Abstract
California killifish (Fundulus parvipinnis) infected with the brain-encysting trematode Euhaplorchis californiensis display conspicuous swimming behaviours rendering them more susceptible to predation by avian final hosts. Heavily infected killifish grow and reproduce normally, despite having thousands of cysts inside their braincases. This suggests that E. californiensis affects only specific locomotory behaviours. We hypothesised that changes in the serotonin and dopamine metabolism, essential for controlling locomotion and arousal may underlie this behaviour modification. We employed micropunch dissection and HPLC to analyse monoamine and monoamine metabolite concentrations in the brain regions of uninfected and experimentally infected fish. The parasites exerted density-dependent changes in monoaminergic activity distinct from those exhibited by fish subjected to stress. Specifically, E. californiensis inhibited a normally occurring, stress-induced elevation of serotonergic metabolism in the raphae nuclei. This effect was particularly evident in the experimentally infected fish, whose low-density infections were concentrated on the brainstem. Furthermore, high E. californiensis density was associated with increased dopaminergic activity in the hypothalamus and decreased serotonergic activity in the hippocampus. In conclusion, the altered monoaminergic metabolism may explain behavioural differences leading to increased predation of the infected killifish by their final host predators.
Keywords: parasites, fish, dopamine, serotonin, monoamine, trematode
1. Introduction
Certain parasites manipulate their host's behaviour in dramatic ways. Typically, altered behaviours appear to increase parasite fitness by enhancing the likelihood of transmission between hosts in the parasite's life cycle (Holmes & Bethel 1972; Barber et al. 2000; Combes 2001; Moore 2002; Kuris 2003; reviews in Thomas et al. 2005). Predator hosts often consume a higher proportion of infected prey—whose manipulated behaviours render them easier to catch—a phenomenon termed parasite-increased trophic transmission (PITT; Carney 1969; Holmes & Bethel 1972; Brassard et al. 1982; Hoogenboom & Dijkstra 1987; Lafferty 1992; Lafferty & Morris 1996; Bakker et al. 1997; Thomas & Poulin 1998; Lafferty 1999; McCurdy et al. 1999; Berdoy et al. 2000; Knudsen et al. 2001). From both a proximate and an ultimate perspective, it remains of considerable interest whether the behavioural changes of the infected prey result from the side effects of general debilitation, orwhether the parasites actively target the host's neuroendocrine systems (reviewed in Thomas et al. 2005).
Recent studies have revealed possible neurobiological mechanisms of behaviour modification by parasites (Kavaliers et al. 1999; Thomas et al. 2005). The manipulation of invertebrate hosts involves parasite-secreted molecules (Beckage 1993; de Jong-Brink et al. 2001; Biron et al. 2005), and neuroanatomical and monoaminergic disruption (Adamo & Shoemaker 2000; Helluy & Thomas 2003; Thomas et al. 2003; Rojas & Ojeda 2005; Tain et al. 2006), including reduced activity of monoaminergic neurons (Rosenberg et al. 2006). There are fewer studies on vertebrates (see Klein (2003) for a review). Rodents infected with Toxoplasma gondii show increased exploratory behaviour and an attraction to cat odours, which could greatly increase T. gondii transmission to its feline final host (Berdoy et al. 2000; Vyas et al. 2007). T. gondii-infected mice display increased brain dopamine (DA) levels (Stibbs 1985), and administration of a specific DA receptor agonist can induce similar behaviours in uninfected mice (Skallova et al. 2006). Stickleback fishes infected with larval tapeworms (Schistocephalus solidus) show increased serotonin metabolism in their hypothalamus and brainstem (Øverli et al. 2001). However, S. solidus infections can be quite debilitating, and the neuroendocrine responses of infected fishes are consistent with those seen in chronically stressed fishes (Winberg & Nilsson 1993).
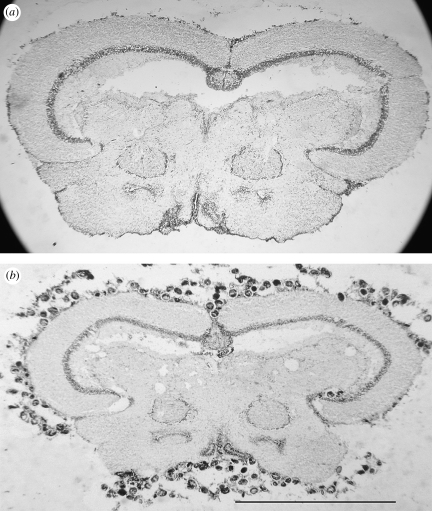
In the present paper, we explore the potential mechanisms a trematode uses to manipulate the behaviour of the California killifish, Fundulus parvipinnis. This classic example of PITT involves F. parvipinnis and its brain parasite, Euhaplorchis californiensis (Lafferty & Morris 1996). The California killifish is one of the most common fishes in Southern California and Baja California estuaries (West & Zedler 2000; Allen et al. 2006). Infected populations occur where the California horn snail, Cerithidea californica, is also present in the estuary. The trematode E. californiensis, one of the most abundant trematode parasites in these habitats (Martin 1955; Hechinger et al. 2007), uses three hosts in its life cycle: horn snails (C. californica); killifish; and several species of piscivorous birds (Martin 1950). Larval E. californiensis cercariae swim out of their first intermediate snail host, penetrate the skin of F. parvipinnis and migrate to the brain, presumably following blood vessels or nerve tracts (McNeff 1978; Hendrickson 1979; Haas et al. 2007). Once inside the braincase, cercariae encyst on the pial surface of the brain as metacercariae. Infected killifish display four times as many conspicuous swimming behaviours as uninfected ones, rendering them 10–30 times more likely to be eaten by a bird, the parasite's final host (Lafferty & Morris 1996). Prevalence of E. californiensis reaches 100 per cent in most localities, such that all killifish over a minimum size are infected (Shaw 2007). Although this infection pattern prevents a comparison of infected and uninfected individuals from the same population, the killifish–E. californiensis system provides an ideal model to investigate the specific neurobiological mechanisms of behaviour modification. Infected killifish appear otherwise healthy and school normally, despite hundreds to thousands of cysts packed around their brains (figure 1). Specific neurochemical alteration provides a plausible hypothesis for the modification of killifish behaviour by E. californiensis when considering the lack of gross pathology and the parasite's direct contact with the brain surface.
Figure 1.
Cross sections through the midbrains (diencephalons) of killifish both (a) uninfected and (b) infected with E. californiensis (metacercaricae surrounding the outer surface).
Monoamine neurotransmitters include the indoleamine serotonin and the catecholamines DA, adrenaline and noradrenaline and play an important role in shaping vertebrate social, locomotory and reproductive behaviours (Winberg & Nilsson 1993; Fabre-Nys 1998; Summers & Winberg 2006). These substances are considered neuromodulators, because they have long-term neuronal effects in addition to their brief synaptic actions (Adamo 2002; Libersat & Pflueger 2004). DA and serotonin (5-hydroxytryptamine, 5-HT) in particular influence fish social behaviour and swimming activity (Winberg & Nilsson 1993; Mok & Munro 1998; Höglund et al. 2001; Clements & Schreck 2004; Øverli et al. 2004). Considering that monoamines affect such fundamental behaviours, it is no surprise these neuromodulators respond quickly to external stimuli. Physical and social stressors can rapidly affect monoamine levels and neurotransmission in teleosts and other non-mammalian vertebrates (Winberg & Nilsson 1993; Winberg et al. 1993; Øverli et al. 1999; Höglund et al. 2001; Summers & Winberg 2006; Watt et al. 2007). Consequently, investigations of brain monoaminergic activity must be interpreted in the context of an organism's stress levels during the experiment and sampling.
