Abstract
Muscle fatigue, a reduction in force as a consequence of exercise, is an important factor for any animal that moves, and can result from both peripheral and/or central mechanisms. Although much is known about whole-limb force generation and activation patterns in fatigued muscles under sustained isometric contractions, little is known about the in vivo dynamics of limb muscle function in relation to whole-body fatigue. Here we show that limb kinematics and contractile function in the lateral (LG) and medial (MG) gastrocnemius of helmeted guineafowl (Numida meleagris) are significantly altered following fatiguing exercise at 2 m s−1 on an inclined treadmill. The two most significant findings were that the variation in muscle force generation, measured directly from the muscles' tendons, increased significantly with fatigue, and fascicle shortening in the proximal MG, but not the distal MG, decreased significantly with fatigue. We suggest that the former is a potential mechanism for decreased stability associated with fatigue. The region-specific alteration of fascicle behaviour within the MG as a result of fatigue suggests a complex response to fatigue that probably depends on muscle–aponeurosis and tendon architecture not previously explored. These findings highlight the importance of studying the integrative in vivo dynamics of muscle function in response to fatigue.
Keywords: biomechanics, locomotion, muscle physiology, muscle fatigue, stability, sonomicrometry
1. Introduction
For over a century, scientists have been captivated and challenged by the mechanisms and effects of muscle fatigue (Mosso 1891; Giulio et al. 2006). Muscle fatigue can be defined as a reduction in maximum muscle force during exercise (Barry & Enoka 2007), but there are several complex mechanisms that involve both central (neural) and peripheral factors (Bigland-Ritchie & Woods 1984; Fitts 1994). Given that muscle function is essential for executing a broad range of motor behaviours, fatigue probably has significant implications for the fitness of an individual. Because a drop in the maximum force-generating capabilities of a muscle (i.e. fatigue) occurs shortly after the onset of exercise (Barry & Enoka 2007), the level of muscle fatigue depends strongly on the duration and intensity of exercise. Fatigue need not result in the inability to perform a task, which has led researchers to examine muscle fatigue as it relates to ‘task failure’ (the point at which a task cannot be maintained; Barry & Enoka 2007). This permits an evaluation of fatigue at a stage that is standardized among individuals, and potentially species.
Muscle fatigue is commonly assessed in animal models under either in vitro or in situ conditions (Askew et al. 1997; Callister et al. 2004; Syme & Tonks 2004). Studies examining in vivo muscle fatigue in humans have used both controlled (e.g. Babault et al. 2006; Rudroff et al. 2007) and dynamic (e.g. Lorentzon et al. 1988; Lee et al. 2000; Loscher & Nordlund 2002; Dimitrov et al. 2006; Ratel et al. 2006) exercise conditions. Although these studies provide vital information about muscle fatigue, little is known about the changes in the in vivo forces and mechanics of individual muscles associated with fatigue during strenuous activity. In addition, controlled exercise conditions ignore the functional complexity associated with common behaviours, where dynamic movement is essential (Syme & Tonks 2004). When running to fatigue, limb bone strain and limb kinematics are significantly altered (Yoshikawa et al. 1994; Fyhrie et al. 1998; Mizrahi et al. 2000; Christina et al. 2001; Milgrom et al. 2007). For example, muscle fatigue in humans results in changes in lower limb joint angles during running, which in turn cause an increased loading rate of the (vertical) impact ground reaction force (Christina et al. 2001). Thus, it is clear that several aspects of locomotion are altered with the onset of whole-body fatigue. A thorough examination of how muscle function responds to whole-body fatigue is essential for understanding the physiological ramifications of high-intensity exercise.
