Abstract
Maternal experience before and during pregnancy is known to play a key role in offspring development. However, the influence of social cues about disease in the maternal environment has not been explored. We indirectly exposed pregnant mice to infected neighbours by housing them next to non-contagious conspecifics infected with Babesia microti. We examined the effect of this indirect immunological exposure on both the females and their adult offspring. Exposed females had higher levels of serum corticosterone and increased kidney growth compared with those with uninfected neighbours. These exposed females subsequently produced offspring that as adults showed an accelerated immune response to B. microti and less aggression in social groups. We suggest that ambient information regarding disease is used adaptively to maximize offspring survival and reproductive success in a challenging environment. Our results shed light on the impact of social information and maternal effects on life histories, and have important consequences for our understanding of epidemiology and individual disease susceptibility in humans and other animals. They also lead us to question the suitability of some laboratory housing conditions during experimental procedures, which may impact negatively upon both animal welfare and the validity of animal science.
Keywords: life history, host–parasite, maternal effect, disease susceptibility, trade-off
1. Introduction
Ambient immunological information, such as that detected from the odours and behaviour of diseased individuals, is increasingly understood to play a key role in both immunity and behaviour (Zinkernagel 2000; Kilpimaa et al. 2004; Brennan & Zufall 2006; Zala et al. 2008b). The presence of diseased individuals is shown to cause dramatic and wide-ranging behavioural and physiological responses in neighbouring conspecifics (Kavaliers et al. 2003; Gelperin 2008). For example, female mice show preferences for the odours of unparasitized males in mate choice experiments (Penn et al. 1998; Ehman & Scott 2002; Kavaliers et al. 2003), while healthy rats (Rattus norvegicus) mimic the organ growth and hormone production of immunologically challenged neighbours (Fernandes 2000). In humans, body odour has long been used by physicians to diagnose diseases such as typhoid (baked bread odour) and yellow fever (meaty odour) (Penn & Potts 1998). It is known that many species of animals, including ourselves, are able to detect disease status in others, particularly in social odours (Kavaliers et al. 2005; Zala et al. 2008a). It is not known, however, if perception of this information benefits the receiver in any way, how it is used by individuals to make assessments about the risk of current disease in the immediate environment, and how it is exploited subsequently to maximize reproductive success.
The study of the transgenerational induction of traits is also gaining prominence. This occurs when information in the environment of the mother causes a change in the phenotype of the offspring, and is commonly known as a maternal effect (Agrawal et al. 1999; Marshall & Uller 2007; Rickard & Lummaa 2007). It is accepted that certain cues in the maternal environment, e.g. the prevalence of predators, can lead to behavioural or morphological changes in the following generation (Agrawal et al. 1999). It is also accepted that direct maternal infection with pathogens can have immunological consequences for offspring (Grindstaff et al. 2006). The adaptive nature of these consequences is still being actively debated (Groothuis et al. 2005a,b; Marshall & Uller 2007).
Here, we combine these two growing fields of investigation to test the hypothesis that cues about the disease status of neighbours are exploited by female mice in reproductive decision making. Specifically, we tested the idea that ambient immunological information is used to gauge the threat of disease to self and offspring, and that the capacity to resist infection in offspring in subsequent adult life is adjusted accordingly.
We tested our hypothesis using BKW laboratory mice and the intraerythrocytic parasite Babesia microti, a well-established host–parasite system used in our laboratories (Barnard et al. 2005; Barnard & Behnke 2006). Babesia species are tick-borne haemoprotozoan parasites that infect virtually all mammalian species, with significant economic consequences in domestic animals and human health implications (Kjemtrup & Conrad 2000; Kim et al. 2007). In mice, B. microti induces high but transient parasitaemias, which are quickly cleared (clearance beginning approx. 10 days after infection; Homer et al. 2000). Antibodies can block B. microti sporozoites from invading erythrocytes shortly following infection. If this fails, natural killer cells and macrophages act to limit the extent of parasitaemia, by production of gamma-interferon (IFN-γ), tumour necrosis factor-alpha (TNF-α), nitrous oxide (NO) and reactive oxygen species (Homer et al. 2000). There is also suggested involvement of the recently discovered MetHb-pseudoperoxidase pathway (Bogdan 2007; Jiang et al. 2007). Ultimately, a resolution stage begins, with parasitaemia levels peaking and then rapidly declining due to the action of CD4+T cells and IFN-γ (Igarashi et al. 1999; Homer et al. 2000). Following primary infection, mice are protected against future infection by the action of CD4+T cells and IFN-γ, with little or no requirement for B-cells or antibodies (Igarashi et al. 1999).
Our experiment involved housing pre-mated dams, opposite groups of stimulus males (figure 1), which had either been infected with B. microti, or subject to one of four control treatments. We used partitioned cages that were designed such that the dams could receive auditory, visual and olfactory information from their neighbours, but had no direct contact with them beyond potentially touching whiskers. Direct transmission of B. microti to our dams could not occur as it is dependent on a tick vector (Randolph 1991), which was absent, or direct exchange of blood, which was not possible. To test our hypothesis, we examined the effect of experimental treatment on maternal physiology and behaviour, and then adult offspring social behaviour and immune response to disease challenge.
Figure 1.
Partitioned cages used during stimulus phase. Dams were housed on one side of the divided cage, separated from stimulus males by a perforated Perspex partition.
