Abstract
Using baited camera landers, the first images of living fishes were recorded in the hadal zone (6000–11 000 m) in the Pacific Ocean. The widespread abyssal macrourid Coryphaenoides yaquinae was observed at a new depth record of approximately 7000 m in the Japan Trench. Two endemic species of liparid were observed at similar depths: Pseudoliparis amblystomopsis in the Japan Trench and Notoliparis kermadecensis in the Kermadec Trench. From these observations, we have documented swimming and feeding behaviour of these species and derived the first estimates of hadal fish abundance. The liparids intercepted bait within 100–200 min but were observed to preferentially feed on scavenging amphipods. Notoliparis kermadecensis act as top predators in the hadal food web, exhibiting up to nine suction-feeding events per minute. Both species showed distinctive swimming gaits: P. amblystomopsis (mean length 22.5 cm) displayed a mean tail-beat frequency of 0.47 Hz and mean caudal : pectoral frequency ratio of 0.76, whereas N. kermadecensis (mean length 31.5 cm) displayed respective values of 1.04 and 2.08 Hz. Despite living at extreme depths, these endemic liparids exhibit similar activity levels compared with shallow-water liparids.
Keywords: liparid, macrourid, Pseudoliparis amblystomopsis, Notoliparis kermadecensis, hadal zone, Pacific Ocean
1. Introduction
Exploration of the deep-sea has been characterized by an extension of the known depth ranges occupied by different taxa, following the speculation by Edward Forbes in 1844 that life may be absent at depths greater than 550 m (Anderson & Rice 2006). Demonstration of the occurrence of life throughout the abyssal depths (3000–6000 m) was popularized by expeditions such as the Challenger expedition (1872–1876) and the description of communities of animals living at the hadal depths (6000 to approx. 11 000 m) from the Danish Galathea and Russian Vityaz expeditions in the 1950s (Wolff 1960; Malyutina 2004). In contrast to the abyssal zone, which extends over more than 50 per cent of the planet's surface, the hadal zone occupies a very small area (approx. 0.56% of planet surface area) but does account for 45 per cent of the world's depth range. The hadal zone is fragmented into 22 deep trenches or depressions that are typically isolated from one another (Angel 1982).
Life in the trenches is dependent on two distinct pathways of energy supply: particulate detritus from overlying productivity and carrion falls. Blankenship & Levin (2007) described the partitioning of these two sources among hadal scavenging amphipods, which are the dominant natant fauna, and hypothesized that carrion feeding is relatively more important beneath oligotrophic waters. Indeed, Beliaev (1989) commented that carrion falls are sufficient to support mass populations of bottom-living scavengers even at 10.5 km depth, in oligotrophic trenches such as the Marianas Trench. It is now evident that hadal trenches support significant communities of animals, with over 50 per cent of species endemic to these depths (Beliaev 1989), but the role of fishes remains unclear; Wolff (1960) regarded them as ‘very insignificant’ in the hadal zone. The vivid report of a flatfish sighting from the ports of the manned bathyscaph Trieste in 1960, at 10 911 m depth in the Challenger Deep (Piccard & Dietz 1961), is now generally discounted (Wolff 1961), and the world's deepest recorded fish is recognized as Abyssobrotula galatheae, trawled from 8370 m (Nielson 1977). There is a logarithmic decline in the number of species of fishes with depth (Priede et al. 2006), with shallow-water species presumably excluded by adverse effects of pressure on cellular processes (Somero 1992; Macdonald 2001) and problems of increasing remoteness from surface-derived food resources. Extrapolating species number plots with depth, Chondrichthyes (sharks, rays and chimaeras) become theoretically extinct at 3850 m, and the maximum depth for Osteichthyes (bony fish) is 8350 m (Priede et al. 2006). Hitherto the hadal ichthyofauna has been known exclusively from a small number of preserved trawled specimens now sequestered as type specimens in various museums around the world. While major advances have been made in knowledge of the behaviour of abyssal bottom-living fishes using baited camera systems, with the deepest recordings down to 5900 m (King & Priede 2008), no data are available at depths beyond 6000 m in the hadal regions. This study documents the first in situ observations of living fishes at the hadal depths, giving indications of their activity and feeding behaviour.
2. Material and methods
(a) Study sites
Autonomous baited camera landers were deployed by free fall at four study sites in the Pacific Ocean: two in the northwest (Japan Trench and Marianas region) and two in the southwest (Kermadec and Tonga Trenches). Hadal lander A was deployed to 6945 m in the northern Japan Trench, 5469 m in the Marianas region, 6007, 6890 and 7966 m in the Kermadec Trench and 8798 and 9729 m in the Tonga Trench. Hadal lander B was only deployed at 5469 m in the abyssal Marianas region (table 1).
Table 1.
List of deployments of the baited landers. (The video system was used in all deployments except 2*, in which a digital still camera was deployed. Depth in decibars was converted to metres, assuming a salinity of 34.7 ppt (Saunders 1981).)
| station | depth | latitude | longitude | region | date | first arrival (min) | fish species | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (decibars) | (m) | temp (°C) | duration (hr.min) | Crustacea | fish | ||||||
| 1 | 5575 | 5469 | 18° 49.2′ N | 149° 50.6′ E | Marianas | 29 Nov | 1.5 | 08.55 | 20 | 52 | Coryphaenoides yaquinae |
| 2* | 5575 | 5469 | 18° 49.1′N | 149° 51.0′ E | Marianas | 29 Nov | 1.5 | 19.15 | 18 | 60 | Coryphaenoides yaquinae |
| 148 | Ophidiidae | ||||||||||
| 3 | 6133 | 6007 | 26° 43.9′ S | 175° 11.3′ W | Kermadec | 7 Jul | 1.2 | 04.27 | 21 | — | — |
| 4 | 7049 | 6890 | 28° 48.7′ S | 175° 18.1′ W | Kermadec | 8 Jul | 1.3 | 06.02 | 20 | 136 | Notoliparis kermadecensis |
| 5 | 7115 | 6945 | 40° 15.3′ N | 144° 30.8′ E | Japan | 30 Oct | 1.8 | 09.26 | 10 | 104 | Coryphaenoides yaquinae |
| 194 | Pseudoliparis amblystomopsis | ||||||||||
| 6 | 8170 | 7966 | 26° 55.0′ S | 175° 30.7′ W | Kermadec | 10 Jul | 1.5 | 10.07 | 5 | — | — |
| 7 | 9036 | 8798 | 24° 08.1′ S | 175° 11.0′ W | Tonga | 14 Jul | 1.6 | 09.05 | 15 | — | — |
| 8 | 10 015 | 9729 | 24° 16.4′ S | 175° 09.3′ W | Tonga | 12 Jul | 1.8 | 08.44 | 5 | — | — |
The three trenches studied exhibit a typical V-shaped topography with soft sediment on the eastern flank (oceanic plate) and steeper slopes on the western flank (continental plate). All deployments were made on the eastern flanks.