Not only do muscles interact with one another to generate an overall force at a joint (Higham et al. 2008), but also different parts of a single muscle can exhibit variation in strain (Pappas et al. 2002; Ahn et al. 2003; Soman et al. 2005; Higham & Biewener 2008; Higham et al. 2008), force generation (Carrasco et al. 1999) and motor unit recruitment (English 1984). These layers of complexity could be important during fatigue, as certain muscles (within a group of synergists) or certain parts of a muscle might be more affected by fatigue, and could thus constrain overall muscle function during a demanding behaviour such as running. This is probably the case given that muscles often exhibit regional differences in fibre type composition (Chanaud et al. 1991; Wang & Kernell 2000), and these fibre types exhibit different physiological characteristics (Kanda & Hashizume 1992; Fitts 1994). Understanding how these inherent physiological differences within a muscle play a role in the response of different muscle regions to fatigue is imperative for understanding how the musculoskeletal system adapts to physiological demands.
To address this issue, we studied the effects of fatigue on skeletal muscle activation patterns and mechanics under dynamic running conditions in the helmeted guineafowl, Numida meleagris. We tested the hypothesis that fatigue has similar effects on the function of two hindlimb muscle synergists, the lateral (LG) and medial (MG) gastrocnemius. In addition, we determined whether fatigue influences the functional heterogeneity within the MG of guineafowl, which has been shown to exhibit substantial regional differences in length change and activation patterns during running (Higham et al. 2008).
2. Material and methods
(a) Animals and surgical procedures
Four helmeted guineafowl (N. meleagris), with an average mass of 2.3±0.2 kg, were trained to run on a motorized treadmill. Once trained, each bird was initially anesthetized using a mixture of ketamine and xylazine, and then maintained on 1–2% isoflurane. To measure fascicle length changes, 2 mm sonomicrometry crystal pairs were implanted parallel to the fascicles of the LG, proximal MG (pMG) and distal MG (dMG), and the two crystals of a pair were spaced approximately 11–12 mm apart. Fine-wire electromyography (EMG) electrodes were implanted in the regions of the sonomicrometry crystals. To measure force, E-type stainless steel tendon buckle force transducers were attached to the individual tendons emerging from the LG and MG. Extensive details of these methods can be found elsewhere (Daley & Biewener 2003). The locations of the sonomicrometry crystals and EMG electrodes were verified after experiments via dissection. Each muscle was weighed and dissected to verify transducer alignment and to determine fascicle length and pennation angle. Muscle physiological cross-sectional area (PCSA) was determined (as in Powell et al. 1984) to calculate muscle stress (force/PCSA) from muscle–tendon force measurements. Force buckles were calibrated in situ immediately following experiments as described elsewhere (Daley & Biewener 2003).
(b) Fatigue protocol
Each bird ran on an inclined (14°) motorized treadmill until it was unable to maintain the speed of 2 m s−1, which was considered task failure. At this point, the birds were unable to maintain a standing posture on the treadmill, clearly indicating a significant level of whole-body fatigue. This combination of speed and incline approximates the maximal rate of oxygen consumption for guineafowl (Ellerby et al. 2003), which would be expected to produce fatigue. The duration of incline exercise ranged from 5 to 8 min. The five strides immediately preceding task failure were considered to represent a fatigued state and included in the analyses. Change in mean power frequency (MPF) of each EMG signal was also determined. To assess the variability of force generation during normal versus fatigued locomotion, we calculated the coefficient of variation (CV).
(c) Muscle immunohistochemistry
Freshly dissected muscle blocks (approx. 1 cm3) were frozen in isopentane (cooled in liquid N2), sectioned on a cryotome, and processed using standard immunohistochemistry and histochemistry methods (Serrano et al. 1996; slow twitch, type 1: positive to S58 anti-slow antibody and preincubated pH 4.3 ATPase stain; fast twitch, type IIb: positive to MY32 anti-fast antibody, negative to preincubated pH 4.3 ATPase stain, and positive to pH 10.3 ATPase stain; and fast twitch, type IIa: negative to S58 and MY32 and positive to NADH stain).
