Abstract
AIM
To investigate the time course of the hypotensive interaction between sildenafil and glyceryl trinitrate (GTN).
METHODS
Two double-blind, placebo-controlled, randomized, crossover studies were performed. Subjects were challenged with sublingual GTN 400 µg at different times after oral sildenafil 100 mg. After each GTN challenge frequent measures of blood pressure (BP) were made. In the first study GTN was given 1–48 h after sildenafil/placebo to 33 healthy men. In the second study GTN was given 1–8 h after sildenafil/placebo to 20 men with stable angina.
RESULTS
In healthy men there was a greater mean maximum reduction in BP with sildenafil/GTN than with placebo/GTN only at 1 h. In angina patients, there was a greater mean maximum reduction in BP with sildenafil/GTN than with placebo/GTN for up to 8 h. The mean (95% confidence interval) differences in maximum systolic BP reduction (mmHg) at 1, 4, 6 and 8 h were −16 (−12, −21), −12 (−4, −20), −6 (1, −12) and −9 (−3, −15), all P < 0.05 except at 6 h (NS). At 6 and 8 h the interaction was not more than additive, and hypotensive symptoms did not occur.
CONCLUSIONS
In men with angina there is an interaction on BP reduction between sildenafil and GTN for ≥ 8 h after sildenafil administration, but this is no more than additive from 6 h. These data may be helpful to clinicians who are considering the use of GTN in patients presenting with angina who have received sildenafil within 24 h.
Keywords: blood pressure, glyceryl trinitrate, hypotension, phosphodiesterase type 5 inhibition, sildenafil citrate
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Co-administration of inhibitors of phosphodiesterase type 5 with organic nitrates can cause profound hypotension.
For this reason, it is recommended that sildenafil and organic nitrates are not administered within 24 h of one another.
However, the time course of the interaction between sildenafil and sublingual glyceryl trinitrate (GTN) has not previously been investigated.
WHAT THIS STUDY ADDS
In men with angina there is an interaction between oral sildenafil and sublingual GTN for ≥8 h, but from 6 h this interaction is not more than additive.
These data may be helpful to clinicians who are considering the use of GTN in patients presenting with angina who have received sildenafil within 24 h.
Introduction
Nitric oxide (NO) causes vasodilation by stimulating vascular smooth muscle soluble guanylate cyclase to convert guanosine 5′-triphosphate to guanosine 3′,5′-cyclic monophosphate (cGMP) [1], which leads to a reduction in intracellular calcium concentration [2]. cGMP is degraded by cGMP-specific, cGMP-binding phosphodiesterase 5 (PDE5) [3], and inhibition of this enzyme enhances vascular smooth muscle relaxation. By stimulating vasodilation within the corpora cavernosa during sexual stimulation, PDE5 inhibitors, such as sildenafil citrate, facilitate penile erection and are useful treatments of male erectile dysfunction [4]. Inhibitors of PDE5 are also vasodilators in the systemic circulation. In hypertension, regular sildenafil monotherapy reduces ambulatory blood pressure (BP) by 10/6 mmHg [5].
The organic nitrates, such as glyceryl trinitrate (GTN), are anti-anginal drugs that dilate arteries and veins through their action as NO donors [6]. The simultaneous provision of exogenous NO from organic nitrates and inhibition of cGMP breakdown with PDE5 inhibition can result in substantial BP reduction. For sildenafil, this interaction has been demonstrated in healthy subjects [7] and in men with angina [7, 8]. It is recommended that, because of the potential for harm from hypotension, sildenafil and organic nitrates are not co-administered [9]. The minimum interval within the licence is 24 h, but this is not firmly evidence based.
There are currently no data on the time course of the interaction between sildenafil and organic nitrates. Previous clinical studies have concentrated on the maximum potential for interaction early after administration of sildenafil [7, 8]. In keeping with its plasma half-life of 3–5 h, blood concentrations of sildenafil are very low 24 h after a single dose. Therefore, it is possible that organic nitrates could safely be administered at a shorter interval after sildenafil than the 24 h that is currently recommended. We have characterized the time course of the pharmacodynamic interaction on BP between sildenafil and GTN, initially in healthy men and subsequently in men with stable angina.