2. Material and methods
(a) Mice and housing
The subjects were 300 mice of the BKW strain (supplied by B & K Universal Ltd, Hull, UK). This number comprised 50 subject dams (seven weeks old); 200 stimulus males (three to five weeks old); and 50 sire males (nine weeks old). We kept subjects in standard polypropylene cages (48×15×13 cm: model M3, North Kent Plastics, UK) except during the stimulus phase of the experiment when stimulus male groups and subject females each had one-half of a large divided cage (figure 1; 28×45×13 cm: model MB1, adapted specifically for this experiment). All cages and cage sections contained wood shavings as a floor substrate, a cotton nestlet for bedding material and a cardboard tube. Subjects had ad libitum access to standard laboratory rodent food pellets and water. Room temperature was maintained between 20 and 22°C and humidity between 45 and 55 per cent. All animals were maintained under a 12 : 12 h reversed light : dark cycle with lights on at 20.00 h, and illuminated by a dim red light during the dark cycle to facilitate observations.
(b) Mating
We introduced dams to the home cages of randomly allocated single-housed sire males, and kept them in these pairs for 6 days. This method was used to minimize sire–dam aggression, and maximize likelihood of impregnation (Koyama 2004), while still leaving sufficient gestation time for the stimulus phase.
(c) Stimulus phase
Following the mating phase, we separated each dam from her sire and rehoused her in a divided cage. The divided cage also housed four stimulus males, from which she was separated by a clear perforated Perspex partition. Stimulus males had been equally, randomly divided into five treatment groups. Each group contained 40 subject males: (A) Babesia treatment: infection with 5×107 red blood cells harbouring B. microti (infection treatment); (B) Sham Babesia: sham infection of B. microti, comprising only the vehicle used to suspend erythrocytes in treatment A (infection vehicle control treatment); (C) SRBC treatment: inoculation with 5×107 sheep red blood cells (immune activity treatment); (D) sham SRBC: sham inoculation of SRBCs, comprising only the vehicle used to suspend the SRBCs (immune activity vehicle control treatment); (E) complete control: no treatment (unmanipulated control). Immediately following treatment, stimulus males were housed in within-treatment groups of four on one side the divided cages. Multiple stimulus males per dam were used to standardize the stimulus exposure to each dam (i.e. to minimize the effects of variance among males). Four stimulus males would allow for this standardization, without increasing the risk of inter-male aggression (high in groups of two or three) or causing overcrowding (Sherwin 2002; Van Loo et al. 2003). Subject dams were divided into one of five indirect exposure groups, with 10 females per group. We kept dams in this stimulus phase for 10 days. At the end of the stimulus phase, we transferred the dams to a new clean cage to give birth.
(d) Pre-weaning phase
At 11 days of age, all pups were sexed and each litter was reduced to four males (three males in the case of three litters that had only three males each). This was to enable manageable sample sizes for individual observations both pre-weaning and in subsequent phases. Male pups were chosen because previous work has shown important dominance–resistance trade-offs in male mice (Barnard et al. 1997a,b, 1998). A total of 145 male pups were included in the following stages of the experiment, from 37 females (13 dams did not become pregnant in the mating phase, but the likelihood of becoming pregnant was not affected by treatment (Χ2-test: X2=1.663, n=50, p=0.197)). We marked the male pups in an individually distinctive pattern using black eyelash dye (Colorsport, Brodie and Stone Plc, London, UK). At 24 days of age, all mothers were removed from litters, and pups (subject males) were left in their fraternal groups until 50 days old.
(e) Social grouping and single housing phases
At 50 days of age, all subject males were separated from their siblings. Approximately two-thirds of the subject males (88 mice) were rehoused with three novel males from the same treatment group, with whom they were allowed to establish dominance hierarchies. Continuous behavioural observations totalling 175 min (10 or 15 min per day) per group were carried out over the following 16 days. All observations were carried out during the active dark cycle of the day, at regular intervals between 0800 and 1900 h. Various social interactions were recorded, including the number of attacks, mounts and aggressive allogrooms, in order to determine social rank within these groups. The remaining third (57 mice) were housed singly to act as a non-socialized treatment. No effect of socializing treatment on infection profile was found (repeated-measures general linear model, GLM: F5,134=0.612, p=0.691; PC1 score GLM: F1,143=0.364, p=0.547), so data from socially and singly housed males were pooled in subsequent analysis. At the end of this phase, all males were removed and housed singly in new clean cages.
(f) Infection phase
At 70 days of age all of the adult offspring, all now housed singly, were injected with 5×107 red blood cells infected with B. microti. The time course of the infection was closely monitored by taking blood samples every other day until clearance. No other infection or immune challenge was given to the adult offspring.
(g) Technical procedures
All inoculations, infections and sham manipulations involved a single intra-peritoneal injection of 200 μl Hanks' solution, containing the appropriate inoculants. Stimulus treatment group E received no injection. All monitoring, sampling and handling for stimulus treatments A and C were repeated accordingly in sham treatments B, D and E. Sham infection and inoculation involved the introduction of Hanks' solution only. All subject males were infected in a random order with 5×107 infected red blood cells of Babesia. The King's 67 strain of B. microti was used throughout, and frozen stock was first passaged five times in BKW mice before being used on stimulus or subject males.