(d) Muscle work
Changes in muscle fascicle length were multiplied by force measurements to obtain values of work as a function of time. Values of lengthening and shortening work were summed to obtain the shortening (positive) and lengthening (negative) work done by each muscle and muscle region (pMG and dMG were assumed to exert similar stresses at the muscle's tendon). Values of work for a given muscle were divided by muscle mass to obtain mass-specific work.
3. Results and discussion
Following 5–8 min of intense exercise on an inclined treadmill (at 2 m s−1), several important functional shifts in hindlimb movements and muscle dynamics occurred as a result of whole-animal fatigue. Stride frequency was significantly lower in the fatigued (2.8±0.04 Hz) trials compared with the non-fatigued (3.1±0.03 Hz) trials (p<0.05, t-test). This reduction in stride frequency also occurs in humans (Mizrahi et al. 2000) and horses (Wickler et al. 2006) running steadily to fatigue. In addition, we found a non-significant decrease in EMG MPF for the LG (p=0.06, range: 89–93% relative to non-fatigue), pMG (p=0.12, range: 93–97% relative to non-fatigue) and dMG (p=0.2, range: 95–98% relative to non-fatigue), which is a comparable decrease to that found in a recent study of fatigue in dogs (Yoshikawa et al. 1994). The slowing of stride frequency and the reduction in EMG MPF indicate that the muscles are becoming fatigued. In studies of fatigue, the rate of force development of the muscles typically decreases (Fitts 1994). However, this interpretation is often drawn from measures of whole-limb force generation, rather than direct measures of muscle force. By directly measuring muscle force from the individual distal tendons, we found that the time to peak force increased significantly for the MG (p=0.03, 10 ms increase, 118% of non-fatigued) and non-significantly for the LG (p=0.06, 7 ms increase, 116% of non-fatigued) of guineafowl under dynamic in vivo conditions, partially supporting prior indirect predictions.
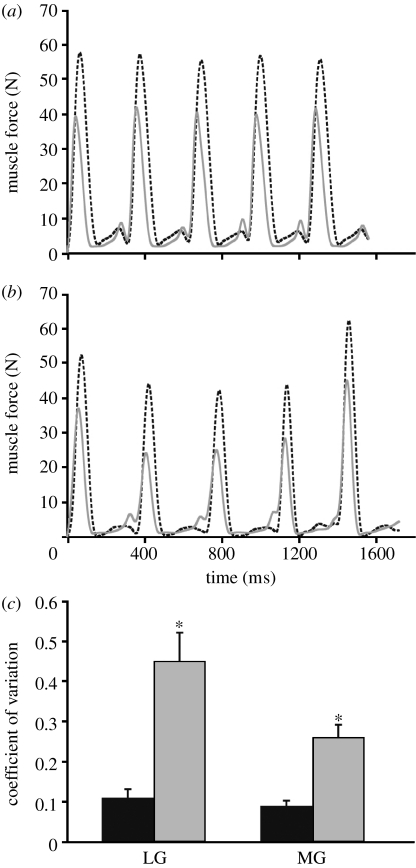
Peak force generation was more variable (measured by the CV) for fatigued strides in both the LG and MG compared with the non-fatigued strides (figure 1; p<0.05, t-test), indicating that the forces exerted by the muscles were more variable even though the animals were maintaining a steady running speed of 2 m s−1. Despite this, mean peak force did not significantly differ (LG: p=0.78; MG: p=0.83, t-tests) between the non-fatigued (LG=31.9±2.7 N; MG=36.6±3.2 N) and fatigued (LG=31.0±1.4 N; MG=37.5±2.4 N) trials. Owing to an increased variability, higher (and lower) forces relative to non-fatigued values are generated at a comparable level of exercise (figure 1a,b). The increased variability in peak muscle force generation we observe here could be a general feature of whole-body fatigue, and could reduce the ability of an animal to maintain stability and/or accuracy, which could lead to injury. Indeed, postural stability in humans decreases following fatigue of lower extremity muscles (Salavati et al. 2007). While explanations for decreased stability associated with fatigue include proprioceptive deficiencies, increased muscle reaction time and inappropriate efferent muscle responses (Salavati et al. 2007), our in vivo results suggest that a key mechanism underlying decreased stability is increased variability in muscle force generation, reflecting diminished motor control. Altered tendon compliance, which has been observed in studies of human muscle following repeated contractions (Maganaris et al. 2002), might also be a contributing mechanism to the increased force variability. Maganaris et al. (2002) suggested that altered fascicular shortening (due to tendon creep) could affect the firing of Ia afferents (sensory afferents from muscle spindle stretch receptors), leading to potential errors in muscle activation. Future work combining measures of in vivo muscle function with aponeurosis–tendon strain is needed to gain further insight into the relationship between fatigue-induced force variability and locomotor stability.
