Abstract
Immature epithelial cells generated in the crypt base undergo differentiation while progressing to the villus tip, where the cells upon apoptosis are detached from the underlying muscular tissue. We previously reported that lipid peroxidation might be involved in the turnover of enterocytes across the crypt–villus axis in rat intestine (Dig Dis Sci 52:1840–1844, 2007). To examine whether long-term feeding of fat with different fatty-acid composition influences this process, in the present study we investigated the effect of feeding fish oil (n – 3) and corn oil (n – 6) polyunsaturated fatty acids on lipid peroxidation and anti-oxidant systems in different epithelial cell fractions isolated in rat intestine. Feeding fish oil or corn oil markedly enhanced lipid peroxidation levels of enterocytes throughout villus height compared with control, but there was no difference in the distribution profile of pro- and anti-oxidant enzyme systems and lipid per-oxidation across the crypt–villus axis under these conditions. Analysis of lipid peroxidation levels in different cell fractions revealed that the thiobarbituric acid reactive substance were 9- to 11-fold higher at the villus tip compared with at the crypt base. The activities of glutathione reductase and glutathione-S-transferase were 2- to 5-fold higher in villus tip compared to the crypt region. However, the activities of superoxide dismutase and catalase were 6- to 8-fold high at the crypt base compared with at villus tip cells. Immunocytolocalization of superoxide dismutase showed high staining in crypt base compared with that in villus, tip cells. These findings further suggest that generation of reactive oxygen species in enterocytes across the crypt–villus axis may be involved in turnover of enterocytes across the crypt–villus unit in rat intestine.
Keywords: Fat feeding, Pro-oxidant enzymes, Lipid peroxidation, Rat intestine
Introduction
The gastrointestinal tract is lined by a simple epithelium that undergoes constant renewal involving cell division, differentiation, and cell death. Enterocytes have a limited lifespan, before being replaced by cells derived from the crypt region. Surviving cells undergo apical migration, limited cell replication, and differentiation [1]. The process of differentiation is gradual, characterized by the accumulation of cell-specific products in the upper crypt region and attainment of the mature phenotype in the lower to middle-villus region [2].
In small intestine, enterocytes generated from stem cells in the crypt base differentiate into absorptive cells and are finally lost from the tips of villi, resulting in replacement of lining cells every 2–3 days. Apoptotic cells are normally observed at the tips of villi, but there is also evidence of apoptosis within the crypt, presumably due to excess cellular proliferation, cytotoxicity or genomic damage [3]. In certain inflammatory conditions, such as celiac disease and nematode infections, the number of apoptotic nuclei is reported to be enhanced in villus epithelial cells, indicating that apoptosis plays important roles not only in physiological replacement of villus epithelial cells but also in pathological conditions [4–6]. Molecular oxygen is converted into cytotoxic byproducts referred to as reactive oxygen species (ROS), which have the potential to induce indiscriminate damage to all biological molecules, macromolecules, and cell structures [7]. Previously we reported [8] a correlation between the shedding of fully mature enterocytes at the villus tip and elevated levels of lipid peroxides across the crypt–villus unit in rat intestine. These experiments have been further extended in animals fed fat-containing n – 3 or n – 6 poly unsaturated fatty acid (PUFA), since feeding of dietary fats modulates changes in the lipid.
These findings suggest that feeding n – 3 (fish oil) or n – 6 (corn oil) PUFA-containing fats showed high levels of induced or uninduced in vitro lipid peroxidation in the villus tip cells as compared with in the crypt base. The activities of the pro-oxidants glutathione transferase (GST) and glutathione reductase (GR) are 2- to 5-fold higher in the villus tip, while the reverse was the case with the free-radical scavenger enzymes catalase and superoxide dismutase (SOD) in rat intestine. Immunostaining of SOD across the crypt further confirmed the enzyme protein distribution in animals fed fish oil.
Materials and Methods
Reagents
All chemicals used were of analytical grade. Thiobarbituric acid (TBA), Tris–HCl, reduced glutathione, oxidized glutathione, 1-chloro-2,4-dinitrobenzene (CDNB), reduced nicotinamide adenine dinucleotide phosphate (NADPH), and nitroblue tetrazoliun (NBT) were purchased from Sigma Chemical Company, Saint Louis, MO, USA. Rabbit polyclonal SOD antibody was purchased from Abcam Inc., Cambridge MA, USA. Corn oil (Safola) and fish oil (Sharkovit) were obtained from Marico Industries Ltd. (Mumbai), India.