Here, we examine whether E. californiensis infections are associated with changes in brain monoaminergic activity in experimentally infected killifish. We conducted experimental infections to (i) control for possible population differences between uninfected and naturally infected fish (which do not co-occur naturally), and (ii) isolate the effects of E. californiensis, as naturally infected killifish are usually infected with multiple trematode species (Yoshino 1971; Shaw et al. 2005). We also implemented a stress treatment in order to differentiate parasite effects from those of generalized stressors, since parasitic infection can cause chronic stress in certain hosts (Barber et al. 2000).
2. Material and methods
(a) Animal care and experimental infection
We used minnow traps and seines to collect adult uninfected F. parvipinnis (mean TL=62.7 mm, s.d.=6.3) from the University of California, Santa Barbara (UCSB) campus lagoon (34°24′32′ N, 119°50′50′ W) in May and June 2005. Fish were housed in 38 l seawater tanks (16–18°C) at UCSB and fed commercial flakes once a day. Tank water was changed daily with aged seawater until fish were killed 2 months later. We experimentally infected half of the uninfected fish with E. californiensis cercariae. Briefly, cercariae were stimulated to shed from C. californica by holding snails dry and dark for at least 1 week, followed by immersion in seawater for 2–3 h under an incandescent lamp. Cercariae were then collected, pooled and quantified following procedures described in Sandland & Goater (2000). Fish were exposed to cercariae or sham-exposed to seawater in buckets with 8 l seawater for 2 h. Although E. californiensis metacercariae are infective to definitive hosts after 2 weeks, we allowed 4 weeks for infections to mature before sampling (Martin 1950). All animal care and research was conducted in accordance to policies of the UCSB Institutional Animal Care and Use Committee.
(b) Stress treatment and sampling
The experimental set-up consisted of a blind design that pooled uninfected and experimentally infected fish together in tanks containing four individuals each. E. californiensis infections are not visible externally and cannot be transmitted horizontally. To sample non-stress tanks, we netted all fish simultaneously and killed them immediately in MS-222 (5 g l−1). Fish heads were removed immediately, frozen on dry ice and stored at −70°C. We recorded the body mass, gonad and liver mass and drew blood samples from the caudal vein using EDTA-coated syringes. For stress treatment tanks, the water levels were lowered to a dorsal fin height (approx. 1 cm) 1 h before sampling. Each fish was netted and held in the air for 5 s, every 15 min for 1 h, after which fish were killed as described above. For the plasma cortisol, we centrifuged blood samples at 10 000 r.p.m. for 5 min at 4°C, separated the plasma and stored it at −70°C.
(c) Micropunch dissection
We chose the following brain regions for analysis based on the behavioural and neuroendocrine significance, stress response and putative homologies to mammalian systems (Northcutt 1983; Parent 1983; Winberg et al. 1993; Salas et al. 2003; Wullimann & Mueller 2004; Butler & Hodos 2005): hippocampus (dorsal lateral area dorsalis telencephali, Dl); striatum (central and dorsal areas ventralis telencephali, Vc, Vd); hypothalamus (preoptic and paraventricular nuclei, NPO and NPP); and raphe nuclei (inferior and superior, IR and SR; abbreviations from Peter et al. 1975 and Wullimann et al. 1996). Frozen whole heads were sectioned at 300 μm on a cryostat at −20°C. Brain regions were identified with stereotaxic atlases (Peter et al. 1975; Wullimann et al. 1996; Anken & Bourrat 1998) and microdissected with a 300 μm diameter punch (sensu Renner & Luine 1984). Microdissection did not dislocate metacercariae, which occurred on the outer surface of the brains. We quantified E. californiensis intensity from brain sections after microdissection.
(d) Monoamine and plasma hormone analyses
We measured DA, its metabolite DOPAC (3,4-dihydroxyphenylacetic acid), serotonin (5-HT), and its metabolite 5-HIAA (5-hydroxyindoleacetic acid) using high-performance liquid chromatography (HPLC) with electrochemical detection, as described by Korzan et al. (2000). See the electronic supplementary material, §1 for HPLC details. To assess the protein content for each sample, we dissolved the tissue pellet in 100 μl of cold NaOH buffer (0.8 g NaOH pellets, 50 ml deionized H2O) and analysed each sample using the Bradford method. We assessed cortisol using a direct enzyme-linked immunosorbent assay kit from Assay Designs, Inc. Details are provided in the electronic supplementary material, §2.
(e) Statistical analysis
Unequal sample sizes, resulting from mortalities and HPLC sample loss (table 1), prevented the use of groupwise comparisons. Instead, we analysed brain monoaminergic activity and plasma cortisol using general linear models (GLMs) and regression. We used the ratio of monoamine metabolite/monoamine as an index of monoaminergic activity (Shannon et al. 1986; James et al. 1989). Data were log (5-HIAA/5-HT in hippocampus and hypothalamus) or logit transformed (DOPAC/DA in striatum) as needed to achieve normality of residuals. Model parameters included stress treatment (non-stressed or stressed) and sex as factors, metacercarial density, fish body mass and condition factor (K, ((wet body mass/total length3)×105)) as covariates, and all first-order interactions. We used parasite density (number of metacercariae/fish body mass) as the independent variable to analyse parasite effects on physiological variables, since parasite numbers tend to scale with killifish body mass (Shaw 2007). We ran an additional regression analysis when metacercarial density was a significant factor, in order to assess the density dependence; regressions were examined separately within each stress treatment group. To identify possible health differences resulting from experimental infections, we compared gonadosomatic (GSI; ((gonad mass/total length)×100)) and hepatosomatic (HSI, ((liver mass/total length)×100)) indices between infection groups separately for each sex, using GLMs with metacercarial density as a factor. For all models, we sequentially removed non-significant main effects (p>0.1) and interactions (p>0.05), conducted post hoc analyses using Tukey's HSD and corrected for multiple comparisons using the false discovery rate (Benjamini & Hochberg 1995). We performed all analyses using JMP 6.0, SAS Institute Inc.
Table 1.