Figure 1.
Variation in muscle force in relation to fatigue. (a) Medial (MG; dashed black curve) and lateral (LG; solid grey curve) gastrocnemius muscle force for five consecutive non-fatigued strides. (b) MG (dashed black curve) and LG (solid grey curve) muscle force for the five consecutive strides immediately prior to task failure. (c) CV in peak muscle force for the MG and LG. Values for (c) are mean±s.e.m. *p<0.05 for differences due to fatigue (black bars, non-fatigued; grey bars, fatigued).
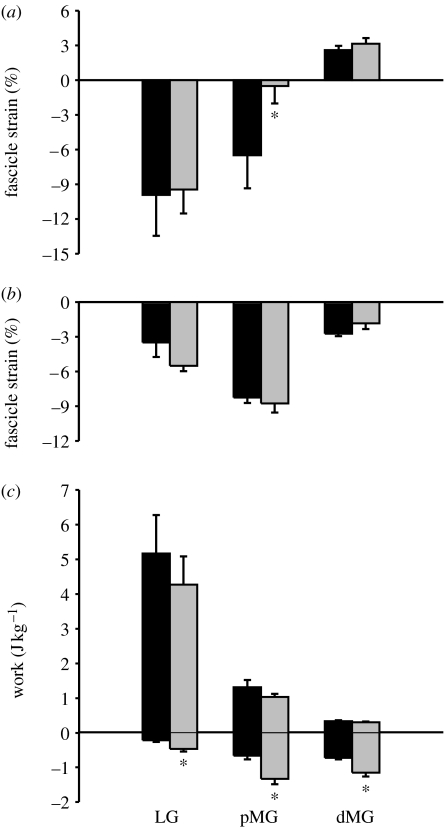
Net fascicle shortening from the onset of force to maximum force in the pMG also decreased significantly from 6.49±2.7% to 0.50±1.5% as a result of fatigue (p<0.05, t-test), while strain in the dMG was not altered (figure 2a). This is the first evidence that in vivo mechanical changes due to fatigue may vary between muscle regions, suggesting that certain parts of muscles are more susceptible to fatigue than others. This is not surprising given that the pMG and dMG of guineafowl respond differently (in terms of in vivo function) to the changes in locomotor demand (Higham et al. 2008). The differential response to fatigue might be facilitated by the higher proportion of fast-twitch fibres in the pMG relative to the dMG (100% versus 58%; J. W. Hermanson, T. E. Higham & A. A. Biewener 2007, unpublished data). In addition, the relatively stiff distal aponeurosis of the guineafowl MG probably constrains fascicle shortening (Higham et al. 2008), potentially shielding this region from changes in strain as a result of fatigue. Although tendon creep has been observed following repeated voluntary contractions, Maganaris et al. (2002) found no significant change in aponeurosis strain of the human MG, supporting this as a possible interpretation of our results here.
Figure 2.