Experimental Design
Male Albino rats (Wistar strain) weighing 90–100 g obtained from the central animal house of Panjab University, Chandigarh, were kept on standard rat pellet diet (Hindustan Lever Ltd., Ghaziabad), with free access to water. The rats were randomized into three groups with 8–10 animals in each group. Animals in the control group were administered 1 ml normal saline intragastrically by using Ryles tube without anesthesia. Animals in group II and group III were given 1 ml each of fish oil or corn oil, respectively, daily for 30 days, between 9 and 10 am. Body weights of the animals in various groups were recorded on alternate days. After overnight fasting, the animals were sacrificed on the 31st day at 10 am, by cervical dislocation. Starting from the ligament of Treitz, proximal 20 cm of intestine was removed, washed with ice cold saline, and used for various biochemical analyses.
Ethical Clearance
The experimental protocol was approved by the Ethical Committee of the Institute on the use of laboratory animals. Experiments on animals were performed in accordance with guidelines for use of laboratory animals, approved by the Indian Council of Medical Research, New Delhi.
Preparation of Cell Fractions
Epithelial cells from intestine were isolated, following the method of Weiser [9]. Intestine was rinsed thoroughly with a solution containing 0.154 M NaCl and 1 mM dithiothretol and incubated at 37°C for 15 min. The fluid was discarded and intestine was refilled with 1.5 mM ethylenediamine tetraacetic acid (EDTA) and phosphate buffer saline (PBS, pH 7.2). By a series of incubations and washings of intestinal loop at 37°C for various time intervals and sequential fractions of epithelial cells were isolated. The cell fractions were suspended in 50 mM sodium maleate buffer (pH 6.8) and homogenized in the same buffer. Cell fractions were characterized by the assay of marker enzymes sucrase and alkaline phosphatase and were used for various biochemical analyses.
Assay of Enzymes
Alkaline phosphatase [E.C.3.1.3.1] activity was assayed using p-nitrophenylphosphate as a substrate by the method of Bergmeyer [10]. Sucrase [E.C.3.2.1.48] activity was assayed by measuring D-glucose liberated from sucrose hydrolysis using glucose-oxidase peroxidase followed by color development using 4-aminoantipyrene as a chromogen as described earlier by Dahlqvist [11]. Glutathione reductase [E.C.1.6.4.2] activity was measured as described by Horn and Brun [12]. Glutathione-S-transferase [E.C.2.5.1.18] activity was assayed by the method of Habig et al. [13]. Superoxide dismutase [E.C. 1.15.1.1] activity was assayed by the method of Yasuhisa Kono [14]. The reaction mixture contained 1.3 ml sodium carbonate with EDTA (pH 10.0), 0.5 ml NBT, and 0.1 ml TritonX-100. The reaction was initiated by the addition of 0.1 ml hydroxylamine hydrochloride (pH 6.0) to the reaction mixture and the rate of reduction of NBT in the absence of the enzyme source was recorded for about 30 s. Then the appropriate amount of enzyme source was added to the test cuvet as well as the reference cuvet, which did not contain hydroxylamine hydrochloride. Catalase [E.C.1.11.1.6] activity was assayed by monitoring the disappearance of H2O2 at 240 nm as described by Luck [15]. Three milliliters of reaction mixture contained potassium phosphate buffer (pH 7.0) H2O2 and an appropriate amount of enzyme source. A blank, lacking H2O2, was also run simultaneously. The decrease in absorbance at 240 nm was measured.
Lipid per-oxidation was measured by the thiobarbituric acid color reaction for malondialdehyde (MDA) formation following the method of Beuge and Aust [16]. In vitro lipid peroxidation under induced (Fe2+/ascorbate) or uninduced conditions was measured as described by Dudeja and Brasitus [17].
Protein content was determined using the method of Lowry et al. [18] using bovine serum albumin (BSA) as the standard. All enzyme activities are expressed as units per milligram protein. One enzyme unit is defined as the amount of enzyme that transformed 1 μmol substrate to product per minute under standard assay conditions.