Sample size and morphometric data for experimental groups. Gonadosomatic (GSI) and HSI indices given as mean±1 s.d., with the exception of the single experimentally infected, non-stressed female. F, females; M, males; N, sample size.
| experimental group | sex | N | body mass (g) | GSI | HSI |
|---|---|---|---|---|---|
| uninfected, non-stressed | F | 2 | 9.7±5.1 | 16.2±2.0 | 2.3±0.4 |
| M | 15 | 9.1±2.3 | 2.4±0.4 | 2.5±0.6 | |
| uninfected, stressed | F | 4 | 10.3±3.0 | 18.6±4.2 | 2.3±0.7 |
| M | 9 | 9.0±2.2 | 2.4±0.3 | 2.3±0.7 | |
| experimentally infected, non-stressed | F | 1 | 10.4 | 8.7 | 2.8 |
| M | 2 | 8.6±1.6 | 2.6±0.1 | 2.8±0.4 | |
| experimentally infected, stressed | F | 3 | 9.1±1.8 | 24.1±5.0 | 3.2±0.5 |
| M | 2 | 7.8±3.2 | 1.8±0.5 | 3.2±0.5 |
3. Results
Parasite density-dependent changes in monoaminergic activity occurred in the hippocampus and raphe nuclei (tables 2 and 3). In particular, E. californiensis density was associated with increased dopaminergic activity in the raphe nuclei and decreased serotonergic activity in the hippocampus and raphe nuclei. We present DA and 5-HT data in uninfected and experimentally infected fish, for each brain region below, including only statistically significant factors and interactions (see tables 2 and 3 for all statistics). Experimental infections resulted in much lower parasite intensities than those found in natural infections (Shaw 2007), yet these infections still produced density-dependent changes in raphe serotonergic activity. The infection treatment did not cause significant changes in GSI or HSI in males or females (table 1). We present mean monoaminergic activity by brain region, for uninfected and experimentally infected fish in the electronic supplementary material, §4.
Table 2.
GLM results for serotonin (5-HT) activity, measured as 5-HIAA/5-HT, in uninfected and experimentally infected killifish. (Brain region codes defined in §2. Parasite effect indicates the effect of increasing metacercarial density on 5-HT activity. Arrows indicate increased or decreased 5-HT activity, EI, experimentally infected; K, body condition. An asterisk (*) denotes significance after correction for multiple comparisons using FDR. D.f.=1 for all main effects and interactions.)
| serotonin activity | |||||
|---|---|---|---|---|---|
| SS | F | p-value | parasite effect | stress effect | |
| hippocampus (Dl) overall model: SS=0.374, F5,36=6.6, p=0.0003, R2=0.5 | |||||
| metacercarial density | 0.150 | 13.3 | 0.0010* | note interaction | |
| stress | 0.077 | 6.8 | 0.0100* | ||
| sex | 0.021 | 1.9 | 0.1820 | ||
| metacercarial density×stress | 0.173 | 15.4 | 0.0005* | ↓ in non-stressed fish | |
| stress×sex | 0.115 | 10.2 | 0.0032* | ↑ in males | |
| striatum (Vc, Vd) overall model: SS=0.076, F4,37=4.1, p=0.008, R2=0.3 | |||||
| stress | 0.045 | 9.6 | 0.004* | note interaction | |
| sex | 0.027 | 5.9 | 0.020* | ||
| K | 0.009 | 1.9 | 0.180 | ||
| stress×K | 0.036 | 7.8 | 0.009* | ↑ with ↑ K | |
| hypothalamus (NPO, NPP) overall model: SS=0.040, F1,35=18.3, p=0.0001, R2=0.4 | |||||
| stress | 0.040 | 18.3 | 0.0001* | ↑ overall | |
| raphe (IR, SR) overall model: SS=0.540, F4,35=36.2, p=<0.0001, R2=0.8 | |||||
| metacercarial density | 0.080 | 21.6 | <0.0001* | ↓ overall | |
| stress | 0.510 | 136.6 | <0.0001* | note interaction | |
| body mass | 0.016 | 4.4 | 0.0448* | ||
| stress×body mass | 0.016 | 4.4 | 0.0436* | ↑ in large fish | |
Table 3.
GLM results for DA activity, measured as DOPAC/DA, in uninfected and experimentally infected killifish. (Brain region codes defined in § 2. Parasite effect indicates the effect of increasing metacercarial density on DA activity. Arrows indicate increased or decreased DA activity; EI, experimentally infected fish; K, body condition. An asterisk (*) denotes significance after correction for multiple comparisons using FDR. D.f.=1 for all main effects and interactions.)
| dopamine activity | |||||
|---|---|---|---|---|---|
| SS | F | p-value | parasite effect | stress effect | |
| striatum (Vc, Vd) overall model: SS=4.687, F6,37=4.3, p=0.003, R2=0.5 | |||||
| stress | 1.389 | 7.7 | 0.009* | note interaction | |
| sex | 0.597 | 3.3 | 0.080 | ||
| body mass | 0.132 | 0.7 | 0.400 | ||
| K | 0.004 | 0.02 | 0.900 | ||
| stress×K | 2.004 | 11.1 | 0.002* | ↑ with ↑ K | |
| sex×body mass | 0.821 | 4.6 | 0.041 | ↑ in males | |
| hypothalamus (NPO, NPP) overall model: SS=0.646, F6,36=4.1, p=0.004, R2=0.5 | |||||
| stress | 0.0004 | 0.02 | 0.900 | note interaction | |
| sex | 0.092 | 3.4 | 0.070 | ||
| body mass | 0.058 | 2.2 | 0.150 | ||
| K | 0.014 | 0.5 | 0.470 | ||
| stress×sex | 0.220 | 8.4 | 0.007* | ↑ in males | |
| body mass×K | 0.123 | 4.7 | 0.040 | ||
| raphe (IR, SR) overall model: SS=0.046, F5,36=3.9, p=0.007, R2=0.4 | |||||
| metacercarial density | 0.010 | 4.5 | 0.04* | ↑ overall | |
| stress | 0.018 | 7.6 | 0.01* | note interaction | |
| sex | 0.000 | 0.0 | 0.93 | ||
| body mass | 0.018 | 7.5 | 0.01* | ||
| stress×sex | 0.018 | 7.6 | 0.01* | ↓ in females | |
(a) Hippocampus
The parasite effect in the suggested hippocampus homologue (Dl) varied according to stress treatment. Serotonergic activity decreased with increasing metacercarial density in non-stressed fish only, whereas there was no significant change observed in stressed fish (metacercarial density×stress; table 2). There was no significant relationship between metacercarial density and hippocampal serotonergic activity (p=0.1, 0.9 for unstressed and stressed groups, respectively). The stress treatment, by contrast, increased serotonergic activity in males but not females (stress×sex; table 2). DA activity was not measurable in this brain region owing to poor separation and low concentrations of the DA metabolite DOPAC.
(b) Striatum
We did not observe significant parasite effects on monoaminergic activity in this brain region. However, stress and sex significantly affected striatal serotonergic activity. The effect of stress also varied with body condition, so that striatal serotonergic activity was greater in the stressed fish with better condition, whereas non-stressed fish showed no significant change (stress×K; table 2). Stress and sex also affected dopaminergic activity in the suggested striatum homologue (Vc, Vd). The effect of stress varied with the body condition, such that the fish with better body condition had higher dopaminergic activity (stress×K; table 3). Males displayed higher dopaminergic activity than females, a trend that increased in heavier individuals (sex×body mass; table 3).