Effects of fatigue on fascicle strain and mass-specific muscle work. (a) Mean fascicle strain from the period of muscle force onset to maximum muscle force for the LG, pMG and dMG under non-fatigued (black bars) and fatigued (grey bars) conditions. (b) Mean fascicle strain from the period of maximum muscle force to muscle force offset for the LG, pMG and dMG under non-fatigued and fatigued conditions (symbols are the same as given in (a)). (c) Mean mass-specific muscle work (both negative and positive) per stride cycle for the LG, pMG and dMG under non-fatigued and fatigued conditions (symbols are the same as given in (a)). Values are mean±s.e.m. *p<0.05 for differences due to fatigue.
The reduced shortening of the pMG indicates that the shortening velocity of fascicles in this region of the muscle decreased. Owing to the force–velocity properties of muscle (Hill 1938), fewer motor units would therefore need to be recruited to generate the same amount of force. As overall MG force generation was not influenced by fatigue, this would suggest that fewer motor units are recruited to generate the same amount of force, resulting in decreased amplitude of EMG activity. However, mean spike amplitude was not affected by fatigue for any of the muscle regions in our study. One possible explanation is that factors downstream of the neuromuscular junction in the muscle fibres themselves became impaired during fatigue, despite the motor drive to the muscle (detected by the EMG signal) still being maintained at the same level. In this case, EMG amplitude and whole-muscle force generation could remain unchanged despite a reduction in the force-generating capacity of each fibre. Whole-muscle force would be maintained because the reduced shortening of the fascicles in the pMG would result in higher forces per fibre.
Lengthening (negative) work, but not shortening (positive) work, increased significantly for all three muscle regions (LG: 200% of non-fatigued; pMG: 206% of non-fatigued; dMG: 177% of non-fatigued) with fatigue (figure 2; p<0.05, t-test). A fatigued muscle is more susceptible to injury (Mair et al. 1996), and lengthening (eccentric) contractions can damage sarcomeres (Morgan 1990). In addition, the tension produced during a lengthening contraction is higher than either isometric or concentric contractions (Lindstedt et al. 2001), which could potentially increase skeletal force levels, consistent with the increased skeletal loading observed during fatigue in dogs (Yoshikawa et al. 1994). The increased lengthening work in the LG and MG is probably associated with increased peak force variability and increased peak forces observed during fatigue in these muscles.
The in vivo changes in muscle force and length mechanics resulting from whole-body fatigue documented here for running guineafowl, and recently for in vitro work-loop studies of frog muscle (Syme & Tonks 2004), suggest that studies examining fatigue under sustained isometric contractions may miss important changes in muscle function that occur under natural (i.e. dynamic) conditions. Our two main findings, that muscle force becomes highly variable as an animal becomes fatigued and fatigue has region-specific effects on muscle function, are probably widespread among animals. The former could play significant roles in sustained behaviours such as running, swimming or chewing, and warrants further investigation. The heterogeneous effects of fatigue on MG function highlight the complexity that can exist within a single muscle during fatigue. Future work teasing apart the mechanisms underlying this heterogeneity will be important for linking muscle–tendon architecture to a muscle's contractile mechanics and its neuromotor control, which are relevant to an animal's physiological performance and evolutionary success.
Acknowledgments
The surgical and experimental protocols were approved by the Harvard University Institutional Animal Care and Use Committee.
Insightful comments from two reviewers greatly enhanced this manuscript. Financial support for this research was provided by a grant (R01-AR047679) from the National Institutes of Health (A.A.B.). We thank Pedro Ramirez for animal care. The authors declare no competing financial interests.