The oligotrophic Kermadec and Tonga Trenches are within the South Pacific subtropical gyre (SPSG) province that has low export rate of primary production to the sea floor of 87 g C m−2 yr−1 (Longhurst et al. 1995). By contrast, the Japan Trench situated in the Kuroshio Current Province has higher primary production export rate of 193 g C m−2 yr−1. The abyssal Marianas region site (5469 m), situated to the east of the southern Marianas Trench within the North Pacific tropical gyre is the most oligotrophic of the three sites (primary production rate=59 g C m−2 yr−1).
No deep water is formed in the North Pacific as flow is predominantly driven by the thermohaline circulation (Stommel 1958; Warren & Owens 1985). The water masses are Antarctic in origin, having travelled northwards and clockwise along the western boundary of the South Pacific (Johnson 1998), making the Kermadec and Tonga trenches some of the coldest in the world (Beliaev 1989). Upon exiting these trenches via the Samoan Passage the water enters the Marianas region from the east before heading northwards into the Japan Trench, eventually turning in the Aleutian Trench. A steady flow of water is present at the bottom of these trenches and the bottom water masses are well ventilated (Johnson 1998). Beliaev (1989) reported temperatures between 1 and 2°C, no significant effects of salinity (34–35 ppt) and sufficient oxygen to support life in these trenches (4.5 ml l−1).
(b) Equipment
Two free-fall autonomous baited camera landers, rated to 12 000 m operational depths, were used to image the sea floor and scavenging fauna intercepting bait. The landers comprised a basic delivery system and scientific payload. The delivery system consisted of a mooring line with positively buoyant floatation modules coupled to it 10 m apart. The floatation modules were pairs of 17′ glass spheres rated to 12 000 m (Nautilus Marine Services, Germany). Attached to the end of the mooring was a 2.5 m high aluminium tripod supporting and protecting the scientific payload. Two purpose-built titanium acoustic releases (Oceano 2500 Ti-Ultimate Depth AR861B2T IXSEA, France) could be acoustically triggered from the ship to jettison three steel ballast weights. This action initiated ascent to the surface by virtue of the positive buoyancy at 35 m min−1.
The first lander, hadal lander A, was equipped with a 3CCD video camera (HV-D30; Hitachi, Japan) positioned 1 m above the sea floor looking vertically down at bait (approx. 1 kg of tuna). The camera and two 50 W lamps were focused on a horizontal 10 mm diameter bar in contact with the sea floor to which the bait was secured in the centre of a total field of view of 68×51 cm (0.35 m−2). The video was controlled and recorded autonomously by a modified DVR Inspector with an Opti-base encoder card (type MPG9005) providing a screen resolution of 704×576 pixels (NetMc Marine, UK) and powered by a 24 V lead–acid battery (Seabattery; DSP&L, USA). The video camera and control/logging system were housed in stainless steel 255 pressure housings rated to 12 000 m operational depth. The system can record up to 3 h in MPEG2 format, which was time-lapsed to record 1 min of footage every 5 min, allowing up to 15 hours observation time on the sea floor. Three baited invertebrate funnel traps were attached to the footpads of the lander to collect scavenging amphipods.
The second lander (hadal lander B) had the same basic delivery system but with a scientific payload comprising a five megapixel digital still camera (OE14-208) and single flash gun (OE11-242; Kongsberg Maritime, Norway). The camera was capable of taking over 1000 images per deployment in JPEG format. On both landers, temperature and pressure were recorded every 30 s throughout the deployment by SBE-39 loggers (Seabird Electronics, USA). Near-bed current velocity estimates were made by tracking particles in the water resuspended by the activity of bottom fauna.
(c) Data analysis
Each 1 min video sequence was manually analysed. Individual fish of the same species were identified by body lengths and exterior condition. Body lengths were measured by a comparison with the in situ scale bar in the field of view. To measure tail-beat frequency, the video sequences (25 fps) were played back frame by frame permitting the number of full stroke tail beats per second (Hz) to be calculated.
The relative abundance of amphipods at each site was assessed by counting the number of visible amphipods swimming around the bait. At the midpoint of each sequence, the video was played back frame by frame and the number of individuals recognized as swimming was counted within the field of view. However, the resolution of the video camera did not permit exact counts and identification of small individuals (less than approx. 5 mm). Thus, the counts do not indicate the absolute density of individual amphipod species but the relative abundance of individual amphipods of more than approximately 5 mm swarming over the bait. This methodology was preferred on the basis of indicating a significant fraction of amphipod biomass present at each site. Since this was employed at all sites, between-site comparisons are consistent in this study.
Regression analysis was performed using SPSS (SPSS Inc., USA) to assess the role of depth gradient in explaining the observed variability in (i) amphipod first arrival time, (ii) amphipod maximum abundance and (iii) fish first arrival time.
3. Results
(a) Environmental characteristics
Bottom temperature varied between 1.16°C at station 3 in the Kermadec Trench at 6007 m and, with the evidence of slight adiabatic heating with depth, 1.78°C at 9729 m (station 8) in the Tonga Trench (table 1). Temperatures in the Northern Hemisphere at corresponding depths were slightly warmer (1.77°C in the Japan Trench at station 5), which is attributed to the warming of bottom water moving northwards from its Antarctic origin. At all sites the substrate was sedimentary, with softer sediments found at the 5500–8000 m sites, indicated by slight burial of the bait arm. At the two deep Tonga Trench sites, the sediment appeared harder, with the bait arm fully exposed. Near-bed current velocity estimates were the strongest at the Kermadec Trench locations (approx. 8–14 cm s−1), in contrast to the other sites (3–7 cm s−1, Japan Trench; 5–9 cm s−1 Marianas region). The current speeds in the Tonga Trench were too low to estimate with confidence.