(c) Hypothalamus
We could not separate killifish preoptic and paraventricular nuclei when using a 300 μm punch needle—the minimum volume for obtaining detectable monoamine and metabolite levels. Hence, the data reported here reflect both areas combined in one punch volume. We did not analyse the remainder of the hypothalamus. We did not observe significant parasite effects on monoaminergic activity in this brain region. Serotonergic activity increased in stressed fish (table 2). The dopaminergic activity was not significantly affected by any factors, although significant interactions suggest potential effects of stress, sex, body mass and condition. Stress caused males to exhibit higher dopaminergic activity than stressed females (stress×sex; table 3). The effect of body mass on dopaminergic activity depended on the body condition, where the dopaminergic activity increased with better condition in small fish but decreased with better condition in large fish (body mass×K).
(d) Raphe nuclei
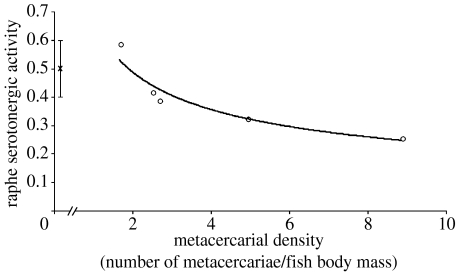
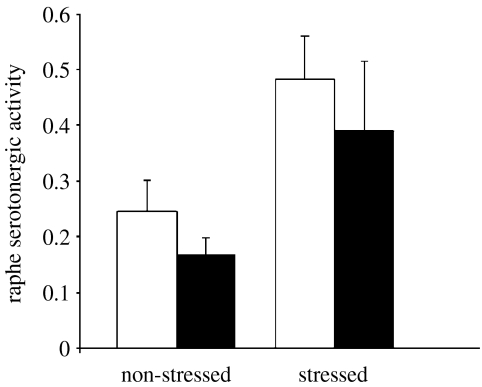
Increasing parasite density was associated with decreased serotonergic activity in experimentally infected fish (table 2). The regression analysis revealed this trend to be driven by stressed, experimentally infected fish (figure 3), although unstressed individuals also showed significant parasite density dependence (p=0.04). Stress also caused a significant increase in serotonergic activity in both uninfected and experimentally infected groups (figure 2). The effect of stress also varied with body size, so that large fish exhibited increased serotonergic activity when stressed (stress×body mass; table 2). In contrast to the results on serotonergic activity, higher parasite density was associated with increased dopaminergic activity in experimentally infected fish (table 3). There was no significant relationship between metacercarial density and raphe dopaminergic activity (p=0.1, 0.3 for unstressed and stressed groups, respectively). The effect of stress on dopaminergic activity varied according to sex. Dopaminergic activity decreased in response to stress in females but not males (stress×sex; table 3).
Figure 3.
Regression of raphe serotonergic activity (5-HIAA/5-HT) in stressed, experimentally infected killifish against E. californiensis density. Means for stressed uninfected (0.5±0.01, n=13) are shown for comparison only and not included in regression (circles, experimentally infected; cross, uninfected (mean±1 s.d.); y=0.672×−0.4637; R2=0.9434; p=0.009.)
Figure 2.
Killifish raphe serotonergic activity (5-HIAA/5-HT) is significantly higher in stressed individuals (open bars, uninfected; filled bars, experimentally infected; F1,35=136.6, p<0.0001; see table 2 for full model).
Interestingly, experimentally infected fish showed drastic changes in raphe monoaminergic activity (decreased 5-HT and increased DA), despite relatively low infection intensities. This suggests that a relatively small number of parasites were able to exert a powerful effect in this brain region. One explanation could be that the majority of metacercariae in experimentally exposed fish were found concentrated near the brainstem. Of the total metacercariae found in the experimentally infected fish, an average of 52 per cent were found on the brainstem (rhombencephalon), 41 per cent on the cerebellum and 18 per cent on the optic lobes (mesencephalon). Only one experimentally infected fish had metacercariae located on the telencephalon (5%; regions defined in Wullimann et al. 1996). We were unable to compare these data with those of naturally infected fish, whose brains are usually covered completely by hundreds to thousands of metacercariae.
(e) Plasma cortisol
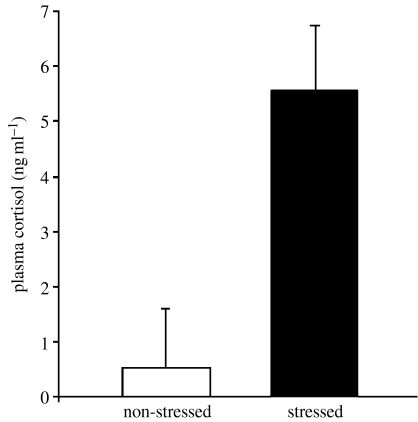
Cortisol increased in stressed killifish (F3,36=68.2, R2=0.9, p<0.0001; figure 4), verifying our stress treatment. There was no significant parasite effect on the cortisol levels. Full model details are provided in electronic supplementary material, §3.
Figure 4.
Plasma cortisol in stressed (n=20) and non-stressed (n=32) killifish (F1,36=141.4, p<0.0001). Values are adjusted (least-squares) means that correct for the effects of infection, sex and body condition (full GLM details in electronic supplementary material, §3). Error bars represent+1 s.d.
(f) Gonadosomatic and hepatosomatic indices
There was no significant effect of metacercarial density on GSI for males (F1,27=0.8, R2=0.03, p=0.4; table 1) or females (F1,9=3.4, R2=0.3, p=0.1; table 1). However, metacercarial density did significantly affect HSI. The HSI of infected females decreased as parasite density increased (F1,8=6.6, R2=0.5, p=0.04; table 1), while the HSI of infected males increased with a corresponding increase in parasite density (F1,27=4.8, R2=0.2, p=0.04; table 1).
4. Discussion
Increasing density of E. californiensis correlated with altered monoaminergic activity in the killifish hippocampus, hypothalamus and raphe nuclei, despite the fact that our experimental infections resulted in parasite intensities much lower than those found in naturally occurring infections. Trematodes can adapt locally to host genotypes from the same locale (Ballabeni & Ward 1993), perhaps explaining in part why our experimental infections resulted in low intensities. Nevertheless, experimentally infected fish still exhibited strong parasite density-dependent changes.
The serotonergic profiles of stressed, uninfected killifish were consistent with those of other stressed teleosts (Winberg & Nilsson 1993). However, experimentally infected fish differed by exhibiting a parasite density-dependent decrease in raphe serotonergic activity—a reaction opposite to those of most stressed teleosts (figure 2). It seems plausible then that a parasite-induced suppression of this innate stress response could lead to different behaviour during times of stress, say, in the presence of avian predators.