References
- Ahn A.N., Monti R.L., Biewener A.A. In vivo and in vitro heterogeneity of segment length changes in the toad semimembranosus. J. Physiol. 2003;549:877–888. doi: 10.1113/jphysiol.2002.038018. doi:10.1113/jphysiol.2002.038018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew G.N., Young I.S., Altringham J.D. Fatigue of mouse soleus muscle, using the work loop technique. J. Exp. Biol. 1997;200:2907–2912. doi: 10.1242/jeb.200.22.2907. [DOI] [PubMed] [Google Scholar]
- Babault N., Desbrosses K., Fabre M.S., Michaut A., Pousson M. Neuromuscular fatigue development during maximal concentric and isometric knee extensions. J. Appl. Physiol. 2006;100:780–785. doi: 10.1152/japplphysiol.00737.2005. doi:10.1152/japplphysiol.00737.2005 [DOI] [PubMed] [Google Scholar]
- Barry B.K., Enoka R.M. The neurobiology of muscle fatigue: 15 years later. Integr. Comp. Biol. 2007;47:465–473. doi: 10.1093/icb/icm047. doi:10.1093/icb/icm047 [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B., Woods J.J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. 1984;7:691–699. doi: 10.1002/mus.880070902. doi:10.1002/mus.880070902 [DOI] [PubMed] [Google Scholar]
- Callister R.J., Sesodia S., Enoka R.M., Nemeth P.M., Reinking R.M., Stuart D.G. Fatigue of rat hindlimb motor units: biochemical-physiological associations. Muscle Nerve. 2004;30:714–726. doi: 10.1002/mus.20158. doi:10.1002/mus.20158 [DOI] [PubMed] [Google Scholar]
- Carrasco D.I., Lawrence J., English A.W. Neuromuscular compartments of cat lateral gastrocnemius produce different torques about the ankle joint. Motor Control. 1999;3:436–446. doi: 10.1123/mcj.3.4.436. [DOI] [PubMed] [Google Scholar]
- Chanaud C.M., Pratt C.A., Loeb G.E. Functionally complex muscles of the cat hindlimb. V. The roles of hitochemical fiber-type regionalization and mechanical heterogeneity in differential muscle activation. Exp. Brain Res. 1991;85:300–313. doi: 10.1007/BF00229408. [DOI] [PubMed] [Google Scholar]
- Christina K.A., White S.C., Gilchrist L.A. Effect of localized muscle fatigue on vertical ground reaction forces and ankle joint motion during running. Hum. Movement Sci. 2001;20:257–276. doi: 10.1016/s0167-9457(01)00048-3. doi:10.1016/S0167-9457(01)00048-3 [DOI] [PubMed] [Google Scholar]
- Daley M.A., Biewener A.A. Muscle force-length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J. Exp. Biol. 2003;206:2941–2958. doi: 10.1242/jeb.00503. doi:10.1242/jeb.00503 [DOI] [PubMed] [Google Scholar]
- Dimitrov G.V., Arabadzhiev I.T., Mileva K.N., Bowtell J.L., Chrichton N., Dimitrova N.A. Muscle fatigue during dynamic contractions assessed by new spectral indices. Med. Sci. Sports Exerc. 2006;38:1971–1979. doi: 10.1249/01.mss.0000233794.31659.6d. doi:10.1249/01.mss.0000233794.31659.6d [DOI] [PubMed] [Google Scholar]
- Ellerby D.J., Cleary M., Marsh R.L., Buchanan C.I. Measurement of maximum oxygen consumption in guinea fowl Numida meleagris indicates that birds and mammals display a similar diversity of aerobic scopes during running. Physiol. Biochem. Zool. 2003;76:695–703. doi: 10.1086/376430. doi:10.1086/376430 [DOI] [PubMed] [Google Scholar]
- English A.W. An electromyographic analysis of compartments in cat lateral gastrocnemius muscle during unrestrained locomotion. J. Neurophysiol. 1984;52:114–125. doi: 10.1152/jn.1984.52.1.114. doi:10.1159/000163244 [DOI] [PubMed] [Google Scholar]