(b) Crustacea
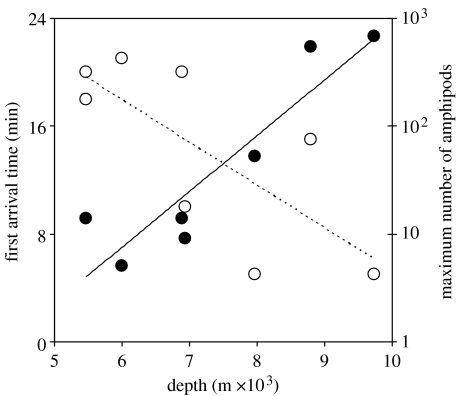
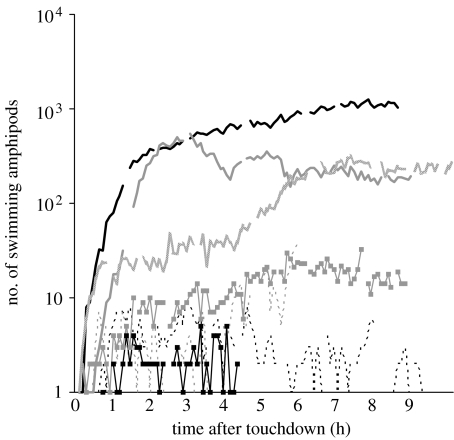
In all localities, amphipods were the first to intercept the bait, with the first individual appearing 5–21 min after touchdown and a trend of shorter delays at the greatest depths (table 1; figure 1, dotted line). As more individuals arrived, dense aggregations of amphipods accumulated around the bait, particularly towards the deepest sites (figure 2), with the highest number of 680 observed during the first 4.5 hours of deployment (based on the shortest observation period of station 3) at 9729 m in the Tonga Trench compared with less than 10 individuals typically present at the shallowest stations (figure 3a–c). There was a trend of exponential increase in numbers with depth (figure 1, solid line). Arrival times at baits can also be used to estimate population densities of deep-sea amphipods (Sainte-Marie & Hargrave 1987) and fishes (Priede et al. 1990), assuming an inverse square law relationship between abundance per unit area N and arrival time tarr,
Applying this to the arrival time data indicates a 10-fold increase in the abundance of amphipods between 6000 and 10 000 m depths. The resolution of the video camera relative to the small body size did not permit a positive identification of amphipods to species level; however, studies by Blankenship & Levin (2007) in the Tonga Trench and Kermadec Trench indicate these are lysianassoid amphipods, Eurythenes gryllus, at the shallowest stations; Scopelocheirus schellenbergi at 6500–8000 m; and there is an increased dominance of Hirondellea dubia from the middle to the deepest stations. Specimens were captured in auxiliary traps for identification and preliminary sorting, indicating the presence of these and further new species. In addition, the natantian decapod, Benthesicymus crenatus (Bate 1881), was observed at stations 1–5 (Jamieson et al. in press), as were isopods Storthyngura sp. in the Japan Trench at station 5 (figure 3d–f).
Figure 1.
Arrivals and maximum number of amphipods at bait as a function of depth in the western North and South Pacific Ocean. Open circles and dotted line represent time from touchdown to arrival of the first individual and fitted line (tarr=−0.0032D+37.057, n=8, R2=0.55, F=7.34, p<0.05). Filled circles and solid line represent the maximum number of swimming amphipods observed up to 4 : 27 hours (based on the shortest observation period of station 3) and fitted line (Y=0.00058e0.00119D, n=7, R2 =0.84, F=25.37, p<0.01).
Figure 2.
Relative abundance of swimming amphipods at bait during the deployment in the western North and South Pacific Ocean. Thick black line, 9729 m Tonga; thick grey line, 8798 m Tonga; grey dotted line, 7966 m Kermadec; black dashed line, 6945 m Japan; grey dashed line, 6890 m Kermadec; black line with squares, 6007 m Kermadec; grey line with squares, 5469 m Marianas.
Figure 3.
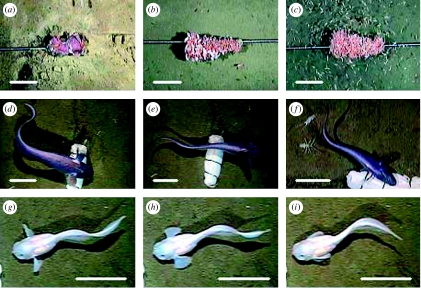
Images of hadal fauna taken from a baited video lander. All scale bars are 10 cm long. Views of the bait showing amphipods at 3 hours after touchdown at: (a) station 3, 6007 m depth, Kermadec Trench; (b) station 6, 7966 m depth, Kermadec Trench; (c) station 8, 9729 m depth, Tonga Trench. (d–f) Images of macrourid, putatively C. yaquinae and isopods Storthyngura sp. at station 5, 6945 m depth Japan Trench. (g–i) Images of liparid, P. amblystomopsis at station 5, 6945 m depth Japan Trench. Sequence of images 0.3 s apart showing synchrony in between pectoral caudal fins during one-half cycle.
(c) Fishes
Fishes arrived after much longer time delays than the Crustacea (table 1). The macrourid C. yaquinae (Iwamoto & Stein 1974) was the first species to arrive, at 5469 m in the Marianas region. An ophidiid, cusk-eel, was also present at station 2 at 3, 12 and 18 hours after touchdown. Two C. yaquinae were also observed at 6945 m in the Japan Trench, a new maximum depth record for macrourid fishes and the first observation from hadal depths (figure 3d–f).