To monitor Babesia infection, a peripheral tail vein was nicked and a single drop of blood was transferred to a glass microscope slide every other day during the infection. The drop was immediately smeared to give monolayer of erythrocytes, then fixed and stained. For staining, fixed slides were placed in a solution of one part Giemsa stain to three parts Sorenson's buffer for 40 min, before rinsing in Sorenson's buffer and drying in air. Larger blood samples (50 μl) were also collected in heparinized haematocrit tubes, on no more than three occasions per animal, and never twice within a two-week period. Dams were sampled at the start and end of the stimulus phase. Offspring had 50 μl blood samples taken at weaning, prior to and at the end of social grouping. An additional blood sample was taken from all animals during autopsy.
The larger blood samples were assayed for testosterone (males only), corticosterone and total IgG (used as a bystander measure of immunocompetence; Barnard et al. 1996) using kits or reagents supplied by IDS Ltd, Tyne and Wear, UK (testosterone); R & D Systems Europe Ltd, Abington, UK (corticosterone); and Universal Biological, Cambridge, UK (IgG). All plates were processed using Micro plate Manager v. 5.2. In all cases, blood samples were anonymized and analysed in a random order, so that it was not possible for investigators to know the treatment group or relatedness of individual samples. In a number of cases, limited serum volumes precluded reliable estimates for all three serum factors at all time points from certain individuals. As a consequence, sample sizes of some analyses vary.
Autopsies were conducted in random order on subject males between the ages of 90 and 100 days, and on dams following weaning, when dams were approximately 100 days old. Autopsy involved the removal and weighing of the thymus, heart, spleen, left and right kidneys, left and right adrenal glands (males and females), uterus (females), left and right testes, seminiferous tubules and left and right preputial glands (males).
(h) Statistical analysis
Analysis was carried out using SPSS 15 (SPSS Inc., Chicago, IL, USA). Alpha was set at 0.05 and all tests were two-tailed. All analyses were based on pooled control treatment groups B–E as no significant differences were found between these groups (see the electronic supplementary material). Where appropriate, values were nested within dam (which was included as a random factor), or averaged per dam to avoid within-litter pseudoreplication. Principal components analysis conducted on five correlated variables describing individual infection profiles generated a principal component (PC1) with an eigenvalue of 2.652. Individual scores for this first component were then used in further analysis. Where appropriate, body mass was fitted as a covariate. As mentioned above, limited serum volumes precluded reliable estimates of serum factors for certain individuals. As a consequence, sample sizes and degrees of freedom vary for some analyses. A priori power tests were conducted to determine minimum sample sizes necessary to detect treatment effects in order to minimize animal use.
3. Results
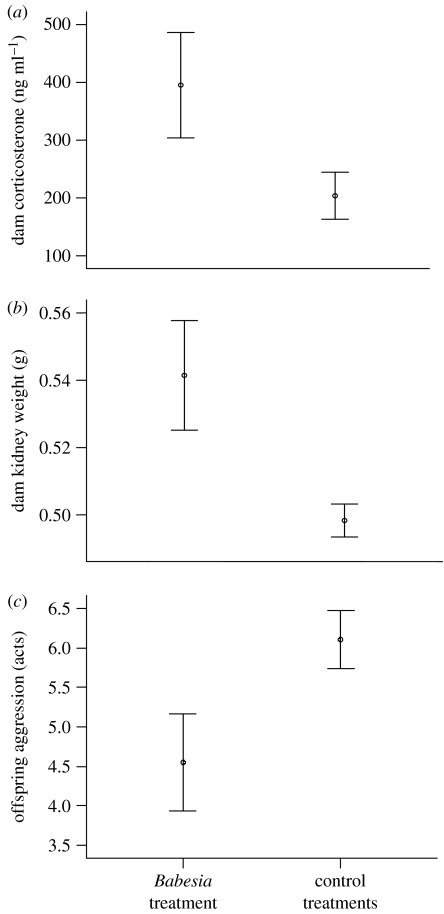
During the stimulus phase, we found that the pregnant dams housed opposite infected males (Babesia treatment) had blood serum levels of corticosterone twice the values recorded from dams housed opposite control males (absolute corticosterone following stimulus phase, GLM: F1,20=4.897, p=0.039; change in corticosterone over the stimulus phase: figure 2a, GLM: F1,20=4.931, p=0.038). Also, dams from the Babesia treatment had kidneys that were 8 per cent larger at autopsy than those of dams in control treatments (figure 2b, GLM: F1,34=9.391, p=0.004). There was no effect of treatment on dam IgG (repeated-measures GLM: F2,33=0.819, p=0.494) or spleen size (GLM: F1,34=1.537, p=0.224).
Figure 2.
Treatment effects on dam physiology and offspring behaviour. Differences between dams that had shared partitioned cages with males infected with B. microti and those that shared with control males in (a) change in dam corticosterone across stimulus phase (n=23 dams), (b) weight of both the kidneys of dams at autopsy (n=37), and (c) adult offspring aggression, measured as the total observed acts in novel social groups (n=88 male offspring from 37 dams). Error bars represent ±1 standard error (s.e.).
Following the stimulus phase, all dams were transferred to new clean cages to give birth. There was no effect of treatment on litter size at birth (F1,35=0.612, p=0.439) or litter sex ratio (F1,35=0.837, p=0.367). At 11 days of age, all litters were standardized to four males. When re-housed with novel males as adults, offspring from Babesia-treatment dams were significantly less aggressive than those from dams in control treatments (figure 2c, GLM: F1,42=5.508, p=0.024). While testosterone levels in offspring did predictably (Barnard et al. 1994) correlate with aggression (Pearson's correlation: n=86, r=0.295, p=0.006), experimental treatment was not found to have a significant effect on this hormone (repeated-measures GLM: F3,33=1.708, p=0.184). There was no effect of treatment on offspring IgG (repeated-measures GLM: F3,33=1.021, p=0.397).