- Fitts R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Fyhrie D.P., Milgrom C., Hoshaw S.J., Simkin A., Dar S., Drumb D., Burr D.B. Effect of fatiguing exercise on longitudinal bone strain as related to stress fracture in humans. Ann. Biomed. Eng. 1998;26:660–665. doi: 10.1114/1.103. doi:10.1114/1.103 [DOI] [PubMed] [Google Scholar]
- Giulio C.D., Daniele F., Tipton C.M. Angelo Mosso and muscular fatigue: 116 years after the first congress of physiologists: IUPS commemoration. Adv. Physiol. Educ. 2006;30:51–57. doi: 10.1152/advan.00041.2005. doi:10.1152/advan.00041.2005 [DOI] [PubMed] [Google Scholar]
- Higham T.E., Biewener A.A. Integration within and between muscles during terrestrial locomotion: effects of incline and speed. J. Exp. Biol. 2008;211:2303–2316. doi: 10.1242/jeb.016139. doi:10.1242/jeb.016139 [DOI] [PubMed] [Google Scholar]
- Higham T.E., Biewener A.A., Wakeling J.M. Functional diversification within and between muscle synergists during locomotion. Biol. Lett. 2008;4:41–44. doi: 10.1098/rsbl.2007.0472. doi:10.1098/rsbl.2007.0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. B. 1938;126:136–195. doi:10.1098/rspb.1938.0050 [Google Scholar]
- Kanda K., Hashizume K. Factors causing difference in force output among motor units in the rat medial gastrocnemius-muscle. J. Physiol. Lond. 1992;448:677–695. doi: 10.1113/jphysiol.1992.sp019064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.C., Becker N.C., Binder-Macleod S.A. Activation of human quadriceps femoris muscle during dynamic contractions: effects of load on fatigue. J. Appl. Physiol. 2000;89:926–936. doi: 10.1152/jappl.2000.89.3.926. [DOI] [PubMed] [Google Scholar]
- Lindstedt S.L., LaStayo P.C., Reich T.E. When active muscles lengthen: properties and consequences of eccentric contractions. News Physiol. Sci. 2001;16:256–261. doi: 10.1152/physiologyonline.2001.16.6.256. [DOI] [PubMed] [Google Scholar]
- Lorentzon R., Johansson C., Sjostrom M., Fagerlund M., Fugl-Meyer A.R. Fatigue during dynamic muscle contractions in male sprinters and marathon runners: relationships between performance, electromyographic activity, muscle cross-sectional area and morphology. Acta Physiol. Scand. 1988;132:531–536. doi: 10.1111/j.1748-1716.1988.tb08361.x. doi:10.1111/j.1748-1716.1988.tb08361.x [DOI] [PubMed] [Google Scholar]
- Loscher W.N., Nordlund M.M. Central fatigue and motor cortical excitability during repeated shortening and lengthening actions. Muscle Nerve. 2002;25:864–872. doi: 10.1002/mus.10124. doi:10.1002/mus.10124 [DOI] [PubMed] [Google Scholar]
- Maganaris C.N., Baltzopoulos V., Sargeant A.J. Repeated contractions alter the geometry of human skeletal muscle. J. Appl. Physiol. 2002;93:2089–2094. doi: 10.1152/japplphysiol.00604.2002. [DOI] [PubMed] [Google Scholar]
- Mair S.D., Seaber A.V., Glisson R.R., Garrett W.E., Jr The role of fatigue in susceptibility to acute muscle strain injury. Am. J. Sports Med. 1996;24:137–143. doi: 10.1177/036354659602400203. doi:10.1177/036354659602400203 [DOI] [PubMed] [Google Scholar]
- Milgrom C., Radeva-Petrova D.R., Finestone A., Nyska M., Mendelson S., Benjuya N., Simkin A., Burr D.B. The effect of muscle fatigue on in vivo tibial strains. J. Biomech. 2007;40:845–850. doi: 10.1016/j.jbiomech.2006.03.006. doi:10.1016/j.jbiomech.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Mizrahi J., Verbitsky O., Isakov E., Daily D. Effect of fatigue on leg kinematics and impact acceleration in long distance running. Hum. Movement Sci. 2000;19:139–151. doi:10.1016/S0167-9457(00)00013-0 [Google Scholar]