Methods
Both studies were approved by the Lothian Research Ethics Committee and were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects. Both studies were of randomized, double-blind, placebo-controlled crossover design. Subjects refrained from alcohol for at least 24 h and caffeinated drinks and smoking for at least 12 h before each visit. In study 1, subjects also refrained from eating for 12 h before each visit. Studies were conducted in a quiet room kept at 22–24°C.
Study 1
Subjects
Inclusion criteria were: healthy male; age 35–65 years; weight between 60 and 100 kg. Exclusion criteria were: any clinically significant disease; history of drug hypersensitivity; supine or standing systolic BP < 100 or >165 mmHg, or supine or standing diastolic BP < 55 or >95 mmHg; orthostatic hypotension (>20 mmHg reduction in systolic BP on standing up); taken any medicine, except paracetamol, within 3 weeks of the start of the study; evidence of alcohol or drug abuse.
Protocol
Subjects were divided into two groups, A and B. The study was performed in four sequential phases. The aim was to maximize safety by giving GTN at decreasing intervals after sildenafil. Subjects rested supine for at least 30 min prior to sildenafil or placebo administration. Single measures of sitting and standing BP and heart rate (HR) were made 30 min, 15 min and immediately before sildenafil or placebo and also immediately before each GTN administration. After each GTN administration, sitting measurements were made every 3 min for 27 min followed, from 30 min, by sitting and standing measurements every 15 min for a further 90 min. The protocol is illustrated in Figure 1.
Figure 1.
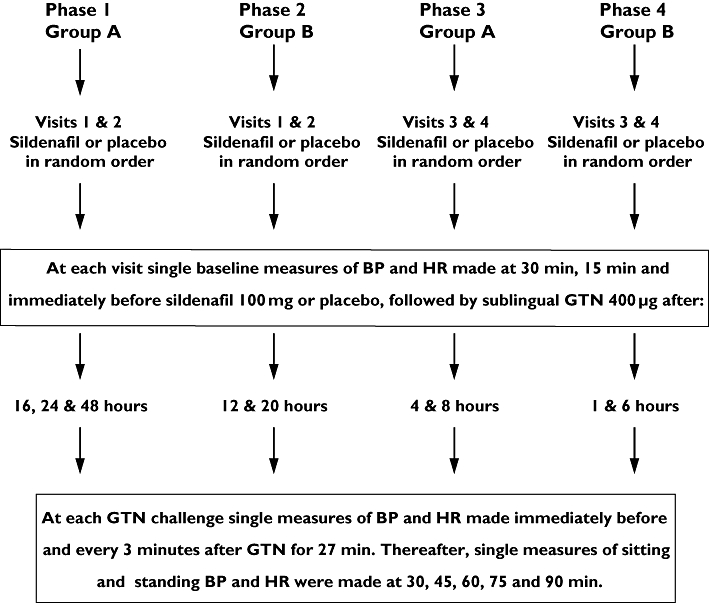
Study 1 was performed in four separate phases. Group A attended phases 1 and 3 and group B attended phases 2 and 4. As a safety measure, phases 2, 3 and 4 were performed only after review of the preceding phase. At each phase subjects attended on two occasions and received, in random order, sildenafil or matched placebo (the alternative therapy was administered after a wash-out of at least 7 days). Sublingual glyceryl trinitrate (GTN) was subsequently administered, after sildenafil or placebo, at the times indicated. Subjects remained in the department throughout the duration of each phase that they attended
Study 2
Subjects
Inclusion criteria were: male; age 30–80 years; weight between 60 and 100 kg; stable angina with one of a classical history of exertional angina, a previous diagnostic exercise treadmill test or angiographic evidence of coronary artery disease. Exclusion criteria were: regular treatment with long-acting nitrates or nicorandil where these could not be withdrawn; myocardial infarction, unstable angina, stroke or transient cerebral ischaemia within 3 months; systolic BP < 100 or >170 mmHg, or diastolic BP < 60 or >100 mmHg; orthostatic hypotension (>20 mmHg fall in systolic BP on standing); diabetes treated with oral hypoglycaemic agents or insulin; any clinically significant disease other than stable angina, excepting other cardiovascular disease risk factors such as smoking, hypercholesterolaemia and diet-controlled diabetes; taking any drug that significantly interacts with sildenafil; evidence of alcohol or drug abuse.