Immunofluorescent Staining in Rat Intestine
For immunocytolocalization of superoxide dismutase, tissues were fixed in 10% formalin and embedding in paraffin, and immunostaining was performed by using standard avidin-biotin-peroxidase complex system, as described earlier [19]. Immunofluorescent studies were carried out on 4-μm paraffin sections of rat ileum tissues mounted on (3-aminopropyl)triethoxysilane-treated slides. The slides were placed at 60°C for 30 min, deparaffinized in xylene for 20 min to remove the embedding media, and washed in absolute ethanol for 5 min. The slides were then gradually rehydrated gently in a series of alcohol washes, including 96%, 85%, and 50%, and placed in distilled water for 5 min each. Antigen retrieval was used for tissue sections submerged in 0.1 M citrate buffer, pH 6.0 for 30 min at 100°C in a steamer. Slides were allowed to cool to room temperature (RT) and rinsed in Wash Buffer [Tris-buffered saline containing 0.1% Triton X-100 (TBST)]. The slides were then incubated for 1 h at RT in 5% normal goat serum (NGS) in TBS to block nonspecific antibody binding. This was followed by incubation of the slides with rabbit polyclonal SOD antibody (Abcam Inc, Cambridge MA, USA), diluted 1:100 (4°C, overnight). Cells were then stained with anti-goat fluorescein isothiocyanate antibody diluted at 1:100 and rhodamine-phalloidin (Molecular Probes Inc., USA) diluted 1:40 and Hoechst 33342 (Invitrogen Corp) for 60 min. After final washes, slides were mounted with a coverslip using Permount and sealed with nail polish [20].
Image acquisition
Microscopy was performed using Carl Zeiss® LSM 510 laser scanning confocal microscope equipped with a 25× water-immersion objective. Beams of 488 nm and 534 nm from an Ag/Kr laser and 361 nm from an ultraviolet (UV) laser were used for excitation. Green and red fluorescence emissions were detected through LP505 and 585 filters, respectively. The two different fluorochromes were scanned sequentially by using multitracking function to avoid any bleed through among these fluorescent dyes. For control experiments, the sections were incubated without the primary antibody.
Statistical analysis of data was done using Student’s t-test.
Results
The isolated cell fractions were characterized by assaying the activity of marker enzymes, sucrase, and alkaline phosphatase (AP) in rat intestine [8]. The activity of sucrase (fourfold) and alkaline phosphatase (sixfold) were higher in villus tip (VT) cells compared with in the crypt base (CB). Feeding fish oil or corn oil elevated (P ≤ 0.001) alkaline phosphatase activity, all along the villus length, compared with the control group. AP activity was increased by 85–107% in villus tip cells, whereas the corresponding changes in mid villus and crypt cells were 111–138% and 111–156%, respectively, in groups fed fish oil or corn oil. A similar, albeit small, increase in sucrase activity was also observed in oil-fed groups at the villus tip (16–27%), mid villus (39–61%), and crypt region (63–84%) compared with control.