- Morgan D.L. New insights into the behavior of muscle during active lengthening. Biophys. J. 1990;57:209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosso A. Fratelli Treves; Milano, Italy: 1891. La Fatica. [Google Scholar]
- Pappas G.P., Asakawa D.S., Delp S.L., Zajac F.E., Drace J.E. Nonuniform shortening in the biceps brachii during elbow flexion. J. Appl. Physiol. 2002;92:2381–2389. doi: 10.1152/japplphysiol.00843.2001. [DOI] [PubMed] [Google Scholar]
- Powell P.L., Roy R.R., Kanim P., Bello M.A., Edgerton V.R. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J. Appl. Physiol. 1984;57:1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- Ratel S., Duche P., Williams C.A. Muscle fatigue during high-intensity exercise in children. Sports Med. 2006;36:1031–1065. doi: 10.2165/00007256-200636120-00004. doi:10.2165/00007256-200636120-00004 [DOI] [PubMed] [Google Scholar]
- Rudroff T., Christou E.A., Poston B., Bojsen-Moller J., Enoka R.M. Time to failure of a sustained contraction is predicted by target torque and initial electromyographic bursts in elbow flexor muscles. Muscle Nerve. 2007;35:657–666. doi: 10.1002/mus.20752. doi:10.1002/mus.20752 [DOI] [PubMed] [Google Scholar]
- Salavati M., Moghadam M., Ebrahimi I., Arab A.M. Changes in postural stability with fatigue of lower extremity frontal and sagittal plane movers. Gait Posture. 2007;26:214–218. doi: 10.1016/j.gaitpost.2006.09.001. doi:10.1016/j.gaitpost.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Serrano A.L., Petrie J.L., Rivero J.-L.L., Hermanson J.W. Myosin isoforms and muscle fiber characteristics in equine gluteus medius muscle. Anat. Rec. 1996;244:444–451. doi: 10.1002/(SICI)1097-0185(199604)244:4<444::AID-AR3>3.0.CO;2-V. doi:10.1002/(SICI)1097-0185(199604)244:4<444::AID-AR3>3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- Soman A., Hedrick T.L., Biewener A.A. Regional patterns of pectoralis fascicle strain in the pigeon Columba livia during level flight. J. Exp. Biol. 2005;208:771–786. doi: 10.1242/jeb.01432. doi:10.1242/jeb.01432 [DOI] [PubMed] [Google Scholar]
- Syme D.A., Tonks D.M. Fatigue and recovery of dynamic and steady-state performance in frog skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R916–R926. doi: 10.1152/ajpregu.00347.2003. doi:10.1152/ajpregu.00347.2003 [DOI] [PubMed] [Google Scholar]
- Wang L., Kernell D. Proximo-distal organization and fibre type regionalization in rat hindlimb muscles. J. Muscle Res. Cell Motil. 2000;21:587–598. doi: 10.1023/a:1026584307999. doi:10.1023/A:1026584307999 [DOI] [PubMed] [Google Scholar]
- Wickler S.J., Greene H.M., Egan K., Astudillo A., Dutto D.J., Hoyt D.F. Stride parameters and hindlimb length in horses fatigued on a treadmill and at an endurance ride. Equine Vet. J. Suppl. 2006;36:60–64. doi: 10.1111/j.2042-3306.2006.tb05514.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Mori S., Santiesteban A.J., Sun T.C., Hafstad E., Chen J., Burr D.B. The effects of muscle fatigue on bone strain. J. Exp. Biol. 1994;188:217–233. doi: 10.1242/jeb.188.1.217. [DOI] [PubMed] [Google Scholar]