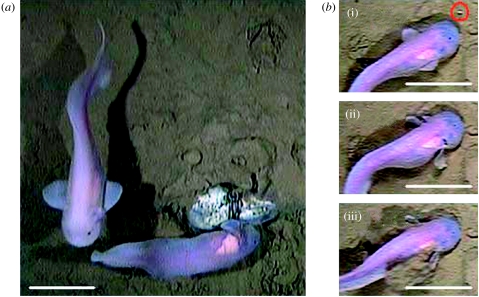
Two species of endemic liparids, both of which had never previously been seen alive, were observed at the hadal depths: a Pseudoliparis amblystomopsis (Andriashev 1955) at 6945 m in the Japan Trench (figure 3g–i) and Notoliparis kermadecensis (Nielson 1964) at 6890 m in the Kermadec Trench (figure 4). The species identification was based on morphological characteristics detailed in holotype descriptions of previously known liparids from each region (Andriashev 1955; Nielson 1964). Identification to species level from video footage cannot be achieved with absolute confidence as there is always a chance of inconspicuous morphological variation, particularly in these rarely studied fishes. However, based on historical findings, taxonomic descriptions and the extremely low fish diversity apparent at these depths, it is highly likely that these fishes are N. kermadecensis and P. amblystomopsis.
Figure 4.
(a) Image of liparid, N. kermadecensis taken from a baited video lander at station 4, 6890 m depth at the Kermadec Trench. (b) Sequence showing the capture of an amphipod by suction feeding: (i) approach to prey indicated by red circle (0.0 s), (ii) prey captured by action of the buccal suction pump (1.0 s), (iii) exhalation of sediment through the opercular openings during ingestion (1.5 s). Scale bars, 10 cm.
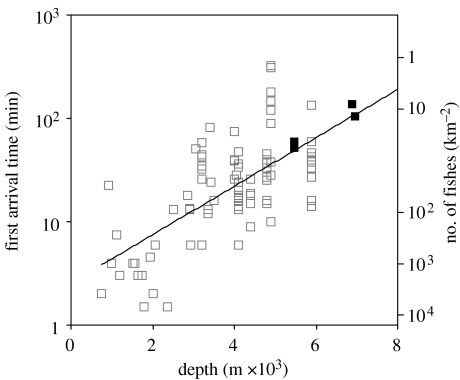
The arrival times of the first fish of each species in this study are plotted in figure 5 as a function of depth and compared with the previous arrival time data from baited camera deployments at depths of 755–5900 m from different oceans (Priede et al. 1990, 1991, 1994a,b; Armstrong et al. 1992; Henriques et al. 2002). An exponential relationship is fitted to these data, reflecting an increase in time delay with depth. The present data lie close to the global line (Priede et al. 2006) when extrapolated to hadal depths. The longer time of arrival of fishes at depth reflects the general decrease in the abundance of scavenging fishes with depth (Priede & Merrett 1996). The relationship between tarr and fish abundance N (km−2) can be defined as
where A is the mean area occupied by one fish (m−2); vw is the water velocity of the current dispersing odour over the sea floor (m s−1); and vf is the velocity of the fishes moving upstream towards the bait following odour interception. Then
Assuming vw=vf =0.05 m s−1, typical of most deep water localities (Priede et al. 1991), and converting the data in figure 2 to predicted fish abundances, results in the relationship
where N is density ind. (km−2) and D is water depth (m). A scale corresponding to this relationship is shown on the right-hand side of figure 5. Predicted abundances of hadal fishes are 11.4 ind. km−2 at 7000 m and 0.43 ind. km−2 at 10 000 m depth.
Figure 5.
Arrivals of fishes at bait as a function of depth. Time from touchdown to arrival of the first individual. White squares are data from previous studies at shallower depths (tarr=2.5537e0.0005393D, n=82, R2=0.43, F=60.03, p<0.0001). Black squares are data from this study. Right-hand scale is the theoretical abundance of fishes calculated from the arrival time data (see text).
(i) Coryphaenoides yaquinae
At abyssal depths (station 1, 5469 m) in the Marianas region, at least 13 different individual C. yaquinae were observed, ranging in total length from 20.8 to 99.7 cm (mean 76.7 cm ±20.9 s.d.), following their first arrival at 52 min. Except for a period when two fish were present at 57 min, only one individual was in the field of view at any one time, indicating a continuous turnover of fish visiting the food source. Mean tail-beat frequency was 0.36 Hz, s.d. =0.11 (n=15). At station 2, using a still camera, the first fish were observed to arrive at 60 min, followed by an early peak of three fish present at 3 hours, the absence of fish at 10 hours and a second peak of three fish at 18 hours. At both stations, C. yaquinae actively attempted to feed on the bait. In the Japan Trench at 6945 m two C. yaquinae were sighted. The first was 36.0 cm long and was present for an entire 1 min video sequence at 104 min (figure 3f). The second was 48.8 cm long and present at 251 min after touchdown (figure 3d,e). Both individuals swam with a mean tail-beat frequency of 0.36 Hz, s.d.=0.07 (n=3), the same as individuals observed at 5469 m, but no feeding activity was observed. At both depths, the macrourids approached the bait from down-current, indicating a typical olfactory search strategy (Wagner 2003).
(ii) Pseudoliparis amblystomopsis
In the Japan Trench at 6945 m, one individual P. amblystomopsis was observed during two video sequences, at 194 and 204 min after touchdown. Total body length was 22.5 cm, and it swam in prevailing currents of 3–7 cm s−1 with a routine tail-beat frequency of 0.47 Hz ±0.01 s.d. (n=2). It used both pectoral and caudal fins for propulsion with a tendency to exhibit 1 : 1 synchrony (figure 3g–i; electronic supplementary material 2). The mean caudal : pectoral frequency ratio was 0.76. Small free-swimming amphipods attracted to the bait were ingested by individually targeted suction feeding at a rate of 2 min−1. No direct attempts to consume the bait were observed.
(iii) Notoliparis kermadecensis
At 6890 m in the Kermadec Trench, three individuals of N. kermadecensis, total body lengths 32.2, 33.3 and 28.7 cm, were observed over a period of 6 hours (figure 4, electronic supplementary material 1). They exhibited an up-current approach to the bait and were capable of swimming against currents of 10–14 cm s−1, with a mean tail-beat frequency of 1.04 Hz ±0.11 s.d. (n=31). The pectoral fins were relatively smaller than that in P. amblystomopsis and the caudal : pectoral frequency ratio was 2.08. The presence of amphipods evoked active swimming and they were captured at rates of up to nine suction feeding events per minute (figure 4a–c). The amphipods consumed by the fish were of similar size (length 1.2 cm ±0.2 s.d., n=8) and moved at a mean speed of 10 cm s−1±4.5 s.d. (n=8). In response to such prey movements, the liparids exhibited fast predatory behaviour with a maximum burst swimming speed of 17.2 cm s−1. Apparent reaction distance of the fish was approximately 2 cm, with prey presumably located by sensory receptors on the snout. No attempts at feeding directly on the bait were observed.