Finally, all offspring were rehoused singly into new clean cages, and infected with B. microti. Offspring from Babesia-treatment dams showed a different response to disease across the period of infection (figure 3a; repeated-measures GLM: F5,30=2.612, p=0.045). This difference reflected an accelerated time course of infection, with offspring from Babesia-treatment dams showing earlier onset, peak and clearance of infection than offspring from control dams (table 1; figure 3b, GLM: F1,34=4.314, p=0.045).
Figure 3.
Treatment effects on adult offspring infection profiles. (a) The time course of infection with B. microti in adult offspring of dams exposed during pregnancy to infected (red dashed line) or control (blue solid line) males. (b) Scores for the first component (PC1) extracted by principal components analysis of five characteristics of the infection profile for offspring from (a) Babesia- and (b) control-treatment dams (see table 1: high positive scores reflect later onset, peak and clearance of infection; n=145 male offspring from 37 dams). Error bars represent ±1 (s.e.).
Table 1.
Principal component analysis of infection profile, showing loadings derived from analysis of the time course of the infection. Scores for different treatments are shown in figure 3b.
| component 1 | |
|---|---|
| eigenvalue | 2.652 |
| percentage of variance | 53.032 |
| loadings | |
| day of onset of infectiona | 0.943 |
| day of peak of infection | 0.921 |
| duration of infection (onset to clearance)b | 0.724 |
| time to clearance (from inoculation) | −0.454 |
| infection level at the first clearance in the population | 0.428 |
Onset measured as the day on which infected red blood cells were first seen in blood smears.
Clearance measured as the first day on which no red blood cells were infected following an infection peak.
4. Discussion
We found that the pregnant dams housed in partitioned cages opposite infected conspecifics were able to detect the effects of infection in those individuals. This information caused physiological changes (elevated levels of serum corticosterone and increased kidney size) in these indirectly challenged dams. Treatment also led to changes in the adult behaviour of sons, with those that had developed in mothers exposed to ambient cues indicating threat of disease showing lower levels of aggression as adults. Most importantly, these sons then showed a different response to infection with B. microti, peaking and clearing infection earlier than individuals that had developed in mothers that did not have diseased neighbours.
Corticosterone is the primary glucocorticoid found in rats and mice, and is homologous to cortisol in humans and many other mammals (Edwards & Burnham 2001). Elevated levels of glucocorticoid reflect physiological changes associated with stressful stimuli (De Kloet et al. 2005), including social information about distress (Boissy et al. 1998) and immune responses (Fernandes 2000) in conspecifics. Therefore, the patterns seen here are consistent with the conclusion that the females had detected disease in neighbours. This detection did not appear to illicit an immune response in the Babesia-treatment dams (there was no effect on IgG production or spleen growth). The unusual kidney growth seen is difficult to explain, but may be the result of the kidney's proximity to the adrenal gland, which was overacting to produce high levels of corticosterone in these females. We did not see any corresponding behavioural indicators of stress in the behaviour of the dams during the stimulus phase (see the electronic supplementary material) and no aversive or avoidance behaviours were observed (O. Curno 2006, personal observation).
As the females in this study were exposed to auditory, behavioural, visual and olfactory information about their neighbours, a number of cues may have been used to detect disease in the stimulus males. There is no known effect of infectious disease on mouse vocalizations, but with increasing understanding of the complexity of mouse ‘song’ (Holy & Guo 2005) such a relationship may emerge, and may have provided an indicator for dams in this experiment. Behavioural cues may also have played a role, but B. microti at the inoculation levels we used has few recorded effects on host behaviour (Barnard et al. 1996), and we found no differences in the behaviour between infected and uninfected stimulus males (see the electronic supplementary material). The eyes and ears of laboratory mice become marginally paler at peak infection due to mild anaemia (Homer et al. 2000), but it is unlikely that this would have been an important cue given the poor eyesight of albino mice. Disease status is perhaps most likely to have been assessed via olfactory cues (Gelperin 2008).
In experiments in which only urine was offered to subjects, mice were able to detect infection in conspecifics (Penn & Potts 1998; Penn et al. 1998; Kavaliers et al. 2003). The underlying mechanism is currently unknown, but there are various hypotheses. Infections can change the composition of commensal microbes that play an important role in individual odour (Penn & Potts 1998; Hurst et al. 2001). Infection also leads to increased expression of MHC molecules (influencing the concentration of volatile acids in the urine) and changes the concentration of excreted endocrine by-products (Penn & Potts 1998). Antigens, antibodies and elevated levels of NO have been found in the urine of malaria patients (a similar intraerythrocytic parasite to Babesia) (Rodriguez-del Valle et al. 1991; Anstey et al. 1996). In our study, females may have also detected components of the parasites shed in the faeces, or subtle changes in the stimulus males' behaviour that we were not able to detect. Thus, the females in this study had access to a broad range of social information with which they could determine the disease status of their neighbours, with important consequences for their own physiology and ultimately the development of their male offspring.