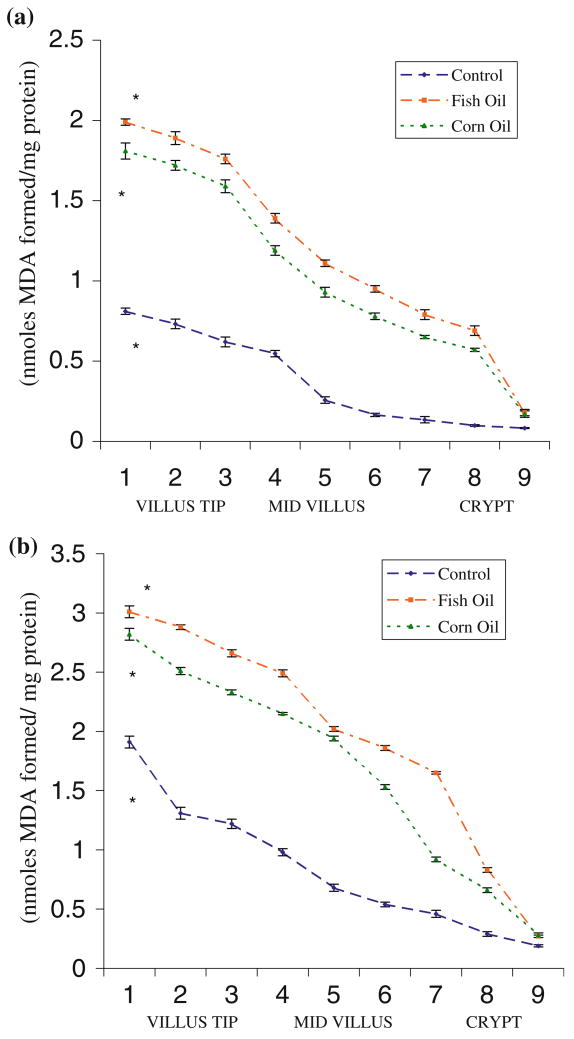
The lipid per-oxidation (LPO) profile of different populations of epithelial cells across villus height, determined by measuring MDA levels, revealed that cells at villus tip had 9- to 11-fold higher MDA levels compared with that in the crypt base (Fig. 1a). Animals fed fish oil showed uninduced lipid peroxidation levels of 1.88 ± 0.03 nmol MDA formed/mg protein in villus tip cells (fractions 1–3) compared with 1.71 ± 0.04 in corn oil fed animals or 0.721 ± 0.03 in control animals. Corresponding values in the crypt region (fractions 7–9) were 0.55 ± 0.03, 0.47 ± 0.01, and 0.106 ± 0.009 nmol MDA/mg protein in rats fed fish oil or corn oil and the control group, respectively. A similar increase in MDA levels (2.85 ± 0.02) of cells at villus tip was observed in oil-fed animals compared with controls (1.48 ± 0.05 nmol/mg protein) under induced lipid per-oxidation conditions.
Fig. 1.
(a) Uninduced lipid peroxidation levels in epithelial cells across the crypt–villus axis in control and fat-fed rat small intestine. Values are mean ± standard deviation (SD) (n = 6). (b) The profile of lipid peroxidation levels induced in vitro (Fe2+/ascorbate) in epithelial cells across the crypt–villus axis in control and fat-fed rat small intestine. Values are mean ± SD (n = 6)
The profile of LPO, determined by MDA measurements, was similar under uninduced or induced (Fe2+/ascorbate) in vitro conditions throughout the length of villi in rat intestine (Fig. 1a, b). The MDA levels were minimum in the crypt base (0.106 ± 0.009 nmol/mg protein), but were high (0.721 ± 0.03 nmol/mg protein) in mature enterocytes at the tip of villi. Feeding fish oil (PUFA n – 3) or corn oil (PUFA n – 6) elevated the lipid peroxidation levels in epithelial cells, all along the villus length.
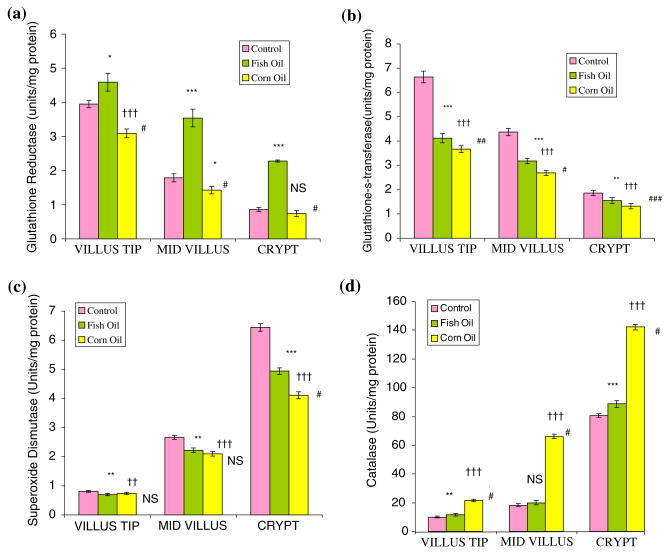
The distribution of glutathione reductase (GR) and glutathione-S-transferase (GST) across the crypt–villus axis in rat small intestine showed that GR and GST activities were 3.95 ± 0.11 and 6.64 ± 0.2 at the villus tip as compared with 0.86 ± 0.06 and 1.86 ± 0.11 (μmol/h/mg protein) in the crypt base region (Fig. 2a). Fish oil feeding elevated GR activity along the villus length, compared with control or corn-oil-fed animals. Fish oil administration to rats enhanced GR activity (4.59 ± 0.26), while corn oil feeding inhibited enzyme activity (3.09 ± 0.13) compared with control (3.95 ± 0.11 μmol/h/mg protein). GR activity was not modified in the crypt base in animals fed corn oil (0.74 ± 0.09 μmol/h/mg protein), while villus tip cells revealed significantly low enzyme levels (3.09 ± 0.13) compared with in control group (3.95 ± 0.11 μmol/h/mg protein).