4. Discussion
Our observations show that both in the Northern Hemisphere (Japan Trench) and Southern Hemisphere (Kermadec Trench) endemic liparid fishes are present and are capable of actively feeding on mobile scavenging amphipods that are abundant in the hadal environment. Pseudoliparis amblystomopsis and N. kermadecensis ingest amphipods by suction feeding on selected individual prey using the classical buccal and opercular pump mechanism characteristic of advanced actinopterygian teleost fishes (Alexander 1967; Muller & Osse 1984). In these liparids, the action of the well-developed opercular pump is clearly important. The question arises as to how, in the darkness of the hadal zone, these fishes can effectively target individual prey in this way. The eyes would not be functional in the bright lights of the camera system owing to the bleaching of retinal pigments (Douglas et al. 1998), but the position of the eyes suggests possible binocular vision, enabling stereoscopic targeting of bioluminescent prey. On the head, P. amblystomopsis and N. kermadecensis have a putative sensory array of suprabranchial, maxillary and mandibular pores (Nielson 1964), which may detect hydrodynamic disturbance in close proximity, enabling ingestion of mobile prey in the absence of vision. These fishes may act as top predators in the hadal food web proposed by Blankenship & Levin (2007). Neither of these fishes, when observed in the Kermadec Trench or Japan Trench, attempted to feed on the bait itself. However, stomach contents of deep-sea liparids, including the N. kermadecensis holotype, have contained cycloid scales of several species of much larger fishes, as well as amphipods of the genera Hirondella, Alicella and Orchomene (Nielson 1964), suggesting facultative necrophagy at carrion falls as well as active predation.
The tail-beat frequency (1.04 Hz) of N. kermadecensis is similar to shallower living liparids (Careproctus sp.) at 1000 m in the Southern Ocean (tail-beat frequency 1.03 Hz ±0.2 s.d., mean body length 13.9 cm ±1.7 s.d., n=6; M. A. Collins, C. Yau, F. Guilfoyle, P. M. Bagley, I. Everson, I. G. Priede & D. Ag 2002, unpublished data), where current speeds were 8–9 cm s−1 (Collins et al. 2002; Yau et al. 2002). It appears that, with a propensity to use caudal rather than pectoral fin propulsion, N. kermadecensis is the most active of the two hadal liparid species able to survive in the higher current regimes observed in the Kermadec Trench (8–14 cm s−1) than the Japan Trench (3–7 cm s−1). Despite the larger body size of N. kermadecensis observed in this study compared with the shallower Careproctus sp., a comparable tail-beat frequency is maintained. Furthermore, a burst swimming speed of 17.2 cm s−1 indicates no reduction in activity level. The use of pectoral fins for locomotion in P. amblystomopsis implies low-speed manoeuvrability within lower current speeds (3–7 cm s−1), as described in the shallow freshwater bluegill sunfish, Lepomis macrochirus (Drucker & Lauder 2000). The low tail-beat frequency of P. amblystomopsis (0.47 Hz) is likely to be a result of the importance of pectoral fin locomotion under low current speeds. The present study suggests that the hadal liparids have no obvious reduction in activity levels when compared with shallower water liparids; however, in the absence of larger comparable datasets on other liparids, these comparisons should be taken as indicative.
One of the most interesting observations is that the delay before the arrival of the first fish in the hadal zone is very close to the predicted value from extrapolation of data from shallower depths. Notwithstanding differences in species, overlying productivity or geographical location, we obtain a highly significant global relationship between first fish arrival time and depth, with 43 per cent of variability accounted for by the single variable of depth. There appears to be no discontinuity in this respect at the 6000 m abyssal–hadal boundary. There are missing values at the deepest stations, where no fish were observed, but, for example, at 9729 m (station 8) the predicted time of arrival from the regression line presented in figure 5 is 9 hours compared with a sea-floor duration of our lander for 8 hours and 44 min; a time that was not long enough, in retrospect, to demonstrate the absence of fish at this depth. Multiple deployments in excess of 24 hours duration on the sea floor will be necessary to unequivocally define the maximum depth of occurrence of hadal fish. There are two possible explanations for longer time delays at deeper depths: slower speeds or lower population densities. However, our videos show that the hadal fish are not any less active compared with fish from shallower depths. Priede et al. (1991) showed that down to the depths of 5000 m (the deepest depth from practical trawling) there is a good correlation between abundance estimated using time delay (mean speeds of 0.05 m s−1) and trawl data. Assuming the speed of 0.05 m s−1 for hadal fishes that appear to have similar activity levels, their arrival time gives us the first estimates of fish abundance in the hadal trenches. Kermadec Trench and Japan Trench have areas of 90 000 and 80 000 km2, respectively, with mean depth of 8000 and 7200 m, respectively (Angel 1982). Assuming a respective mean population density of n=4 and 9 km−2 over the area of these trenches, based on the density equation described in this study, this implies resident hadal fish populations of 350 000 and 700 000 in the Kermadec Trench and Japan Trench, respectively. Although such estimates are highly speculative, these numbers seem sufficiently large to avoid the problems of genetic drift in endemic hadal fish species (Barton et al. 2007) and allow adaptation by natural selection to slightly different conditions between trenches.
Coryphaenoides yaquinae are the dominant macrourid at depths greater than 4700 m in the Pacific Ocean (Wilson & Waples 1983) and have been previously observed in large numbers at baited camera deployments at 5900 m in the North Pacific Ocean (Priede et al. 1990). We recognize that, in the absence of physical specimens, species identification from video images must remain slightly tentative. However, the individuals observed at 6945 m in the Japan Trench represent a major extension of the known depth range of this genus.