Behavioural observations were not conducted in the present study owing to logistical difficulties, and therefore it cannot be concluded that altered brain monoaminergic activity would produce the exact conspicuous swimming behaviours described in Lafferty & Morris (1996). Nevertheless, the lowered serotonergic activity of experimentally infected killifish could still constitute an underlying mechanism mediating parasite-induced behavioural changes. In specific regions of the brain, serotonin (5-HT) inhibits locomotion, aggression and dominance in fishes and other vertebrates (Winberg & Nilsson 1993; Weiger 1997; but see Rueter et al. 1997 and Øverli et al. 2004 for exceptions). Several studies confirm this by reporting the lowest levels of 5-HT levels when swimming activity is the highest (Fenwick 1970; Fingerman 1976; Øverli et al. 1998). Increased brain serotonergic activity is a general physiological response to stress in fishes and other vertebrates (Winberg & Nilsson 1993; Chaouloff 2000; Summers et al. 2005). The resultant movement inhibition, or ‘freezing’ (preferably under cover), is thought to be a primary avoidance response to danger in many fish species (Höglund et al. 2005). The raphe nuclei contain one of the largest populations of serotonin-producing neurons that project extensively throughout the brain and spinal cord (excluding the cerebellum in teleost fishes; Parent et al. 1984; Butler & Hodos 2005). In addition, this brain region is involved in inhibiting aggression (Summers & Winberg 2006). Therefore, we expected the raphe to reflect any changes in 5-HT activity, e.g. the initial rise in serotonergic activity, characteristic of stressed teleosts (Winberg & Nilsson 1993). The strongest effect of E. californiensis was a density-dependent suppression of this natural stress response in the raphe, where parasites accounted for more than 90 per cent of the variation in decreased serotonergic activity (figure 3). Their resulting influence could cause increased locomotion by affecting mid- and forebrain targets responsible for coordinating motor output. Metacercariae aggregated primarily on or near the brainstem in our low intensity, experimental infections. Hendrickson (1979) reported that brain-encysting cercariae migrate along spinal nerves and encounter the brainstem first, which would facilitate encystment near the raphe. It is possible that E. californiensis capitalized on its circumstances, evolving a way to affect host behaviour given its initial default location.
In contrast to the general effects of 5-HT, DA stimulates locomotory, aggressive and dominant behaviours in fishes, reptiles and mammals (Winberg & Nilsson 1993; Mok & Munro 1998; Korzan et al. 2006). Dopaminergic activity in the raphe nuclei regulates midbrain serotonergic activity (Korzan et al. 2001; Aman et al. 2007), whereas serotonergic raphe neurons also project back to stimulate midbrain DA-producing nuclei, forming a reciprocal feedback loop (Gervais & Rouillard 2000; Korzan et al. 2001; Alex & Pehek 2007). Dopaminergic activity in the raphe can also inhibit serotonergic neurons that ascend to the midbrain (Ferré et al. 1994). Given this, it is difficult to make definitive conclusions about the effects of increased raphe dopaminergic activity on killifish behaviour without the knowledge of the specific monoamine receptors involved.
In summary, our study provides an initial look into which neurotransmitter systems may be affected by the brain parasite E. californiensis. Øverli et al. (2001) demonstrated heightened serotonergic activity in sticklebacks infected with larval tapeworms but realized that those results could have been due to chronic stress from infection by a potentially debilitating parasite. Here, we simultaneously examined parasites and stress on killifish monoaminergic activity to disentangle their respective impacts, and we showed that E. californiensis exerted effects separate from those of stress. This pattern was the clearest in the raphe nuclei, where parasites inhibited the stress-induced increase in serotonergic activity in a density-dependent manner. It is unlikely that pathology or general debilitation contributed to any parasite density-dependent effect, particularly since overall body condition (K) alone seldom explained significant variation in the monoaminergic activity. The fact that parasite density did not negatively affect HSI in both sexes suggests that the observed differences may reflect differing energetic demands between sexes, e.g. those required to develop ovaries, rather than resulting directly from parasitic infection.
The exact physiological mechanism of how E. californiensis alters killifish monoaminergic activity—and thereby behaviour—has yet to be determined. We know that E. californiensis metacercariae secrete fibroblast growth factors on the surface of their cysts in vitro, which helps them aggregate (J. La Clair & K. D. Lafferty, unpublished data). It seems plausible then that metacercariae could also secrete chemicals that ultimately modify killifish monoamine activity.
Acknowledgments
All animal care and research was conducted in accordance with the policies of the UCSB Institutional Animal Care and Use Committee.The authors would like to thank Kenneth J. Renner for the HPLC and protein analysis, Jessica Cinkornpumin and Amber Kaplan for their assistance and Ryan Hechinger for his intellectual contribution. R. Hechinger and Valerie McKenzie provided their helpful comments on earlier drafts of the manuscript. The University of California Natural Reserve System provided access to field sites at Carpinteria Salt Marsh. Funding provided by the NSF/NIH Ecology of Infectious Diseases Program (DEB-0224565). Any use of trade, product or firm names in this publication is for descriptive purposes only and does not imply endorsement by the US government.
Supplementary Material
Supplementary methods, analyses, and data for experimental groups
References
- Adamo S.A. Modulating the modulators: parasites, neuromodulators and host behavioral change. Brain Behav. Evol. 2002;60:370–377. doi: 10.1159/000067790. doi:10.1159/000067790 [DOI] [PubMed] [Google Scholar]
- Adamo S.A., Shoemaker K.L. Effects of parasitism on the octopamine content of the central nervous system of Manduca sexta: a possible mechanism underlying host behavioural change. Can. J. Zool. 2000;78:1580–1587. doi:10.1139/cjz-78-9-1580 [Google Scholar]
- Alex K.D., Pehek E.A. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol. Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. doi:10.1016/j.pharmthera.2006.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen L.G., Yoklavich M.M., Cailliet G.M., Horn M.H. Bays and estuaries. In: Allen L.G., Pondella D.J., Horn M.H., editors. The ecology of marine fishes: California and adjacent waters. University of California Press; Berkeley, CA: 2006. pp. 119–148. [Google Scholar]
- Aman T.K., Shen R.Y., Haj-Dahmane S. D-2-like dopamine receptors depolarize dorsal raphe serotonin neurons through the activation of nonselective cationic conductance. J. Pharmacol. Exp. Ther. 2007;320:376–385. doi: 10.1124/jpet.106.111690. doi:10.1124/jpet.106.111690 [DOI] [PubMed] [Google Scholar]
- Anken R., Bourrat F. INRA Editions; Paris: 1998. Brain atlas of the Medakafish Oryzias latipes. [Google Scholar]