Fig. 2.
(a) Glutathione reductase activity in enterocytes across the crypt–villus axis in control and fat-fed rat small intestine, as μmol/h/mg protein. Values are mean ± SD (n = 6). Control versus fish oil: *P ≤ 0.01, **P ≤ 0.05, ***P ≤ 0.001; control versus corn oil: †P ≤ 0.01, ††P ≤ 0.05, †††P ≤ 0.001; fish oil versus corn oil: #P ≤ 0.001, ##P ≤ 0.01, ###P ≤ 0.05, NS: nonsignificant. (b) Glutathione-S-transferase activity in enterocytes across the crypt–villus axis in control and fat-fed rat small intestine, as μmol/h/mg protein. Values are mean ± SD (n = 6). Control versus fish oil: *P ≤ 0.01, **P ≤ 0.05, ***P ≤ 0.001; control versus corn oil: †P ≤ 0.01, ††P ≤ 0.05, †††P ≤ 0.001; fish oil versus corn oil: #P ≤ 0.001, ##P ≤ 0.01, ###P ≤ 0.05, NS: nonsignificant. (c) Superoxide dismutase activity in epithelial cells across the crypt–villus axis in control and fat-fed rat small intestine, as amount of enzyme required to produce 50% inhibition of NBT reduction per min/mg protein. Values are mean ± SD (n = 6). Control versus fish oil: *P ≤ 0.01, **P ≤ 0.05, ***P ≤ 0.001, control versus corn oil: †P ≤ 0.01, ††P ≤ 0.05, †††P ≤ 0.001, fish oil versus corn oil: #P ≤ 0.001, ##P ≤ 0.01, ###P ≤ 0.05, NS: nonsignificant. (d) Catalase activity in enterocytes across the crypt–villus axis in control and fat-fed rat small intestine, as μmol/h/mg protein. Values are mean ± SD (n = 6). Control versus fish oil: *P ≤ 0.01, **P ≤ 0.05, ***P ≤ 0.001, control versus corn oil: †P ≤ 0.01, ††P ≤ 0.05, †††P ≤ 0.001, fish oil versus corn oil: #P ≤ 0.001, ##P ≤ 0.01, ###P ≤ 0.05, NS: nonsignificant
GST activity in villus tip cells was 3- to 4-fold high and gradually decreased towards the mid villus and was nearly constant in the crypt cells. Feeding fish oil or corn oil inhibited GST activity in the villus tip cells, whereas there was essentially no change in enzyme activity in the crypt base under these conditions.
The activities of SOD and catalase showed the opposite pattern; their levels were high (P ≤ 0.001) in crypt base (6.44 ± 0.11) and the least in villus tip cells (0.813 ± 0.03 units/mg protein) (Fig. 2c, d) both in oil-fed and control animals. Results for animals fed fish oil or corn oil for a month showed a similar distribution pattern of the enzymes. There was a 6- to 8-fold decrease in SOD activity in villus tip (0.813 ± 0.03) cells compared with that in the crypt base (6.44 ± 0.11 units/mg protein) in control animals. SOD levels were not different in the control and fat-fed groups in the villus tip or mid-villus regions but there was a significant increase (P ≤ 0.001) in enzyme activity in the crypt base region. There was 7- to 8-fold reduction in catalase activity in the villus tip cells compared with that in the crypt base in rats fed fish oil or corn oil and control (Fig. 2d). However, there was essentially no difference in catalase levels in epithelial cells across the crypt–villus unit in the control or fish-oil-fed groups. Rats fed corn oil showed a marked increase in catalase activity (P ≤ 0.001) in various cell fractions across the villus height compared with control group (Fig. 2d).