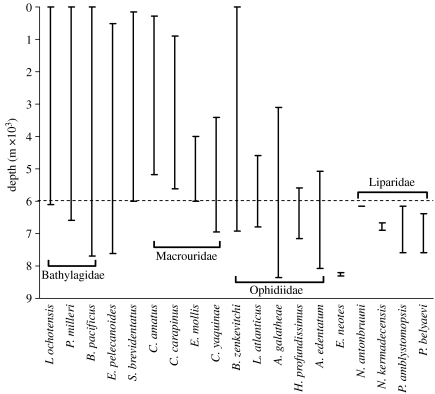
Including the new observation of C. yaquinae in this study, 15 species of fishes have now been recorded at hadal depths greater than 6000 m (figure 6; Froese & Pauly 2008). Except for four species of liparid that are endemic to the trenches, most have extensive bathymetric and geographical ranges and can be regarded as opportunistic vagrants that enter the trenches but are not dependent on this environment. The ophidiid A. galatheae, recognized as the world's deepest-living fish, probably belongs to this category, occurring in both the tropical Pacific and Atlantic Oceans.
Figure 6.
Depth ranges of hadal and near-hadal fishes (minimum and maximum recorded depths). The horizontal line at 6000 m denotes the abyssal–hadal boundary. Soecies are: (Bathylagidae) Lipolagus ochotensis, Pseudobathylagus milleri, Bathylagus pacificus; (Eurypharyngidae) Eurypharynx pelecanoides; (Serrivomeridae) Serrivomer brevidentatus; (Macrouridae) Coryphaenoides armatus, Coryphaenoides carapinus, Echinomacrurus mollis, Coryphaenoides yaquinae; (Ophidiidae) Bassozetus zenkevitchi, Leucicorus atlanticus, Abyssobrotula galatheae, Holcomycteronus profundissimus, Apagesoma edentatum; (Carapidae) Echiodon neotes; (Liparidae) Notoliparis antonbruuni, Notoliparis kermadecensis, Pseudoliparis amblystomopsis, Pseudoliparis belyaevi (Birshtyn & Vinogradov 1955; Nielson 1964, 1977; Masuda et al. 1984; Cohen et al. 1990; Chernova 1998; Nielsen et al. 1999; Chernova et al. 2004; Stein 2005; Froese & Pauly 2008).
The four hadal liparids have never been recorded at depths of less than 6000 m and are confined to their respective trench systems: N. kermadecensis in the Kermadec Trench, Notoliparis antonbruuni (Stein 2005) in the Peru Trench, P. amblystomopsis, in the northwest Pacific trenches (Japan Trench and neighbouring Kuril–Kamchatka Trench; Birshtyn & Vinogradov 1955) and Pseudoliparis belyaevi (Andriashev & Pitruk 1993) in the Japan Trench. These fishes are close to the average maximum size for marine teleost fish (length 26.1 cm, Priede et al. 2006) and, from our observations, show no obvious macroscopic or behavioural adaptations to hadal environments.
Liparids produce relatively few, large eggs, with fecundity in N. kermadecensis of less than 1000, and the eggs are probably produced in small batches (Nielson 1964). Such in situ demersal development enables the formation of isolated populations in each trench, in contrast to the macrourids that produce buoyant pelagic eggs and larvae that develop in the surface layers of the ocean (Merrett 1978; Merrett & Barnes 1996). The Pacific Ocean is regarded as the centre of evolutionary origin of the liparids, with the deeper-living species regarded as the more derived forms (Knudsen et al. 2007). Invasion of the hadal trenches and development of endemism is therefore likely to be a recent phenomenon, but hitherto no hadal or Southern Hemisphere samples have been included in molecular phylogenetic studies.
In this study, we have no direct observations of fishes living deeper than 7000 m, despite over the 28 hours presence across three deployments in two hadal trenches. Although largely based on infaunal invertebrates, Wolff (1960) argued that 6000–7000 m is the zone within which the transition takes place from the abyssal to a truly hadal fauna present at greater than 7000 m. However, owing to their endemic nature, there is no doubt that P. amblystomopsis and N. kermadecensis are truly hadal. The question of whether they occur at greater depths, and possibly to full ocean depth greater than 10 000 m remains open. Since these fish feed on hadal amphipods, it might be logical to presume that they would thrive at the greatest depths, where these crustacean prey are most abundant. On the other hand, it may also be possible to assume that the increasing abundance of amphipods with depth is attributable to decreasing predation pressures from a decreasing number of hadal fishes. Furthermore, amphipods in high abundance may overwhelm and consume living fishes (as occurs in deep-sea traps), thus excluding them from the deepest parts of the world's oceans.
Some of the vagrant species that have been recorded at hadal depths, such as Bathylagus pacificus (0–7700 m), show remarkably wide depth ranges. This species, living around the North Pacific subduction arc, may be an exceptional eurybathic species, with specialized physiology enabling exploitation of shallow to hadal depths when these occur in close proximity. Such proximity, however, also increases the possibility of errors of depth range estimation during sampling resulting from the use of non-closing nets. Depth records should be treated with caution; camera observations with precise depth measurements provide unequivocal evidence of the presence and activity of fishes at extreme depths.
The environmental changes that occur from the sea surface to the deep-sea represent one of the great physical and biological gradients on the planet, prompting much speculation on the trends in physiology, biomass, body size and biodiversity (Herring 2002). Measurements in the hadal zone at the extreme end of that gradient can be very influential, exerting great leverage in regression models. Hadal studies therefore play a key role in understanding global trends. It is interesting to note that fish arrival times (figure 5) are close to the predicted values and that these hadal fishes are close to average size and activity for benthic teleost fishes.
Acknowledgments
We are thankful to Prof. H.-J. Wagner (University of Tubingen, Germany) and Prof. H. Tokuyama (University of Tokyo, Japan) for supporting this work. We thank the crew and company of the FS Sonne, RV Hakuho-Maru and the RV Kairei (in particular Masato Sugano) for their assistance at sea, and are grateful to Dr N. Chernova (Russian Academy of Sciences, Russia) and Mr J. MacLaine (NHM, UK) for their assistance in identifying the fishes. This research was funded jointly by the Natural Environmental Research Council (UK) and the Nippon Foundation (Japan), with additional support from the Sasakawa Foundation (Japan) and the University of Aberdeen (UK). This work complies with the ethical policies of the University of Aberdeen and the University of Tokyo for research on animals and with the national laws of the United Kingdom, Japan and Germany.