Protocol
The study was performed in two phases, with two visits to the research unit in each phase. All subjects attended both phases. On each study day subjects took their regular morning medicines immediately after waking and had a light breakfast before attending the unit at 07.00 h. During phase 1, GTN was administered 4 and 8 h after oral sildenafil (at one visit) or matched placebo (at the other visit), given in random order. During phase 2, GTN was administered 1 and 6 h after sildenafil or placebo, again given in random order between the two visits. Following at least 20 min rest, baseline sitting and standing BP and HR were recorded, in duplicate, 20 min and immediately before sildenafil or placebo administration. Immediately before and every 3 min for 33 min after each GTN dose further measurements of sitting BP and HR were made, duplicate measures before GTN and single measures after GTN. Standing BP and HR were repeated 36 min after each GTN dose. The protocol is illustrated in Figure 2.
Figure 2.
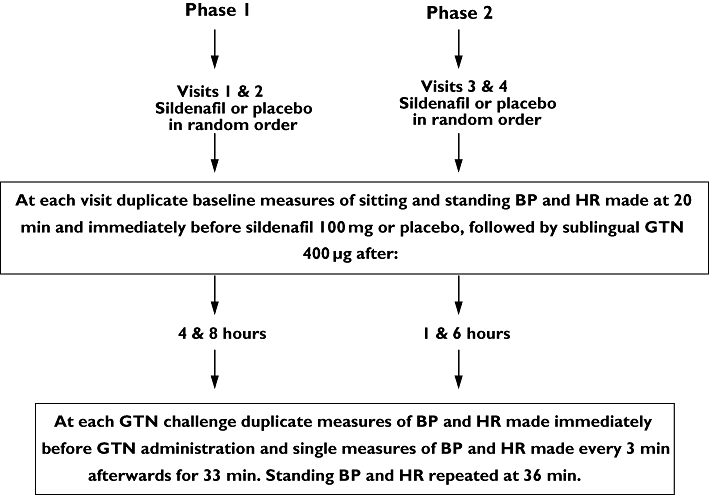
Study 2 was performed in two phases. At each phase subjects attended on two occasions and received, in random order, sildenafil or matched placebo (the alternative therapy was administered after a wash-out of at least 5 days). Sublingual glyceryl trinitrate (GTN) was subsequently administered, after sildenafil or placebo, at the times indicated. Subjects remained in the department throughout the duration of each phase
BP measurement
BP was measured after at least 5 min sitting and after 2 min standing, as indicated in the protocol. BP and HR were measured using Omron HEM-705CP monitors (Omron, Milton Keynes, UK) [10] in study 1 and Dinamap Pro 100 monitors (GE Healthcare, Little Chalfont, UK) [11] in study 2.
Drugs
GTN 400 µg (Nitrolingual Pumpspray®; Merck Pharmaceuticals, West Drayton, UK) was administered as a single spray sublingually. Sildenafil 100 mg and matched placebo (both Pfizer Ltd, Sandwich, UK) were administered orally as single tablets. Administration was directly observed.
Statistical analysis
Baseline values were calculated as the mean of those obtained 30 min, 15 min and immediately before sildenafil or placebo administration for study 1, and as the mean of those obtained 20 min and immediately before sildenafil or placebo administration for study 2. For both studies, the prespecified primary analysis was a comparison of the effects of the combination of sildenafil and GTN and the combination of placebo and GTN on the maximum reduction from baseline in sitting BP and the maximum increase from baseline in HR that occurred in the monitoring period following each GTN challenge. In study 2, effects from pre-GTN values were also assessed for each time point. Pre-GTN BPs and HRs were calculated as the mean of the two measurements made before each GTN challenge. Maximum reductions in BP, maximum increases in HR and areas under the curves (AUCs) for changes in these parameters from pre-GTN values, during the 33 min following each GTN challenge, were compared between sildenafil and placebo phases.
Unless stated, data are presented as means and standard errors of means. All comparisons between sildenafil and placebo were made using a two-period crossover anova model allowing for variation due to subject, treatment and treatment order. Statistical significance was declared if a two-sided P-value was ≤0.05.