Male offspring that developed in the Babesia-treatment environment were less aggressive as adults. Pre- and post-natal maternal corticosterone levels have been shown to affect the behaviour of offspring as adults via in utero exposure through the blood or post partum through milk (Edwards & Burnham 2001). Improved learning, reduced anxiety, sleep disturbances, enhanced fear, reduced social interactions and decreased exploratory behaviour have all been found as a result of endogenously or exogenously elevated corticosterone (or cortisol) (Edwards & Burnham 2001) during development. In humans, maternal stress during pregnancy is associated with a range of physical and behavioural effects in children and adult offspring (Edwards & Burnham 2001; Gluckman et al. 2005; Rickard & Lummaa 2007). Maternal stress during pregnancy may affect offspring development through an effect on foetal hormone production. Maternal stress has been shown to reduce the normal testosterone surge during development in male rat foetuses with downstream effects on adult male sexual behaviour (Pollard & Dyer 1985; Ward et al. 2003). It is therefore possible that the behavioural changes found here among the adult offspring of Babesia-treatment mothers were mediated by maternal corticosterone through foetal testosterone modulation. Interestingly, other studies have also found that offspring behavioural changes due to maternal stress are not evident before weaning in either the mother or offspring, but emerge later when the offspring reaches adulthood (Casolini et al. 1997), as was the case in our study (see the electronic supplementary material).
Aggression in adult mice is associated with social dominance, territory acquisition and maintenance, and consequently increased access to mating opportunities (Barnard et al. 1994; Meagher et al. 2000; Waterman 2007). However, there is evidence that the benefits of aggressive behaviour are counterbalanced by costs associated with reduced resistance to disease (Barnard et al. 1994; Whitacre 2001; Zala et al. 2008b). In our study, male offspring that developed in a Babesia-treatment environment showed both accelerated response to infection (particularly an accelerated resolution phase; figure 3), and reduced aggression in novel social groups. Our results thus support the existence of a trade-off between social dominance and disease resistance. Evidence from other studies strongly implicates testosterone in the mediation of dominance–resistance trade-offs (Folstad & Karter 1992; Barnard et al. 1994; Whitacre 2001; Decristophoris et al. 2007). Of particular relevance in the context of our study, testosterone is known to interact with CD4+T-cells (Roberts et al. 2001), which are important for the resolution phase of B. microti infection (Homer et al. 2000). However, as we were not able to detect a significant effect of treatment on testosterone levels (see the electronic supplementary material), we cannot confirm a direct role for this hormone in the trade-off that we appear to have observed. Nevertheless, our results do point to a role for maternal corticosterone in the response to exposure to diseased conspecifics. Elevated levels of corticosterone may have led to the observed decrease in costly aggressive behaviour and the altered immune response seen in the male Babesia-treatment offspring. These results are consistent with evidence from the literature on birds, where maternal hormones are increasingly recognized to be one of the most important factors mediating transgenerational immune priming (Tschirren et al. 2004; Groothuis et al. 2005b; Müller et al. 2005).
We suggest that our study demonstrates adaptive investment in immunocompetence (Manz et al. 2005; clearing infection sooner) in a situation where the imminent threat of infection has been perceived, possibly at the expense of investment in the acquisition of dominance. Our experimental design limits our ability to speculate on the specificity of the response we have observed, and it would be interesting to investigate in further experiments the extent to which exposure of mothers to infected conspecifics generates a more generalized altered sensitivity to all stressors or infections in their offspring. Whether general or specific, accelerated clearance of infection would return individuals to a competitive (Kilpimaa et al. 2004) and attractive (Hamilton & Zuk 1982; Ehman & Scott 2002) state more quickly, and thus enable them to secure future mating opportunities. Such benefits could outweigh any costs associated with reduced social dominance in an environment, where the risk of disease is high. Alternatively, the responses seen may not have been a response by offspring to a threat of disease specifically, but a generalized adaptation of life-history strategy in the presence of maternal stress. Either way, we conclude that either dams (through strategic maternal investment; Marshall & Uller 2007) or offspring (through individual life-history ‘decisions’) responded adaptively to an ambient threat of infection in order to maximize the chances of offspring survival (Hazel et al. 2000), and ultimately reproductive success.
To our knowledge, this study provides the first evidence for transgenerational regulation of immune response based on social information, and the implications of our findings are wide-reaching. These results are of great significance for our understanding of the role of parasites in the evolution of life histories (Virgin 2007), adding maternal perception of disease risk in the immediate environment to the factors potentially determining future social dominance, and related aspects of fitness, in offspring. Furthermore, the individual differences in disease susceptibility found within many species, including humans (Bateson et al. 2004; Rickard & Lummaa 2007), which are known to detect disease from the odours of others (Penn & Potts 1998), might be explained in part by similar maternal effects (Zinkernagel 2000). Through immunological maternal effects, individual decisions may have population-level consequences in the following generations (Mitchell & Read 2005), adding a new complexity to our understanding of epidemiological processes.
Moreover, our findings have implications for both animal welfare and the validity of scientific procedures. Most animal housing units enable some level of auditory and olfactory interaction between individuals. Here, we have shown that a measurable perception of disease in neighbouring conspecifics occurs, and elicits a stress response in the perceiver. Therefore, our results highlight the potential for substantial and unexpected effects of experimental design on animal welfare. Given this, we support the call for animal welfare sections in our publications (Würbel 2007) with the added suggestion that the welfare of bystanders, as well as experimental subjects, should be considered when planning experimental work. Finally, we begin to question the accuracy of considering co-housed ‘control’ animals as ‘untreated’ in scientific procedures, when in fact we have shown that they may respond in complex physiological and behavioural ways to ambient information from their treated neighbours. Further work to establish the mechanistic basis for our results, and the extent to which they can be generalized, is thus very desirable.