- Bakker T.C.M., Mazzi D., Zala S. Parasite-induced changes in behavior and color make Gammarus pulex more prone to fish predation. Ecology. 1997;78:1098–1104. doi:10.1890/0012-9658(1997)078[1098:PICIBA]2.0.CO;2 [Google Scholar]
- Ballabeni P., Ward P.I. Local adaptation of the tremadote Diplostomum phoxini to the European minnow Phoxinus phoxinus, its 2nd intermediate host. Funct. Ecol. 1993;7:84–90. doi:10.2307/2389870 [Google Scholar]
- Barber I., Hoare D., Krause J. Effects of parasites on fish behaviour: a review and evolutionary perspective. Rev. Fish Biol. Fish. 2000;10:131–165. doi:10.1023/A:1016658224470 [Google Scholar]
- Beckage N.E. Endocrine and neuroendocrine host-parasite relationships. Receptor. 1993;3:233–245. [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate–a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- Berdoy M., Webster J.P., Macdonald D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. B. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. doi:10.1098/rspb.2000.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron D.G., Marche L., Ponton F., Loxdale H.D., Galeotti N., Renault L., Joly C., Thomas F. Behavioural manipulation in a grasshopper harbouring hairworm: a proteomics approach. Proc. R. Soc. B. 2005;272:2117–2126. doi: 10.1098/rspb.2005.3213. doi:10.1098/rspb.2005.3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassard P., Rau M.E., Curtis M.A. Parasite-induced susceptibility to predation in diplostomiasis. Parasitology. 1982;85:495–501. [Google Scholar]
- Butler A.B., Hodos W. Wiley-Liss; New York, NY: 2005. Comparative vertebrate neuroanatomy: evolution and adaptation. [Google Scholar]
- Carney W.P. Behavioral and morphological changes in carpenter ants harboring dicrocoeliid metacercaria. Am. Midl. Nat. 1969;82:605–611. doi:10.2307/2423801 [Google Scholar]
- Chaouloff F. Serotonin, stress and corticoids. J. Psychopharmacol. 2000;14:139–151. doi: 10.1177/026988110001400203. [DOI] [PubMed] [Google Scholar]
- Clements S., Schreck C.B. Evidence that GABA mediates dopaminergic and serotonergic pathways associated with locomotor activity in juvenile chinook salmon (Oncorhynchus tshawytscha) Behav. Neurosci. 2004;118:191–198. doi: 10.1037/0735-7044.118.1.191. doi:10.1037/0735-7044.118.1.191 [DOI] [PubMed] [Google Scholar]
- Combes C. The University of Chicago Press; Chicago, IL: 2001. Parasitism: the ecology and evolutions of intimate interactions. [Google Scholar]
- de Jong-Brink M., Bergamin-Sassen M., Soto M.S. Multiple strategies of schistosomes to meet their requirements in the intermediate snail host. Parasitology. 2001;123:S129–S141. doi: 10.1017/s0031182001008149. doi:10.1017/S0031182001008149 [DOI] [PubMed] [Google Scholar]
- Fabre-Nys C. Steroid control of monoamines in relation to sexual behaviour. Rev. Reprod. 1998;3:31–41. doi: 10.1530/ror.0.0030031. doi:10.1530/ror.0.0030031 [DOI] [PubMed] [Google Scholar]
- Fenwick J.C. Brain serotonin and swimming activity in goldfish, Carassius auratus. Comp. Biochem. Physiol. 1970;32:803–806. doi:10.1016/0010-406X(70)90830-3 [Google Scholar]
- Ferré S., Cortes R., Artigas F. Dopaminergic regulation of the serotonergic raphe-striatal pathway–microdialysis studies in freely moving rats. J. Neurosci. 1994;14:4839–4846. doi: 10.1523/JNEUROSCI.14-08-04839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerman S.W. Circadian rhythms of brain 5-hydroxytryptamine and swimming activity in teleost, Fundulus grandis. Comp. Biochem. Phys. C. 1976;54:49–53. doi: 10.1016/0306-4492(76)90024-1. doi:10.1016/0306-4492(76)90024-1 [DOI] [PubMed] [Google Scholar]
- Gervais J., Rouillard C. Dorsal raphe stimulation differentially modulates dopaminergic neurons in the ventral tegmental area and substantia nigra. Synapse. 2000;35:281–291. doi: 10.1002/(SICI)1098-2396(20000315)35:4<281::AID-SYN6>3.0.CO;2-A. doi:10.1002/(SICI)1098-2396(20000315)35:4<281::AID-SYN6>3.0.CO;2-A [DOI] [PubMed] [Google Scholar]
- Haas W., Wulff C., Grabe K., Meyer V., Haeberlein S. Navigation within host tissues: cues for orientation of Diplostomum spathaceum (Trematoda) in fish towards veins, head and eye. Parasitology. 2007;134:1013–1023. doi: 10.1017/S0031182007002430. doi:10.1017/S0031182007002430 [DOI] [PubMed] [Google Scholar]
- Hechinger R.F., Lafferty K.D., Huspeni T.C., Brooks A., Kuris A.M. Can parasites be indicators of free-living diversity? Relationships between species richness and the abundance of larval trematodes and of local fishes and benthos. Oecologia. 2007;151:82–92. doi: 10.1007/s00442-006-0568-z. doi:10.1007/s00442-006-0568-z [DOI] [PubMed] [Google Scholar]
- Helluy S., Thomas F. Effects of Microphallus papillorobustus (Platyhelminthes: Trematoda) on serotonergic immunoreactivity and neuronal architecture in the brain of Gammarus insensibilis (Crustacea: Amphipoda) Proc. R. Soc. B. 2003;270:563–568. doi: 10.1098/rspb.2002.2264. doi:10.1098/rspb.2002.2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson G.L. Ornithodiplostomum ptychocheilus: migration to the brain of the fish intermediate host, Pimephales promelas. Exp. Parasitol. 1979;48:245–258. doi: 10.1016/0014-4894(79)90106-1. doi:10.1016/0014-4894(79)90106-1 [DOI] [PubMed] [Google Scholar]
- Höglund E., Kolm N., Winberg S. Stress-induced changes in brain serotonergic activity, plasma cortisol and aggressive behavior in Arctic charr (Salvelinus alpinus) is counteracted by L-DOPA. Physiol. Behav. 2001;74:381–389. doi: 10.1016/s0031-9384(01)00571-6. doi:10.1016/S0031-9384(01)00571-6 [DOI] [PubMed] [Google Scholar]
- Höglund E., Weltzien F.A., Schjolden J., Winberg S., Ursin H., Doving K.B. Avoidance behavior and brain monoamines in fish. Brain Res. 2005;1032:104–110. doi: 10.1016/j.brainres.2004.10.050. doi:10.1016/j.brainres.2004.10.050 [DOI] [PubMed] [Google Scholar]
- Holmes J.C., Bethel W.M. Modification of intermediate host behaviour by parasites. In: Canning E.U., Wright C.A., editors. Behavioural aspects of parasite transmission. Academic Press Inc; New York, NY: 1972. pp. 123–150. [Google Scholar]
- Hoogenboom I., Dijkstra C. Sarcocystis cernae–a parasite increasing the risk of predation of its intermediate host, Microtus arvalis. Oecologia. 1987;74:86–92. doi: 10.1007/BF00377350. doi:10.1007/BF00377350 [DOI] [PubMed] [Google Scholar]
- James M.D., Hole D.R., Wilson C.A. Differential involvement of 5-hydroxytryptamine (5HT) in specific hypothalamic areas in the mediation of steroid-induced changes in gonadotropin release and sexual behavior in female rats. Neuroendocrinology. 1989;49:561–569. doi: 10.1159/000125169. doi:10.1159/000125169 [DOI] [PubMed] [Google Scholar]
- Kavaliers M., Colwell D.D., Choleris E. Parasites and behavior: an ethopharmacological analysis and biomedical implications. Neurosci. Biobehav. Rev. 1999;23:1037–1045. doi: 10.1016/s0149-7634(99)00035-4. doi:10.1016/S0149-7634(99)00035-4 [DOI] [PubMed] [Google Scholar]
- Klein S.L. Parasite manipulation of the proximate mechanisms that mediate social behavior in vertebrates. Physiol. Behav. 2003;79:441–449. doi: 10.1016/s0031-9384(03)00163-x. doi:10.1016/S0031-9384(03)00163-X [DOI] [PubMed] [Google Scholar]