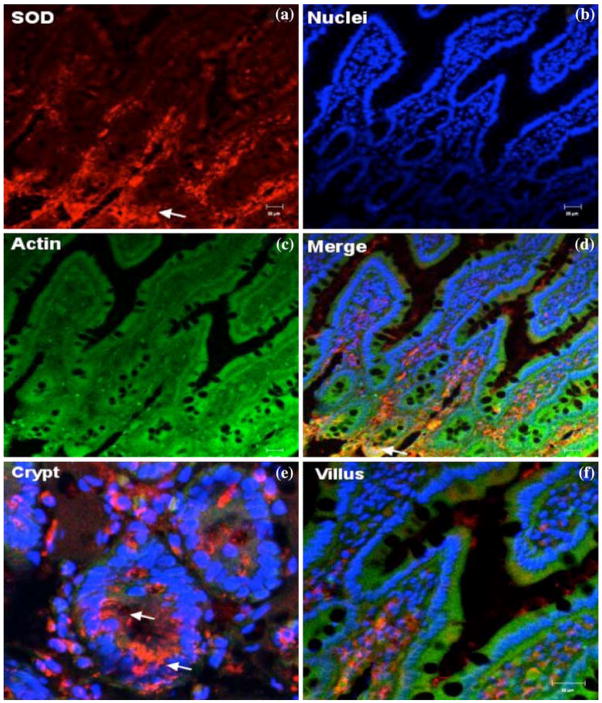
Immunocytolocalization of SOD in fish-oil-fed animals showed intense staining in the crypt-base cells but low intensity of the SOD staining was seen at the upper villus (Fig. 3). These results corroborate the enzyme distribution data in fish-oil-fed animals.
Fig. 3.
Confocal microscopic localization of SOD (red, a); Alexa Fluor 488 phalloidin (actin, green, c); Hoecht dye (Nuclei, blue, b) and an overlay merge image (d). Intenset immunoreactivity for SOD was observed in the crypt region, predominantly in the cytoplasm, while villus tip cells showed faint staining. Representative images of crypt base (e) and villus tip (f) regions are shown. Scale bars, 20 μm
Discussion
Epithelial cells were characterized by analysis of marker enzymes, sucrase, and AP. There was 4- to 6-fold enrichment of the enzymes in villus tip cells compared with that in the crypt base. Analyses of lipid per-oxidation under induced and uninduced conditions measured by determining TBRS showed 9- to 11-fold increase in villus tip cells compared with in the crypt region. The observed increase in lipid peroxidation levels was associated with 2- to 5-fold increase in activities of GST and GR across the crypt–villus unit, the highest activity being observed in the villus tip, and the least in the crypt base. However, the opposite pattern was observed for the distribution of activities of SOD and catalase, which are scavengers of reactive oxygen species. These results are in agreement with those reported earlier [8]. Immunocytolocalization of SOD in crypt–villus regions confirmed high staining due to SOD in the crypt base compared with that at the villus tip region. Cable et al. [21] have described high catalase activity in the crypt base and low levels in villus tip cells using ultrastructural cytochemistry. The present data show high catalase activity in crypt cells, which was reduced by 7- to 8-fold in villus tip cells. Such a profile of pro- and anti-oxidant systems along the villus length supports the contention that high levels of reactive oxygen species may be responsible for the release of enterocytes from villus tip into lumen in rat intestine. The data also showed that crypt cells are characterized by the least amount of free radicals as indicated by low MDA levels and have high catalase and SOD activities compared with nondividing, mature villus tip cells in rat intestine. Fish oil feeding enhanced lipid peroxidation in rat intestine, which is in agreement with results by Kaur et al. [22].
Brasitus and Dudeja [23] described that brush border membrane lining the mature enterocyte at the villus tip are less fluid compared with that of immature cells in the crypt base. Such a difference in membrane fluidity may also be explained by high levels of lipid peroxidation in villus tip cells compared with low levels in crypt base, as indicated by the present data. The balance of ROS generation and antioxidant capacity within the cell determines the oxidative environment. When the ROS exceed the antioxidant capacity of the cell, oxidative stress results. Oxidative stress can initiate and/or mediate a number of signaling cascades, including apoptosis [24]. Thus high levels of lipid peroxidation as a consequence of the generation of free radicals may be implicated in the slough off from of epithelial cells from the villus tip. Basivireddy et al. [25] have suggested that villus tip cells are more prone to the damaging effects of indomethacin, a nonsteroidal anti-inflammatory drug (NSAID), as a consequence of oxidative stress involved in cell damage in the intestine.