Acknowledgments
All the procedures were conducted under UK Home Office License and with approval from the appropriate government and university animal ethics committees (Animals (Scientific Procedures) Act: code of practice for the housing and care of animals used in scientific procedures, 1989).We thank J. Bielby, J. Bradley, J. Gibson, F. Gilbert and A. MacColl for support, advice and comments on the manuscript. We thank H. Travis and A. Lowe for their technical support. This work was supported by the BBSRC and the School of Biology, University of Nottingham.
Supplementary Material
1. No differences were found between the different control treatments. 2. There was no effect of Babesia treatment on various physiological and behavioural responses.
References
- Agrawal A.A., Laforsch C., Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999;401:60–63. doi:10.1038/43425 [Google Scholar]
- Anstey N.M., et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 1996;184:557–567. doi: 10.1084/jem.184.2.557. doi:10.1084/jem.184.2.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard C.J., Behnke J.M. Behaviour, life-history strategies and parasite infection in rodents. In: Morand S.S., Krasnov B., Poulin R., editors. Micromammals and macroparasites: from evolutionary ecology to management. Springer-Verlag Publishing; Tokyo, Japan: 2006. pp. 475–514. [Google Scholar]
- Barnard C.J., Behnke J.M., Sewell J. Social behaviour and susceptibility to infection in house mice (Mus musculus): effects of group size, aggressive behaviour and status-related hormonal responses prior to infection on resistance to Babesia microti. Parasitology. 1994;108:487–496. doi: 10.1017/s0031182000077349. [DOI] [PubMed] [Google Scholar]
- Barnard C.J., Behnke J.M., Sewell J. Environmental enrichment, immunocompetence, and resistance to Babesia microti in male mice. Physiol. Behav. 1996;60:1223–1231. doi: 10.1016/s0031-9384(96)00174-6. doi:10.1016/S0031-9384(96)00174-6 [DOI] [PubMed] [Google Scholar]
- Barnard C.J., Behnke J.M., Gage A.R., Brown H., Smithurst P.R. Immunity costs and behavioural modulation in male laboratory mice (Mus musculus) exposed to the odours of females. Physiol. Behav. 1997a;62:857–866. doi: 10.1016/s0031-9384(97)00249-7. doi:10.1016/S0031-9384(97)00249-7 [DOI] [PubMed] [Google Scholar]
- Barnard C.J., Behnke J.M., Gage A.R., Brown H., Smithurst P.R. Modulation of behaviour and testosterone concentration in immunodepressed male laboratory mice (Mus musculus) Physiol. Behav. 1997b;61:907–917. doi: 10.1016/s0031-9384(97)00011-5. doi:10.1016/S0031-9384(97)00011-5 [DOI] [PubMed] [Google Scholar]
- Barnard C.J., Behnke J.M., Gage A.R., Brown H., Smithurst P.R. Maternal effects on the development of social rank and immunity trade-offs in male laboratory mice (Mus musculus) Proc. R. Soc. B. 1998;265:2087–2093. doi: 10.1098/rspb.1998.0544. doi:10.1098/rspb.1998.0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard C.J., Collins S.A., Daisley J.N., Behnke J.M. Maze performance and immunity costs in mice. Behaviour. 2005;142:241–263. doi:10.1163/1568539053627686 [Google Scholar]
- Bateson P., et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. doi:10.1038/nature02725 [DOI] [PubMed] [Google Scholar]
- Bogdan C. Oxidative burst without phagocytes: the role of respiratory proteins. Nat. Immunol. 2007;8:1029–1031. doi: 10.1038/ni1007-1029. doi:10.1038/ni1007-1029 [DOI] [PubMed] [Google Scholar]
- Boissy A., Terlouw C., Le Neindre P. Presence of cues from stressed conspecifics increases reactivity to aversive events in cattle: evidence for the existence of alarm substances in urine. Physiol. Behav. 1998;63:489–495. doi: 10.1016/s0031-9384(97)00466-6. doi:10.1016/S0031-9384(97)00466-6 [DOI] [PubMed] [Google Scholar]
- Brennan P.A., Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. doi: 10.1038/nature05404. doi:10.1038/nature05404 [DOI] [PubMed] [Google Scholar]
- Casolini P., Cigliana G., Alema G.S., Ruggieri V., Angelucci L., Catalani A. Effect of increased maternal corticosterone during lactation on hippocampal corticosteroid receptors, stress response and learning in offspring in the early stages of life. Neuroscience. 1997;79:1005–1012. doi: 10.1016/s0306-4522(96)00668-9. doi:10.1016/S0306-4522(96)00668-9 [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Joels M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. doi:10.1038/nrn1683 [DOI] [PubMed] [Google Scholar]
- Decristophoris P.M.A., von Hardenberg A., McElligott A.G. Testosterone is positively related to the output of nematode eggs in male Alpine ibex (Capra ibex) feces. Evol. Ecol. Res. 2007;9:1–16. [Google Scholar]
- Edwards H.E., Burnham W.M. The impact of corticosteroids on the developing animal. Pediatr. Res. 2001;50:433–440. doi: 10.1203/00006450-200110000-00003. doi:10.1203/00006450-200110000-00003 [DOI] [PubMed] [Google Scholar]
- Ehman K.D., Scott M.E. Female mice mate preferentially with non-parasitized males. Parasitology. 2002;125:461–466. doi: 10.1017/s003118200200224x. doi:10.1017/S003118200200224X [DOI] [PubMed] [Google Scholar]