- Knudsen R., Gabler H.M., Kuris A.M., Amundsen P.A. Selective predation on parasitized prey—a comparison between two helminth species with different life-history strategies. J. Parasitol. 2001;87:941–945. doi: 10.1645/0022-3395(2001)087[0941:SPOPPA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Korzan W.J., Summers T.R., Summers C.H. Monoaminergic activities of limbic regions are elevated during aggression: influence of sympathetic social signaling. Brain Res. 2000;870:170–178. doi: 10.1016/s0006-8993(00)02420-3. doi:10.1016/S0006-8993(00)02420-3 [DOI] [PubMed] [Google Scholar]
- Korzan W.J., Summers T.R., Ronan P.J., Renner K.J., Summers C.H. The role of monoaminergic nuclei during aggression and sympathetic social signaling. Brain Behav. Evol. 2001;57:317–327. doi: 10.1159/000047250. doi:10.1159/000047250 [DOI] [PubMed] [Google Scholar]
- Korzan W.J., Forster G.L., Watt M.J., Summers C.H. Dopaminergic activity modulation via aggression, status, and a visual social signal. Behav. Neurosci. 2006;120:93–102. doi: 10.1037/0735-7044.120.1.93. doi:10.1037/0735-7044.120.1.93 [DOI] [PubMed] [Google Scholar]
- Kuris A. Evolutionary ecology of trophically transmitted parasites. J. Parasitol. 2003;89:s96–s100. [Google Scholar]
- Lafferty K.D. Foraging on prey that are modified by parasites. Am. Nat. 1992;140:854–867. doi:10.1086/285444 [Google Scholar]
- Lafferty K.D. The evolution of trophic transmission. Parasitol. Today. 1999;15:111–115. doi: 10.1016/s0169-4758(99)01397-6. doi:10.1016/S0169-4758(99)01397-6 [DOI] [PubMed] [Google Scholar]
- Lafferty K.D., Morris A.K. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology. 1996;77:1390–1397. doi:10.2307/2265536 [Google Scholar]
- Libersat F., Pflueger H.J. Monoamines and the orchestration of behavior. Bioscience. 2004;54:17–25. doi:10.1641/0006-3568(2004)054[0017:MATOOB]2.0.CO;2 [Google Scholar]
- Martin W.E. Euhaplorchis californiensis N.G., N. SP., Heterophyidae, Trematoda, with notes on its life cycle. Trans. Am. Microsc. Soc. 1950;69:194–209. doi:10.2307/3223410 [Google Scholar]
- Martin W.E. Essays in the natural sciences in honor of Captain Allan Hancock. University of Southern California Press; Los Angeles, CA: 1955. Seasonal infections of the snail Cerithidea californica Haldeman, with larval trematodes; pp. 203–210. [Google Scholar]
- McCurdy D.G., Forbes M.R., Boates J.S. Evidence that the parasitic nematode Skrjabinoclava manipulates host Corophium behavior to increase transmission to the sandpiper, Calidris pusilla. Behav. Ecol. 1999;10:351–357. doi:10.1093/beheco/10.4.351 [Google Scholar]
- McNeff, L. L. 1978 Marine cercariae from Cerithidea pliculosa Menke from Dauphin Island, Alabama; Life Cycles of Heterophyid and Opisthorchiid Digenea from Cerithidea Swainson from the Eastern Gulf of Mexico. MSc thesis, University of Alabama, USA.
- Mok E.Y.M., Munro A.D. Effects of dopaminergic drugs on locomotor activity in teleost fish of the genus Oreochromis (Cichlidae): involvement of the telencephalon. Physiol. Behav. 1998;64:227–234. doi: 10.1016/s0031-9384(98)00038-9. doi:10.1016/S0031-9384(98)00038-9 [DOI] [PubMed] [Google Scholar]
- Moore J. Oxford series in ecology and evolution. Oxford University Press, Inc; New York, NY: 2002. Parasites and the behavior of animals. [Google Scholar]
- Northcutt R.G. Telencephalic organization in ray-finned fishes. In: Davis R.E., Northcutt R.G., editors. Fish neurobiology. vol. 2. The University of Michigan Press; Ann Arbor, MI: 1983. pp. 203–236. [Google Scholar]
- Øverli Ø., Winberg S., Damsgard B., Jobling M. Food intake and spontaneous swimming activity in Arctic char (Salvelinus alpinus): role of brain serotonergic activity and social interactions. Can. J. Zool. 1998;76:1366–1370. doi:10.1139/cjz-76-7-1366 [Google Scholar]
- Øverli Ø., Harris C.A., Winberg S. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav. Evol. 1999;54:263–275. doi: 10.1159/000006627. doi:10.1159/000006627 [DOI] [PubMed] [Google Scholar]
- Øverli Ø., Pall M., Borg B., Jobling M., Winberg S. Effects of Schistocephalus solidus infection on brain monoaminergic activity in female three-spined sticklebacks Gasterosteus aculeatus. Proc. R. Soc. B. 2001;268:1411–1415. doi: 10.1098/rspb.2001.1668. doi:10.1098/rspb.2001.1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øverli Ø., Korzan W.J., Larson E.T., Winberg S., Lepage O., Pottinger T.G., Renner K.J., Summers C.H. Behavioral and neuroendocrine correlates of displaced aggression in trout. Horm. Behav. 2004;45:324–329. doi: 10.1016/j.yhbeh.2004.01.001. doi:10.1016/j.yhbeh.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Parent A. The monoamine-containing neuronal systems in the teleostean brain. In: Davis R.E., Northcutt R.G., editors. Fish neurobiology. vol. 2. The University of Michigan Press; Ann Arbor, MI: 1983. pp. 285–315. [Google Scholar]
- Parent A., Poitras D., Dube L. Comparative anatomy of central monoaminergic systems. In: Bjorklund A., Hokfelt T., editors. Handbook of chemical neuroanatomy. vol. 2. Elsevier Science Publishers; Amsterdam, NY: 1984. pp. 409–439. [Google Scholar]
- Peter R.E., Macey M.J., Gill V.E. Stereotaxic atlas and technique for forebrain nuclei of killifish, Fundulus heteroclitusy. J. Comp. Neurol. 1975;159:103–127. doi: 10.1002/cne.901590107. doi:10.1002/cne.901590107 [DOI] [PubMed] [Google Scholar]
- Renner K.J., Luine V.N. Determination of monoamines in brain nuclei by high-performance liquid-chromatography with electrochemical detection–young vs middle-aged rats. Life Sci. 1984;34:2193–2199. doi: 10.1016/0024-3205(84)90320-5. doi:10.1016/0024-3205(84)90320-5 [DOI] [PubMed] [Google Scholar]
- Rojas J.M., Ojeda F.P. Altered dopamine levels induced by the parasite Profilicollis antarcticus on its intermediate host, the crab Hemigrapsus crenulatus. Biol. Res. 2005;38:259–266. doi: 10.4067/s0716-97602005000200015. [DOI] [PubMed] [Google Scholar]
- Rosenberg L.A., Pfluger H.J., Wegener G., Libersat F. Wasp venom injected into the prey's brain modulates thoracic identified monoaminergic neurons. J. Neurobiol. 2006;66:155–168. doi: 10.1002/neu.20203. doi:10.1002/neu.20203 [DOI] [PubMed] [Google Scholar]
- Rueter L.E., Fornal C.A., Jacobs B.L. A critical review of 5-HT brain microdialysis and behavior. Rev. Neurosci. 1997;8:117–137. doi: 10.1515/revneuro.1997.8.2.117. [DOI] [PubMed] [Google Scholar]
- Salas C., Broglio C., Rodriguez F. Evolution of forebrain and spatial cognition in vertebrates: conservation across diversity. Brain Behav. Evol. 2003;62:72–82. doi: 10.1159/000072438. doi:10.1159/000072438 [DOI] [PubMed] [Google Scholar]
- Sandland G.J., Goater C.P. Development and intensity dependence of Ornithodiplostomum ptychocheilus metacercariae in fathead minnows (Pimephales promelas) J. Parasitol. 2000;86:1056–1060. doi: 10.1645/0022-3395(2000)086[1056:DAIDOO]2.0.CO;2. doi:10.1645/0022-3395(2000)086[1056:DAIDOO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shannon N.J., Gunnet J.W., Moore K.E. A comparison of biochemical indices of 5-hydroxytryptaminergic neuronal activity following electrical stimulation of the dorsal raphe nucleus. J. Neurochem. 1986;47:958–965. doi: 10.1111/j.1471-4159.1986.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Shaw, J. C. 2007 Neural mechanisms of behavior modification in killifish (Fundulus parvipinnis) by a brain parasite (Euhaplorchis californiensis) and the ecology of the host-parasite relationship. PhD Dissertation, University of California, Santa Barbara, CA, USA.