Ko et al. [26] reported considerably higher levels of the hepatic antioxidant enzymes GR and GST in rats fed fish oil than in those fed corn oil. In the present study, we observed that the pro-oxidant enzyme system respond differently to polyunsaturated fat (fish oil or corn oil) feeding across the crypt–villus axis in rat intestine. Fish oil administration enhanced glutathione reductase activity while corn oil feeding reduced it. These observations are opposite to those reported in hepatic tissue. The observed increase in the activities of anti-oxidant enzymes (GR and GST) and lipid peroxidation after fish oil administration may imply that fish oil is an oxidative stress-causing factor. Diets rich in fish oil as the lipid source enhanced ROS generation in colonocytes [27]. This is not unexpected considering the high degree of unsaturation found in the long-chain (n – 3) PUFA in fish oil. The primary (n – 3) fatty acids in fish oil, eicosapentaenoic acid [20:5(n – 3)] and docosahexaenoic acid [22:6(n – 3)] have up to three times as many double bonds per molecule than the (n – 6) fatty acids found in corn oil, such as linoleic acid [18:2(n – 6)]. This increases the opportunity for oxidant attack and can contribute to the propagation of ROS. Chapkin et al. [27] reported dietary (n – 3) PUFA to be readily incorporated into the mitochondrial membrane [27], predisposing mitochondria to enhanced lipid peroxidation and membrane damage and contributing to the propagation of ROS generated by the mitochondrial electron transport system [28].
The role of lipid peroxidation in the detachment of epithelial cells from the underlying basement membrane in the lung has been described by Johnson et al. [29]. Damage of cellular DNA by lipid peroxidation plays a major role in cellular injury and altered cell functions, leading to apoptosis [30]. Such a role of ROS in epithelial cell turnover may be of physiological significance in intestinal diseases such as celiac disease and nematode infections where the role of ROS has been suggested [4–6]. The present data further reinforce the contention that ROS may play some role during migration and maturation of enterocytes along the villus length. Data on immunocytolocalization of SOD also support the role of lipid peroxides in the exfoliation of fully mature enterocytes at villus tip and elevated levels of lipid peroxides across the crypt–villus unit in rat intestine.
Acknowledgments
Aasma Turan is a recipient of a Senior Research Fellowship from the Indian Council of Medical Research, New Delhi, India.
Abbreviations
- TBRS
Thiobarbituric acid reactive substance
- GST
Glutathione-S-transferase
- GR
Glutathione reductase
- SOD
Superoxide dismutase
- ROS
Reactive oxygen species
References
- 1.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine columnar cell. Am J Anat. 1974;141:461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- 2.Hall PA, Coates PJ, Ansari B. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 3.Potten CS, Grant HK. The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br J Cancer. 1998;78:993–1003. doi: 10.1038/bjc.1998.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss SF, Attia L, Scholes JV, et al. Increased small intestinal apoptosis in coeliac disease. Gut. 1996;39:811–817. doi: 10.1136/gut.39.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyoh Y, Nishida M, Tegoshi T, et al. Enhancement of apoptosis with loss of cellular adherence in the villus epithelium of the small intestine after infection with the nematodeNippostrongylus brasiliensis in rats. Parasitology. 1999;119:199–207. doi: 10.1017/S003118209900462X. [DOI] [PubMed] [Google Scholar]
- 6.Stiiber E, Buschenfld A, Von FA, et al. Intestinal crypt cell apoptosis in murine acute graft versus host disease is mediated by tumour necrosis factor alpha and not by the FasL–Fas interaction: effect of pentoxifylline on the development of mucosal atrophy. Gut. 1999;45:229–235. doi: 10.1136/gut.45.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ames BN, Shigeenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turan A, Mahmood A. The profile of antioxidant and lipid peroxidation across crypt–villus axis in rat intestine . Dig Dis Sci. 2007;52:1840–1844. doi: 10.1007/s10620-006-9633-z. [DOI] [PubMed] [Google Scholar]