- Fernandes G.A. Immunological stress in rats induces bodily alterations in saline-treated conspecifics. Physiol. Behav. 2000;69:221–230. doi: 10.1016/s0031-9384(99)00226-7. doi:10.1016/S0031-9384(99)00226-7 [DOI] [PubMed] [Google Scholar]
- Folstad I., Karter A.J. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992;139:603. doi:10.1086/285346 [Google Scholar]
- Gelperin A. Neural computations with mammalian infochemicals. J. Chem. Ecol. 2008;34:928–942. doi: 10.1007/s10886-008-9483-6. doi:10.1007/s10886-008-9483-6 [DOI] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A., Spencer H.G., Bateson P. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc. R. Soc. B. 2005;272:671–677. doi: 10.1098/rspb.2004.3001. doi:10.1098/rspb.2004.3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff J.L., Hasselquist D., Nilsson J.-A., Sandell M., Smith H.G., Stjernman M. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc. R. Soc. B. 2006;273:2551–2557. doi: 10.1098/rspb.2006.3608. doi:10.1098/rspb.2006.3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis T.G., Eising C.M., Dijkstra C., Müller W. Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol. Lett. 2005a;1:78–81. doi: 10.1098/rsbl.2004.0233. doi:10.1098/rsbl.2004.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis T.G., Muller W., von Engelhardt N., Carere C., Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005b;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. doi:10.1016/j.neubiorev.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D., Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. doi:10.1126/science.7123238 [DOI] [PubMed] [Google Scholar]
- Hazel S.M., Bennett M., Chantrey J., Bown K., Cavanagh R., Jones T.R., Baxby D., Begon M. A longitudinal study of an endemic disease in its wildlife reservoir: cowpox and wild rodents. Epidemiol. Infect. 2000;124:551–562. doi: 10.1017/s0950268899003799. doi:10.1017/S0950268899003799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy T.E., Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. doi:10.1371/journal.pbio.0030386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer M.J., Aguilar-Delfin I., Telford S.R., III, Krause P.J., Persing D.H. Babesiosis. Clin. Microbiol. Rev. 2000;13:451–469. doi: 10.1128/cmr.13.3.451-469.2000. doi:10.1128/CMR.13.3.451-469.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst J.L., Payne C.E., Nevison C.M., Marie A.D., Humphries R.E., Robertson D.H.L., Cavaggioni A., Beynon R.J. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. doi:10.1038/414631a [DOI] [PubMed] [Google Scholar]
- Igarashi I., et al. Roles of CD4+T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infect. Immun. 1999;67:4143–4148. doi: 10.1128/iai.67.8.4143-4148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N., Tan N.S., Ho B., Ding J.L. Respiratory protein-generated reactive oxygen species as an antimicrobial strategy. Nat. Immunol. 2007;8:1114–1122. doi: 10.1038/ni1501. doi:10.1038/ni1501 [DOI] [PubMed] [Google Scholar]
- Kavaliers M., Fudge M.A., Colwell D.D., Choleris E. Aversive and avoidance responses of female mice to the odors of males infected with an ectoparasite and the effects of prior familiarity. Behav. Ecol. Sociobiol. 2003;54:423–430. doi:10.1007/s00265-003-0631-2 [Google Scholar]
- Kavaliers M., Choleris E., Pfaff D.W. Genes, odours and the recognition of parasitized individuals by rodents. Trends Parasitol. 2005;21:423–429. doi: 10.1016/j.pt.2005.07.008. doi:10.1016/j.pt.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Kilpimaa J., Alatalo R.V., Siitari H. Trade-offs between sexual advertisement and immune function in the pied flycatcher (Ficedula hypoleuca) Proc. R. Soc. B. 2004;271:245–250. doi: 10.1098/rspb.2003.2568. doi:10.1098/rspb.2003.2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-Y., et al. First case of human babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine Babesia. J. Clin. Microbiol. 2007;45:2084–2087. doi: 10.1128/JCM.01334-06. doi:10.1128/JCM.01334-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjemtrup A.M., Conrad P.A. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 2000;30:1323–1337. doi: 10.1016/s0020-7519(00)00137-5. doi:10.1016/S0020-7519(00)00137-5 [DOI] [PubMed] [Google Scholar]
- Koyama S. Primer effects by conspecific odors in house mice: a new perspective in the study of primer effects on reproductive activities. Horm. Behav. 2004;46:303–310. doi: 10.1016/j.yhbeh.2004.03.002. doi:10.1016/j.yhbeh.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Manz R.A., Hauser A.E., Hiepe F., Radbruch A. Maintenance of serum antibody levels. Annu. Rev. Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. doi:10.1146/annurev.immunol.23.021704.115723 [DOI] [PubMed] [Google Scholar]
- Marshall D.J., Uller T. When is a maternal effect adaptive? Oikos. 2007;116:1957–1963. doi:10.1111/j.2007.0030-1299.16203.x [Google Scholar]
