Abstract
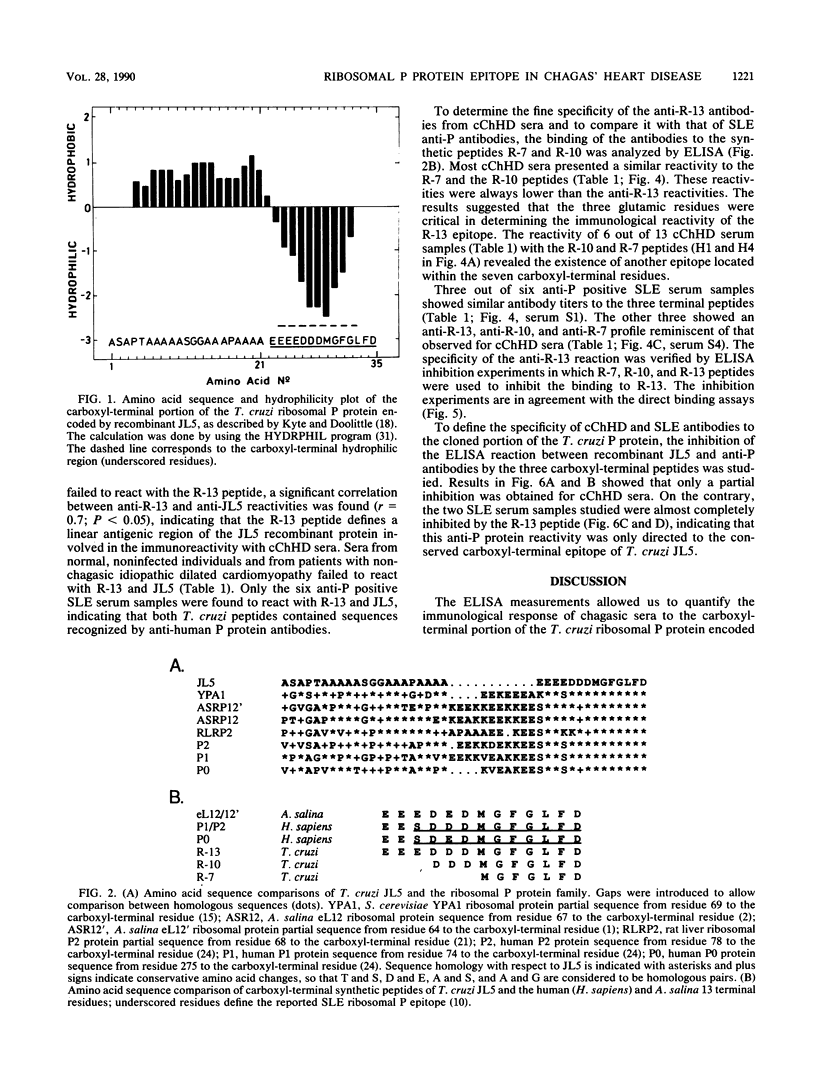
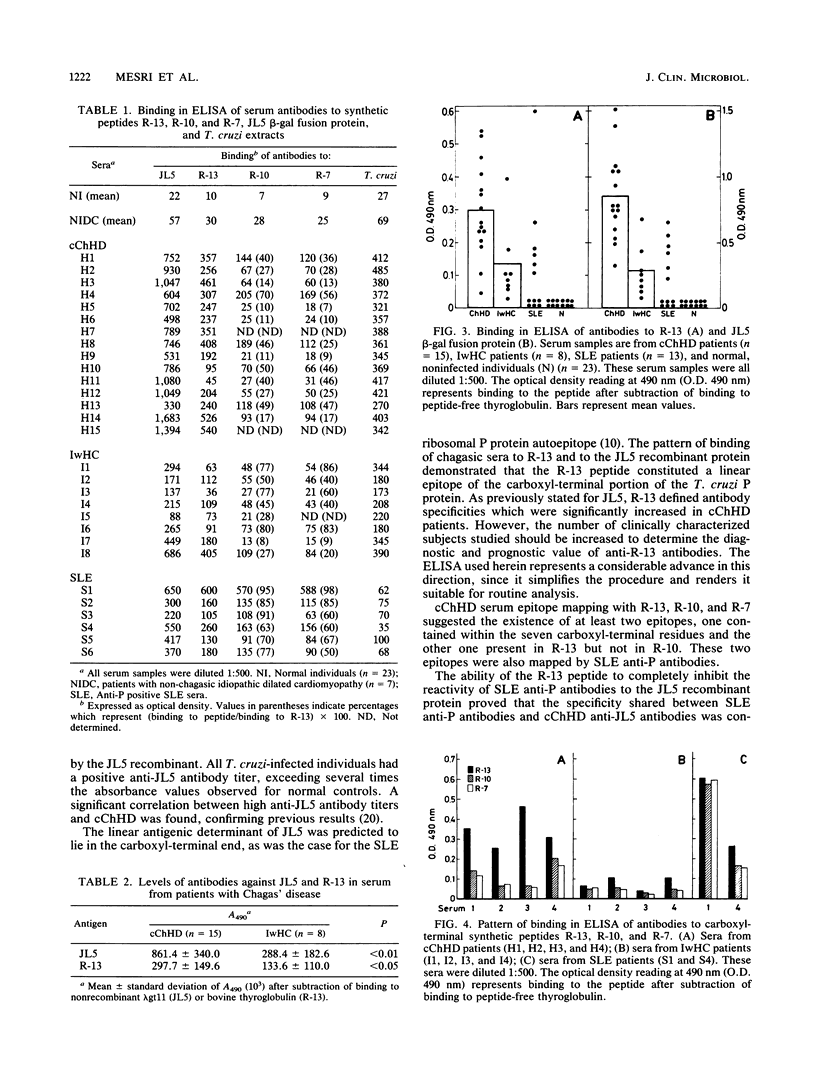
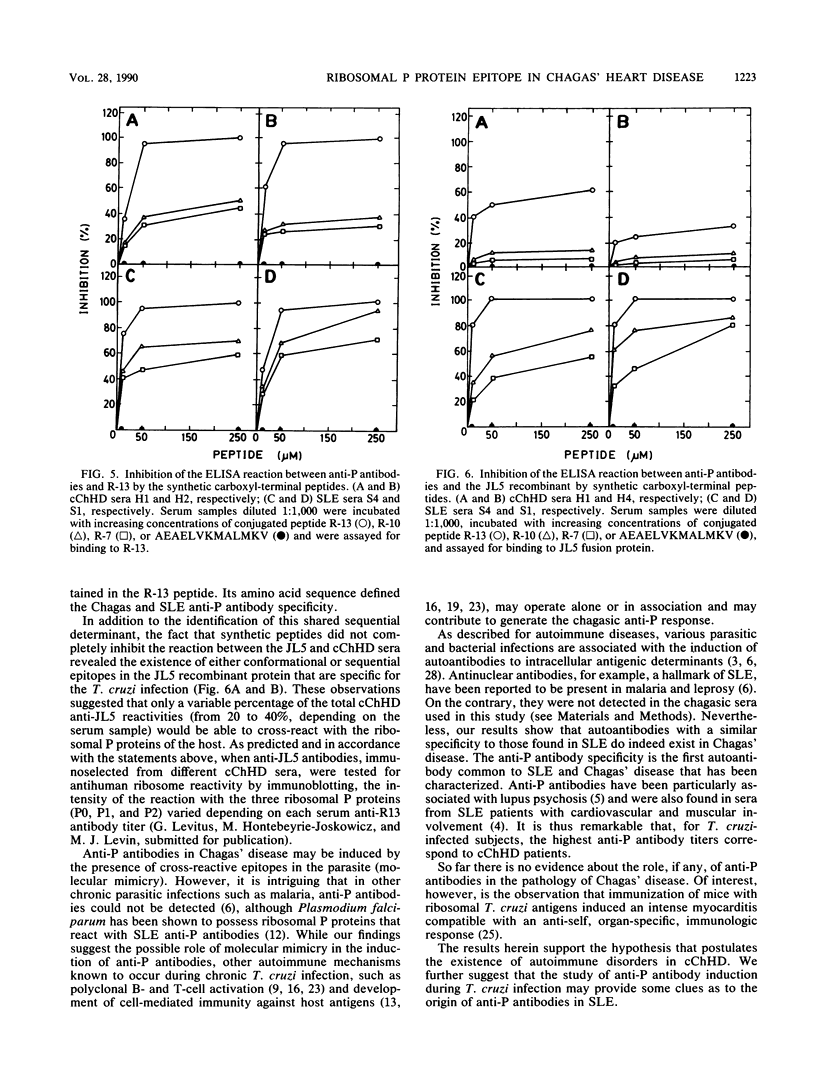
A Trypanosoma cruzi lambda gt11 cDNA clone, JL5, expressed a recombinant protein which was found to react predominantly with chronic Chagas' heart disease sera. The cloned 35-residue-long peptide was identified as the carboxyl-terminal portion of a T. cruzi ribosomal P protein. The JL5 13 carboxyl-terminal residues shared a high degree of homology with the systemic lupus erythematosus (SLE) ribosomal P protein epitope. Synthetic peptides comprising the 13 (R-13), 10 (R-10), and 7 (R-7) carboxyl-terminal residues of the JL5 protein were used to study, by enzyme-linked immunosorbent assay, the specificity of the Chagas' disease anti-JL5 and SLE anti-P antibodies. The R-13 peptide defined a linear antigenic determinant of the JL5 recombinant protein. As was proved for JL5, R-13 defined antibody specificities which were significantly increased in chronic Chagas' heart disease patients. Only SLE anti-P positive sera were found to react with JL5 and R-13. Fine epitope mapping showed that Chagas' disease anti-JL5 and SLE anti-P antibodies define similar epitopes within the R-13 peptide. The binding of the SLE sera to JL5 was completely blocked by the R-13 peptide, indicating that the shared specificity between anti-JL5 and anti-P autoantibodies was exclusively limited to the conserved linear epitope(s) within the R-13 peptide. The prevalence of high anti-R-13 antibody titers in Chagas' heart disease patients supports the hypothesis that postulates the existence of autoimmune disorders in Chagas' heart disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R., Pluijms W., Möller W. The primary structure of ribosomal protein eL12/eL12-P from Artemia salina 80 S ribosomes. FEBS Lett. 1979 Aug 1;104(1):85–89. doi: 10.1016/0014-5793(79)81089-3. [DOI] [PubMed] [Google Scholar]