- 9.Weiser MM. Intestinal epithelial cells surface membrane glycoprotein synthesis. J Biol Chem. 1973;215:2536–2541. [PubMed] [Google Scholar]
- 10.Bergmeyer MVC. Methods of Enzymatic Analysis. New York, USA: Academic; 1963. pp. 783–785. [Google Scholar]
- 11.Dahlqvist A. Method for the assay of intestinal disaccharidases. Anal Biochem. 1964;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- 12.Horn HD, Brun FM. In: Methods of Enzymatic Analysis. Bergmeyer HU, editor. New York, USA: Academic; 1971. pp. 875–881. [Google Scholar]
- 13.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 14.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–195. doi: 10.1016/0003-9861(78) 90479-4. [DOI] [PubMed] [Google Scholar]
- 15.Luck H. Methods of Enzymatic Analysis. New York, USA: Academic; 1971. pp. 885–888. [Google Scholar]
- 16.Beuge JA, Aust SD. Microsomal lipid peroxidation. Metab Enzmol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 17.Dudeja PK, Brasitus TA. Inactivation of rat small intestinal brush-border membrane alkaline phosphatase by oxygen free radicals. Gastroenterology. 1993;105:357–366. doi: 10.1016/0016-5085(93)90708-k. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosenbrough NS, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Mahmood A, Shao J, Alpers DH. Rat enterocytes secrete SLPs containing alkaline phosphatase and cubilin in response to corn oil feeding. Am J Physiol Gastrointest Liver Physiol. 2003;285:G433–G441. doi: 10.1152/ajpgi.00466.2002. [DOI] [PubMed] [Google Scholar]
- 20.War PA, Johnson KJ, Warren JS, Kunkel RG. Immune-complexes, oxygen radical and lung injury. Oxygen Radical and Tissue Injury: Proc. Brook Lodge Symp; Augusta, MI, USA. April 27–29;1987; pp. 107–114. [Google Scholar]
- 21.Cable S, Kedinger M, Dauca M. Peroxisomes and peroxisomal enzymes along the crypt–villus axis of the rat intestine. Differentiation. 1993;54:99–108. doi: 10.1111/j.1432-0436.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaur M, Kaur J, Ojha S, Mahmood A. Ethanol affect on lipid peroxidation and glutathione mediated defense in rat small intestine. Alcohol. 1998;15:65–69. doi: 10.1016/S0741-8329(97)00099-2. [DOI] [PubMed] [Google Scholar]
- 23.Brasitus TA, Dudeja PK. Regional differences in the lipid composition and fluidity of rat colonic brush-border membranes. Biochim Biophys Acta. 1985;819:10–17. doi: 10.1016/0005-2736(85)90189-0. [DOI] [PubMed] [Google Scholar]
- 24.Sanders LM, Henderson CE, Hong MY, et al. An increase in reactive oxygen species by dietary fish oil coupled with the attenuation of antioxidant defenses by dietary pectin enhances rat colonocyte apoptosis. J Nutr. 2004;134:3233–3238. doi: 10.1093/jn/134.12.3233. [DOI] [PubMed] [Google Scholar]
- 25.Basivireddy J, Vasudevan A, Jacob M, Balasubramanian KA. Indomethacin-induced mitochondrial dysfunction and oxidative stress in villus enterocytes. Biochem Pharmacol. 2002;64:339–349. doi: 10.1016/S0006-2952(02)01067-5. [DOI] [PubMed] [Google Scholar]
- 26.Ko Y, Lii CK, Ou CC, Liu JY, Lin WL, Chen HW. Comparison of the effect of fish oil and corn oil on chemical-induced hepatic enzyme-altered foci in rats . J Agric Food Chem. 2000;48:4144–4150. doi: 10.1021/jf0000631. [DOI] [PubMed] [Google Scholar]
- 27.Chapkin R, Hong M, Fan Y, et al. Dietary n – 3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids. 2002;37:193–199. doi: 10.1007/s11745-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 28.Hong MY, Chapkin RS, Barhoumi R, et al. Fish oil increases mitochondrial phospholipid unsaturation, unregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1926. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- 29.Johnson KJ, Wilson BS, Till GO, Ward PA. Acnli lung injury in rat caused by immunoglobulin A immune complex. J Clin Invest. 1984;74:358–368. doi: 10.1172/JCI111431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakson J, Schraeafsfatter IU, Idyslop PA. Role of oxidants in DNA damage: hydroxyl radical the synergistic DNA damaging effect of asbestoses and cigarette smoke. J Clin Invest. 1987;80:1090–1095. doi: 10.1172/JCI113165. [DOI] [PMC free article] [PubMed] [Google Scholar]