Supplementary Material
Video sequence of the liparid Pseudoliparis amblystomopsis at 7000 m in the Japan Trench
Video sequence of the liparid Notoliparis kermadecensis at 7000 m in the Kermadec Trench
References
- Alexander R.M. The functions and mechanisms of protrusible jaws of some acanthopterygian fish. J. Zool. 1967;151:43–64. [Google Scholar]
- Anderson T.R., Rice T. Deserts on the sea floor: Edward Forbes and his azoic hypothesis for a lifeless deep ocean. Endeavour. 2006;30:131–137. doi: 10.1016/j.endeavour.2006.10.003. doi:10.1016/j.endeavour.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Andriashev A.P. A new fish of the snailfish family (Pisces, Liparidae) found at a depth of more than 7 kilometers. Trudy Instituta okeanologii im. P.P. Shirshova v. 1955;12:340–344. [In Russian; English transl. in 1963.] [Google Scholar]
- Angel M.V. Ocean trench conservation. Environmentalist. 1982;2:17. doi:10.1007/BF02340472 [Google Scholar]
- Armstrong J.D., Bagley P.M., Priede I.G. Photographic and acoustic tracking observations of the behaviour of the grenadier, Coryphaenoides (Nematonurus) armatus, the eel, Synaphobranchus bathybius, and other abyssal demersal fish in the North Atlantic Ocean. Mar. Biol. 1992;112:535–544. doi:10.1007/BF00346170 [Google Scholar]
- Barton N.H., Briggs D.E.G., Eisen J.A., Goldstein D.B., Patel N.H. Cold Spring Harbor Laboratory Press; New York, NY: 2007. Evolution. [Google Scholar]
- Beliaev G.M. Nauka; Moscow, Russia: 1989. Deep-sea ocean trenches and their fauna. [Google Scholar]
- Birshtyn Y.A., Vinogradov M.Y. Notes on the feeding of deep-sea fishes in the Kuril–Kamchatka Trench. Zoologicheskij Zhurnal. 1955;34:842–849. [Google Scholar]
- Blankenship L.E., Levin L.A. Extreme food webs: foraging strategies and diets of scavenging amphipods from the ocean's deepest 5 kilometers. Limnol. Oceanogr. 2007;52:1685–1697. [Google Scholar]
- Chernova N.V. Catalogue of the type specimens of snailfish (Liparidae, Scorpaeniformes) in the Zoological Institute of the Russian Academy of Sciences. J. Ichthyol. 1998;38:730–746. [Google Scholar]
- Chernova N.V., Stein D.L., Andriashev A.P. Family Liparidae (Scopoli 1777)—snailfishes. Calif. Acad. Sci. Annot. Checkl. Fish. 2004;31:1–82. [Google Scholar]
- Cohen D.M., Inada T., Iwamoto T., Scialabba N. Gadiform fishes of the World. FAO Fish. Synop. 125. 1990;10:442. [Google Scholar]
- Collins M.A., Yau C., Guilfoyle F., Bagley P.M., Everson I., Priede I.G., Ag D. Assessment of stone crab (Lithodidae) density on the South Georgia slope using baited video cameras. ICES J. Mar. Sci. 2002;59:370–379. doi:10.1006/jmsc.2001.1167 [Google Scholar]
- Douglas R.H., Partridge J.C., Marshall N.J. The eyes of deep-sea fish I: lens pigmentation, tapeta and visual pigments. Prog. Ret. Eye Res. 1998;17:597–636. doi: 10.1016/s1350-9462(98)00002-0. doi:10.1016/S1350-9462(98)00002-0 [DOI] [PubMed] [Google Scholar]
- Drucker E.G., Lauder G.V. A hydrodynamic analysis of fish swimming speed: wake structure and locomotor force in slow and fast labriform swimmers. J. Exp. Biol. 2000;203:2379–2393. doi: 10.1242/jeb.203.16.2379. [DOI] [PubMed] [Google Scholar]
- Froese, R. & Pauly. D. 2008 FishBase. Internet database. See www.fishbase.org
- Henriques C., Priede I.G., Bagley P.M. Baited camera observations of deep-sea demersal fishes of the northeast Atlantic Ocean at 15–28° N off West Africa. Mar. Biol. 2002;141:307–314. doi:10.1007/s00227-002-0833-6 [Google Scholar]
- Herring P.J. Oxford University Press; Oxford, UK: 2002. The biology of the deep ocean. [Google Scholar]
- Jamieson, A. J., Fujii, T., Solan, M., Matsumoto, A. K., Bagley, P. M. & Priede, I. G. In press. First findings of decapod crustacea in the hadal zone. Deep-Sea Res. 1 (doi:10.1016/j.dsr.2008.11.003)
- Johnson G.C. Deep water properties, velocities, and dynamics over ocean trenches. J. Mar. Res. 1998;56:239–347. doi:10.1357/002224098321822339 [Google Scholar]
- King N.J., Priede I.G. Coryphaenoides armatus, the abyssal grenadier: global distribution, abundance and ecology as determined by baited landers. Am. Fish. Soc. Symp. 2008;63:1139–1161. [Google Scholar]
- Knudsen S.W., Møller P.R., Gravlund P. Phylogeny of the snailfishes (Teleostei: Liparidae) based on molecular and morphological data. Mol. Phylogenet. Evol. 2007;44:649–666. doi: 10.1016/j.ympev.2007.04.005. doi:10.1016/j.ympev.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Longhurst A., Sathyendranath S., Patt T., Caverhill C. An estimate of global primary production in the ocean from satellite radiometer data. J. Plankt. Res. 1995;17:1245–1271. doi:10.1093/plankt/17.6.1245 [Google Scholar]
- Macdonald A.G. Effects of high pressure on cellular processes. In: Sperelakis N., editor. Cell physiology sourcebook: a molecular approach. Academic Press; San Diego, CA: 2001. pp. 1003–1023. [Google Scholar]
- Malyutina M. Russian deep-sea investigations of Antarctic fauna. Deep-Sea Res. Part II. 2004;51:1551–1570. doi:10.1016/j.dsr2.2004.07.012 [Google Scholar]
- Masuda H., Amaoka K., Araga C., Uyeno T., Yoshino T. The fishes of the Japanese Archipelago. vol. 1. Tokai University Press; Tokyo, Japan: 1984. [Google Scholar]