- Argov S., Jaffe C. L., Krupp M., Slor H., Shoenfeld Y. Autoantibody production by patients infected with Leishmania. Clin Exp Immunol. 1989 May;76(2):190–197. [PMC free article] [PubMed] [Google Scholar]
- Bonfa E., Elkon K. B. Clinical and serologic associations of the antiribosomal P protein antibody. Arthritis Rheum. 1986 Aug;29(8):981–985. doi: 10.1002/art.1780290806. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Golombek S. J., Kaufman L. D., Skelly S., Weissbach H., Brot N., Elkon K. B. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987 Jul 30;317(5):265–271. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Llovet R., Scheinberg M., de Souza J. M., Elkon K. B. Comparison between autoantibodies in malaria and leprosy with lupus. Clin Exp Immunol. 1987 Dec;70(3):529–537. [PMC free article] [PubMed] [Google Scholar]
- Briand J. P., Muller S., Van Regenmortel M. H. Synthetic peptides as antigens: pitfalls of conjugation methods. J Immunol Methods. 1985 Apr 8;78(1):59–69. doi: 10.1016/0022-1759(85)90329-1. [DOI] [PubMed] [Google Scholar]
- Elkon K., Bonfa E., Llovet R., Danho W., Weissbach H., Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5186–5189. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K., Skelly S., Parnassa A., Moller W., Danho W., Weissbach H., Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7419–7423. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoeur A. M., Peebles C. L., Heckman K. J., Lee J. C., Tan E. M. Identification of ribosomal protein autoantigens. J Immunol. 1985 Oct;135(4):2378–2384. [PubMed] [Google Scholar]
- Hontebeyrie-Joskowicz M., Said G., Milon G., Marchal G., Eisen H. L3T4+ T cells able to mediate parasite-specific delayed-type hypersensitivity play a role in the pathology of experimental Chagas' disease. Eur J Immunol. 1987 Jul;17(7):1027–1033. doi: 10.1002/eji.1830170720. [DOI] [PubMed] [Google Scholar]