- Shaw J.C., Aguirre-Macedo L., Lafferty K.D. An efficient strategy to estimate intensity and prevalence: sampling metacercariae in fishes. J. Parasitol. 2005;91:515–521. doi: 10.1645/GE-385R2. doi:10.1645/GE-385R2 [DOI] [PubMed] [Google Scholar]
- Skallova A., Kodym P., Frynta D., Flegr J. The role of dopamine in Toxoplasma-induced behavioural alterations in mice: an ethological and ethopharmacological study. Parasitology. 2006;133:525–535. doi: 10.1017/S0031182006000886. doi:10.1017/S0031182006000886 [DOI] [PubMed] [Google Scholar]
- Stibbs H.H. Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann. Trop. Med. Parasitol. 1985;79:153–157. doi: 10.1080/00034983.1985.11811902. [DOI] [PubMed] [Google Scholar]
- Summers C.H., Winberg S. Interactions between the neural regulation of stress and aggression. J. Exp. Biol. 2006;209:4581–4589. doi: 10.1242/jeb.02565. doi:10.1242/jeb.02565 [DOI] [PubMed] [Google Scholar]
- Summers C.H., et al. Does serotonin influence aggression? Comparing regional activity before and during social interaction. Physiol. Biochem. Zool. 2005;78:679–694. doi: 10.1086/432139. doi:10.1086/432139 [DOI] [PubMed] [Google Scholar]
- Tain L., Perrot-Minnot M.J., Cezilly F. Altered host behaviour and brain serotonergic activity caused by acanthocephalans: evidence for specificity. Proc. R. Soc. B. 2006;273:3039–3045. doi: 10.1098/rspb.2006.3618. doi:10.1098/rspb.2006.3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F., Poulin R. Manipulation of a mollusc by a trophically transmitted parasite: convergent evolution or phylogenetic inheritance? Parasitology. 1998;116:431–436. doi: 10.1017/s003118209800239x. doi:10.1017/S003118209800239X [DOI] [PubMed] [Google Scholar]
- Thomas F., Ulitsky P., Augier R., Dusticier N., Samuel D., Strambi C., Biron D.G., Cayre M. Biochemical and histological changes in the brain of the cricket Nemobius sylvestris infected by the manipulative parasite Paragordius tricuspidatus (Nematomorpha) Int. J. Parasitol. 2003;33:435–443. doi: 10.1016/s0020-7519(03)00014-6. doi:10.1016/S0020-7519(03)00014-6 [DOI] [PubMed] [Google Scholar]
- Thomas F., Adamo S., Moore J. Parasitic manipulation: where are we and where should we go? Behav. Process. 2005;68:185–199. doi: 10.1016/j.beproc.2004.06.010. doi:10.1016/j.beproc.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Vyas A., Kim S.-K., Giacomini N., Boothroyd J.C., Sapolsky R.M. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Natl Acad. Sci. USA. 2007;104:6442–6447. doi: 10.1073/pnas.0608310104. doi:10.1073/pnas.0608310104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M.J., Forster G.L., Korzan W.J., Renner K.J., Summers C.H. Rapid neuroendocrine responses evoked at the onset of social challenge. Physiol. Behav. 2007;90:567–575. doi: 10.1016/j.physbeh.2006.11.006. doi:10.1016/j.physbeh.2006.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiger W.A. Serotonergic modulation of behaviour: a phylogenetic overview. Biol. Rev. Camb. Philos. Soc. 1997;72:61–95. doi: 10.1017/s0006323196004975. doi:10.1017/S0006323196004975 [DOI] [PubMed] [Google Scholar]
- West J.M., Zedler J.B. Marsh-creek connectivity: fish use of a tidal salt marsh in southern California. Estuaries. 2000;23:699–710. doi:10.2307/1352896 [Google Scholar]
- Winberg S., Nilsson G.E. Roles of brain monoamine neurotransmitters in agonistic behavior and stress reactions, with particular reference to fish. Comp. Biochem. Phys. C. 1993;106:597–614. doi:10.1016/0742-8413(93)90216-8 [Google Scholar]
- Winberg S., Myrberg A.A., Nilsson G.E. Predator exposure alters brain serotonin metabolism in bicolor damselfish. Neuroreport. 1993;4:399–402. doi: 10.1097/00001756-199304000-00014. doi:10.1097/00001756-199304000-00014 [DOI] [PubMed] [Google Scholar]
- Wullimann M.F., Mueller T. Teleostean and mammalian forebrains contrasted: evidence from genes to behavior. J. Comp. Neurol. 2004;475:143–162. doi: 10.1002/cne.20183. doi:10.1002/cne.20183 [DOI] [PubMed] [Google Scholar]
- Wullimann M.F., Rupp B., Reichert H. Birkhäuser Verlag; Basel; Boston, MA: 1996. Neuroanatomy of the zebrafish brain: a topological atlas. [Google Scholar]
- Yoshino, T. P. 1971 Helminth Parasitism in the Pacific Killifish Fundulus parvipinnis from Goleta Slough. Dissertation, University of California, Santa Barbara, California, MA. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods, analyses, and data for experimental groups