- Merrett N.R. On the identity and pelagic occurrence of larval and juvenile stages of rattail fishes (Family Macrouridae) from 60° N, 20° W and 53° N, 20° W. Deep-Sea Res. 1978;25:147–160. doi:10.1016/0146-6291(78)90002-4 [Google Scholar]
- Merrett N.R., Barnes S.H. Preliminary survey of egg envelope morphology in the Macrouridae and the possible implications of its ornamentation. J. Fish Biol. 1996;48:101–119. doi:10.1111/j.1095-8649.1996.tb01422.x [Google Scholar]
- Muller M., Osse J.W.M. Hydrodynamics of suction feeding in fish. Trans. Zool. Soc. Lond. 1984;37:51–135. [Google Scholar]
- Nielson J.G. Fishes from depths exceeding 6000 meters. Galathea Report. 1964;7:113–124. [Google Scholar]
- Nielson J.G. The deepest living fish Abyssobrotula galathea. A new genus and species of oviparous ophidiids (Pisces, Brotulidae) Galathea Report. 1977;14:41–48. [Google Scholar]
- Nielsen J.G., Cohen D.M., Markle D.F., Robins C.R. Ophidiiform fishes of the world (Order Ophidiiformes). An annotated and illustrated catalogue of pearlfishes, cusk-eels, brotulas and other ophidiiform fishes known to date. FAO Fish. Synop. 125. 1999;18:1–178. [Google Scholar]
- Piccard J., Dietz R.S. Longman; London, UK: 1961. Seven miles down. The story of the bathyscaph ‘Trieste’. [Google Scholar]
- Priede I.G., Merrett N.R. Estimation of abundance of abyssal demersal fishes; a comparison of data from trawls and baited cameras. J. Fish Biol. 1996;49:207–216. doi:10.1111/j.1095-8649.1996.tb06077.x [Google Scholar]
- Priede I.G., Smith K.L., Jr, Armstrong J.D. Foraging behaviour of abyssal grenadier fish: inferences from acoustic tagging and tracking in the North Pacific Ocean. Deep-Sea Res. 1990;37:81–101. doi:10.1016/0198-0149(90)90030-Y [Google Scholar]
- Priede I.G., Bagley P.M., Armstrong J.D., Smith K.L., Merrett N.R. Direct measurement of active dispersal of food-falls by deep-sea demersal fishes. Nature. 1991;351:647–649. doi:10.1038/351647a0 [Google Scholar]
- Priede I.G., Bagley P.M., Smith A., Creasy S., Merrett N.R. Scavenging deep demersal fishes of the Porcupine Seabight (NE Atlantic Ocean); observations by baited camera, trap and trawl. J. Mar. Biol. Assoc. UK. 1994a;74:481–498. [Google Scholar]
- Priede I.G., Bagley P.M., Smith K.L., Jr Seasonal change in activity of abyssal demersal scavenging grenadiers Coryphaenoides (Nematonurus) armatus in the eastern North Pacific Ocean. Limnol. Oceanogr. 1994b;39:279–285. [Google Scholar]
- Priede I.G., Froese R., Bailey D.M., Bergstad O.A., Collins M.A., Dyb J.E., Henriques C., Jones E.G., King N. The absence of sharks from abyssal regions of the world's oceans. Proc. R. Soc. B. 2006;273:1435–1441. doi: 10.1098/rspb.2005.3461. doi:10.1098/rspb.2005.3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainte-Marie B., Hargrave B.T. Estimation of scavenger abundance and distance of attraction to bait. Mar. Biol. 1987;94:431–443. doi:10.1007/BF00428250 [Google Scholar]
- Saunders P.M. Practical conversion of pressure to depth. J. Phys. Oceanog. 1981;11:573–574. doi:10.1175/1520-0485(1981)011<0573:PCOPTD>2.0.CO;2 [Google Scholar]
- Somero G.N. Adaptations to high hydrostatic pressure. Annu. Rev. Physiol. 1992;54:557–577. doi: 10.1146/annurev.ph.54.030192.003013. doi:10.1146/annurev.ph.54.030192.003013 [DOI] [PubMed] [Google Scholar]
- Stein D.L. Descriptions of four new species, redescription of Paraliparis membranaceus, and additional data on species of the fish family Liparidae (Pisces, Scorpaeniformes) from the west coast of South America and the Indian Ocean. Zootaxa. 2005;1019:1–25. [Google Scholar]
- Stommel H. The abyssal circulation. Deep-Sea Res. 1958;5:80–82. doi:10.1016/S0146-6291(58)80014-4 [Google Scholar]
- Wagner H.-J. Volumetric analysis of brain areas indicates a shift in sensory orientation during development in the deep-sea grenadier Coryphaenoides armatus. Mar. Biol. 2003;142:791–797. [Google Scholar]
- Warren B.A., Owens W.B. Some preliminary results concerning deep northern-boundary currents in the North Pacific. Prog. Oceanogr. 1985;14:537–551. doi:10.1016/0079-6611(85)90027-8 [Google Scholar]
- Wilson R.R., Jr, Waples R.S. Distribution, morphology, and biochemical genetics of Coryphaenoides (N.) armatus and C. yaquinae (Pisces: Macrouridae) in the central and eastern North Pacific. Deep-Sea Res. 1983;30:1127–1145. doi:10.1016/0198-0149(83)90092-4 [Google Scholar]
- Wolff T. The hadal community, an introduction. Deep-Sea Res. 1960;6:95–124. doi:10.1016/0146-6313(59)90063-2 [Google Scholar]
- Wolff T. The deepest recorded fishes. Nature. 1961;190:283. doi:10.1038/190283a0 [Google Scholar]
- Yau C., Collins M.A., Bagley P.M., Everson I., Priede I.G. Scavenging by megabenthos and demersal fish on the South Georgia slope. Antarct. Sci. 2002;14:16–24. doi:10.1017/S0954102002000536 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video sequence of the liparid Pseudoliparis amblystomopsis at 7000 m in the Japan Trench
Video sequence of the liparid Notoliparis kermadecensis at 7000 m in the Kermadec Trench