- Itoh T. Primary structure of an acidic ribosomal protein YPA1 from Saccharomyces cerevisiae. Isolation and characterization of peptides and the complete amino acid sequence. Biochim Biophys Acta. 1981 Nov 30;671(1):16–24. doi: 10.1016/0005-2795(81)90088-x. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F. Autoimmunity in Chagas' disease. J Parasitol. 1986 Apr;72(2):201–211. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laguens R. P., Meckert P. C., Chambó J. G. Antiheart antibody-dependent cytotoxicity in the sera of mice chronically infected with Trypanosoma cruzi. Infect Immun. 1988 Apr;56(4):993–997. doi: 10.1128/iai.56.4.993-997.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. J., Mesri E., Benarous R., Levitus G., Schijman A., Levy-Yeyati P., Chiale P. A., Ruiz A. M., Kahn A., Rosenbaum M. B. Identification of major Trypanosoma cruzi antigenic determinants in chronic Chagas' heart disease. Am J Trop Med Hyg. 1989 Nov;41(5):530–538. doi: 10.4269/ajtmh.1989.41.530. [DOI] [PubMed] [Google Scholar]
- Lin A., Wittmann-Liebold B., McNally J., Wool I. G. The primary structure of the acidic phosphoprotein P2 from rat liver 60 S ribosomal subunits. Comparison with ribosomal 'A' proteins from other species. J Biol Chem. 1982 Aug 10;257(15):9189–9197. [PubMed] [Google Scholar]
- Muller S., Couppez M., Briand J. P., Gordon J., Sautière P., van Regenmortel M. H. Antigenic structure of histone H2B. Biochim Biophys Acta. 1985 Mar 1;827(3):235–246. doi: 10.1016/0167-4838(85)90208-0. [DOI] [PubMed] [Google Scholar]
- Petry K., Eisen H. Chagas disease: a model for the study of autoimmune diseases. Parasitol Today. 1989 Apr;5(4):111–116. doi: 10.1016/0169-4758(89)90052-5. [DOI] [PubMed] [Google Scholar]