- Meagher S., Penn D.J., Potts W.K. Male–male competition magnifies inbreeding depression in wild house mice. Proc. Natl Acad. Sci. 2000;97:3324–3329. doi: 10.1073/pnas.060284797. doi:10.1073/pnas.060284797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.E., Read A.F. Poor maternal environment enhances offspring disease resistance in an invertebrate. Proc. R. Soc. B. 2005;272:2601–2607. doi: 10.1098/rspb.2005.3253. doi:10.1098/rspb.2005.3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Groothuis T.G.G., Kasprzik A., Dijkstra C., Alatalo R.V., Siitari H. Prenatal androgen exposure modulates cellular and humoral immune function of black-headed gull chicks. Proc. R. Soc. B. 2005;272:1971–1977. doi: 10.1098/rspb.2005.3178. doi:10.1098/rspb.2005.3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn D.J., Potts W.K. Chemical signals and parasite-mediated sexual selection. Trends Ecol. Evol. 1998;13:391–396. doi: 10.1016/s0169-5347(98)01473-6. doi:10.1016/S0169-5347(98)01473-6 [DOI] [PubMed] [Google Scholar]
- Penn D.J., Schneider G., White K., Slev P., Potts W.K. Influenza infection neutralizes the attractiveness of male odor to female mice (Mus musculus) Ethology. 1998;104:685–694. [Google Scholar]
- Pollard I., Dyer S.I. Effects of stress administered during pregnancy on the development of fetal testes and their subsequent function in the adult rat. J. Endocrinol. 1985;107:241–245. doi: 10.1677/joe.0.1070241. [DOI] [PubMed] [Google Scholar]
- Randolph S.E. The effect of Babesia microti on feeding and survival in its tick vector. Ixodes trianguliceps. Parasitology. 1991;102:9–12. doi: 10.1017/s0031182000060285. [DOI] [PubMed] [Google Scholar]
- Rickard I.J., Lummaa V. The predictive adaptive response and metabolic syndrome: challenges for the hypothesis. Trends Endocrinol. Metab. 2007;18:94–99. doi: 10.1016/j.tem.2007.02.004. doi:10.1016/j.tem.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Roberts C.W., Walker W., Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001;14:476–488. doi: 10.1128/CMR.14.3.476-488.2001. doi:10.1128/CMR.14.3.476-488.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-del Valle M., Quakyi I.A., Amuesi J., Quaye J.T., Nkrumah F.K., Taylor D.W. Detection of antigens and antibodies in the urine of humans with Plasmodium falciparum malaria. J. Clin. Microbiol. 1991;29:1236–1242. doi: 10.1128/jcm.29.6.1236-1242.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin C.M. Comfortable quarters for mice in research institutions. In: Reinhardt V., Reinhardt A., editors. Comfortable quarters for laboratory animals. Animal Welfare Institute; Washington, DC: 2002. pp. 6–17. [Google Scholar]
- Tschirren B., Richner H., Schwabl H. Ectoparasite-modulated deposition of maternal androgens in great tit eggs. Proc. R. Soc. B. 2004;271:1371–1375. doi: 10.1098/rspb.2004.2730. doi:10.1098/rspb.2004.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loo P.L.P., Van Zutphen L.F.M., Baumans V. Male management: coping with aggression problems in male laboratory mice. Lab. Anim. 2003;37:300–313. doi: 10.1258/002367703322389870. doi:10.1258/002367703322389870 [DOI] [PubMed] [Google Scholar]
- Virgin H.W. In vivo veritas: pathogenesis of infection as it actually happens. Nat. Immunol. 2007;8:1143–1147. doi: 10.1038/ni1529. doi:10.1038/ni1529 [DOI] [PubMed] [Google Scholar]
- Ward I.L., Ward O.B., Affuso J.D., Long W.D., French J.A., Hendricks S.E. Fetal testosterone surge: specific modulations induced in male rats by maternal stress and/or alcohol consumption. Horm. Behav. 2003;43:531–539. doi: 10.1016/s0018-506x(03)00061-8. doi:10.1016/S0018-506X(03)00061-8 [DOI] [PubMed] [Google Scholar]
- Waterman J.M. Male mating strategies in rodents. In: Wolff J.O., Sherman P.W., editors. Rodent societies: an ecological and evolutionary perspective. Chicago University Press; Chicago, IL: 2007. pp. 27–41. [Google Scholar]
- Whitacre C.C. Sex differences in autoimmune disease. Nat. Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. doi:10.1038/ni0901-777 [DOI] [PubMed] [Google Scholar]
- Würbel H. Publications should include an animal-welfare section. Nature. 2007;446:257. doi: 10.1038/446257a. doi:10.1038/446257a [DOI] [PubMed] [Google Scholar]
- Zala S.M., Chan B.K., Bilbo S.D., Potts W.K., Nelson R.J., Penn D.J. Genetic resistance to infection influences a male's sexual attractiveness and modulation of testosterone. Brain Behav. Immun. 2008a;22:381–387. doi: 10.1016/j.bbi.2007.09.003. doi:10.1016/j.bbi.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Zala S.M., Potts W.K., Penn D.J. Exposing males to female scent increases the cost of controlling Salmonella infection in wild house mice. Behav. Ecol. Sociobiol. 2008b;62:895–900. doi:10.1007/s00265-007-0513-0 [Google Scholar]
- Zinkernagel R.M. What is missing in immunology to understand immunity? Nat. Immunol. 2000;1:181–185. doi: 10.1038/79712. doi:10.1038/79712 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. No differences were found between the different control treatments. 2. There was no effect of Babesia treatment on various physiological and behavioural responses.