, Anthozoa: Actniaria, Corallimorpharia, Ceriantharia, and Zoanthidea. NOAA Technical report NMFS 141
- Sher D., Fishman Y., Melamed-Book N., Zhang M., Zlotkin E. Osmotically driven prey disintegration in the gastrovascular cavity of the green hydra by a pore-forming protein. FASEB J. 2008;22:207–214. doi: 10.1096/fj.07-9133com. doi:10.1096/fj.07-9133com [DOI] [PubMed] [Google Scholar]
- Shick J.M. Chapman & Hall; New York, NY: 1991. A functional biology of sea anemones. [Google Scholar]
- Thorington G.A., Hessinger D.A. Efferent mechanisms in discharging cnidae. 1. Measurements of intrinsic adherence of cnidae discharged from the tentacles of the sea anemone Aiptasia pallida. Biol. Bull. 1996;190:125–138. doi: 10.2307/1542681. doi:10.2307/1542681 [DOI] [PubMed] [Google Scholar]
- Thorington G.A., Hessinger D.A. Efferent mechanisms in discharging cnidae. 2. A nematocyst release response in sea anemone tentacle. Biol. Bull. 1998;195:145–155. doi: 10.2307/1542822. doi:10.2307/1542822 [DOI] [PubMed] [Google Scholar]
- van Praet M. Nutrition of sea anemones. Adv. Mar. Biol. 1985;22:65–69. doi:10.1016/S0065-2881(08)60050-4 [Google Scholar]
- Yanagihara A.A., Kuroiwa J.M.Y., Oliver L.M., Kunkel D.D. The ultrastructure of nematocysts from the fishing tentacle of the Hawaiian bluebottle, Physalia utriculus (Cnidaria, Hydrozoa, Siphonophora) Hydrobiologia. 2002;489:139–150. doi:10.1023/A:1023272519668 [Google Scholar]
- Yonge C.M. Studies on the physiology of corals. I. Feeding mechanisms and food. Sci. Rep. Great Barrier Reef Exped. 1930;1:13–57. [Google Scholar]
- Yonge C.M. The nature of reef building (hermatypic) corals. Bull. Mar. Sci. 1973;23:1–15. [Google Scholar]