- Rich B. E., Steitz J. A. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol Cell Biol. 1987 Nov;7(11):4065–4074. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A. M., Esteva M., Cabeza Meckert P., Laguens R. P., Segura E. L. Protective immunity and pathology induced by inoculation of mice with different subcellular fractions of Trypanosoma cruzi. Acta Trop. 1985 Dec;42(4):299–309. [PubMed] [Google Scholar]
- Sadigursky M., Acosta A. M., Santos-Buch C. A. Muscle sarcoplasmic reticulum antigen shared by a Trypanosoma cruzi clone. Am J Trop Med Hyg. 1982 Sep;31(5):934–941. doi: 10.4269/ajtmh.1982.31.934. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A. Mycobacteria and autoimmunity. Immunol Today. 1988 Jun;9(6):178–182. doi: 10.1016/0167-5699(88)91294-7. [DOI] [PubMed] [Google Scholar]
- Szarfman A., Terranova V. P., Rennard S. I., Foidart J. M., de Fatima Lima M., Scheinman J. I., Martin G. R. Antibodies to laminin in Chagas' disease. J Exp Med. 1982 Apr 1;155(4):1161–1171. doi: 10.1084/jem.155.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Ramjoué H. P., Kuster H., Liverani D., Gordon J. Monoclonal antibodies against eucaryotic ribosomes. Use to characterize a ribosomal protein not previously identified and antigenically related to the acidic phosphoproteins P1/P2. J Biol Chem. 1982 Nov 10;257(21):12709–12715. [PubMed] [Google Scholar]
- Van Voorhis W. C., Eisen H. Fl-160. A surface antigen of Trypanosoma cruzi that mimics mammalian nervous tissue. J Exp Med. 1989 Mar 1;169(3):641–652. doi: 10.1084/jem.169.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Imperio Lima M. R., Eisen H., Minoprio P., Joskowicz M., Coutinho A. Persistence of polyclonal B cell activation with undetectable parasitemia in late stages of experimental Chagas' disease. J Immunol. 1986 Jul 1;137(1):353–356. [PubMed] [Google Scholar]