Results
Study 1
Subjects
Thirty-four subjects were screened for the study and, of these, one was not suitable. Of the remaining subjects, 16 were recruited to group A and 17 to group B. Overall, the mean age was 49 years (range 38–64) and the mean body weight was 78 kg (range 60–102). Four subjects failed to complete all stages of the study. Two subjects were withdrawn due to non-adherence to the protocol, one withdrew consent and the other was unable to complete the study because of other commitments. None of the discontinuations was related to the study drugs. Fifteen subjects were included in the analysis of step 1, 16 for step 2, 14 for step 3 and 15 for step 4.
Effects of sildenafil alone
The recordings taken before each GTN challenge provided a measure of the effect of sildenafil alone on BP and HR. Compared with placebo, sildenafil reduced sitting diastolic BP at 1 h [mean change from baseline −4.7 mmHg (1.6) with sildenafil vs. 4.0 mmHg (1.3) with placebo, P= 0.01], but not at the other time points. Sitting systolic BP was unaffected by sildenafil at any time point. Sitting HR was reduced by sildenafil compared with placebo at 48 h only [−1.4 bpm (1.8) vs. 6.4 bpm (1.6), P < 0.05], probably a chance finding. There was a greater reduction in standing diastolic BP with sildenafil than with placebo at 1 h [−3.0 mmHg (1.3) vs. 3.8 mmHg (1.5), P < 0.05) and 8 h [−6.6 mmHg (1.2) vs.−2.0 mmHg (1.7), P < 0.05], but standing systolic BP was unaffected by sildenafil. Standing HR was increased by sildenafil at 1 h [2.4 bpm (1.5) vs.−1.8 bpm (1.5), P < 0.05] and 8 h [11.1 bpm (2.4) vs. 6.7 bpm (1.6), P < 0.05] and decreased by sildenafil at 48 h [2.2 bpm (1.7) vs. 7.3 bpm (1.9), P < 0.05].
Effect of GTN with sildenafil
There were greater mean maximum reductions in sitting systolic and diastolic BP with the combination of sildenafil and GTN than with the combination of placebo and GTN at 1 h only. At all other times differences between placebo and sildenafil were not statistically significant (Table 1). Modest but significant differences in sitting HR occurred when GTN was administered 1, 6 and 24 h post sildenafil (Table 1). Similarly, only at 1 h was there a greater mean maximum reduction in standing BP with the combination of sildenafil and GTN than with the combination of placebo with GTN [systolic BP: −23.8 mmHg (2.8) vs.−13.5 mmHg (2.5), P < 0.01; diastolic BP: −14.9 mmHg (2.3) vs.−6.9 mmHg (1.2), P < 0.01]. For standing HR, there was a greater increase when GTN was given 1 h after sildenafil [9.6 bpm (1.7) vs. 3.9 bpm (1.1) with placebo, P < 0.05) and a lesser increase when GTN was given 48 h after sildenafil [7.1 bpm (1.6) vs. 13.3 bpm (2.5) with placebo, P < 0.05].
Table 1.
Mean (SEM) maximum changes from baseline in sitting blood pressure (BP) and heart rate (HR) with glyceryl trinitrate given after sildenafil and placebo in healthy men
| Hours after sildenafil or placebo | Systolic BP (mmHg) | Diastolic BP (mmHg) | HR (bpm) | |||
|---|---|---|---|---|---|---|
| Placebo | Sildenafil | Placebo | Sildenafil | Placebo | Sildenafil | |
| 1 | −16.6 (2.6) | −28.7 (3.5)** | −11.2 (1.9) | −22.7 (2.7)** | 12.0 (1.2) | 16.8 (1.9)* |
| 4 | −17.5 (2.2) | −20 (2.2) | −17 (1.7) | −18.5 (1.6) | 24.3 (2.7) | 25.5 (2.9) |
| 6 | −17.1 (2.1) | −16.2 (2.4) | −14.4 (0.9) | −17.3 (1.6) | 18.6 (1.7) | 24.7 (2.7)* |
| 8 | −17.0 (3.2) | −18.7 (3.1) | −16.4 (2.1) | −18.5 (1.4) | 23.7 (2.5) | 25.6 (2.9) |
| 12 | −24.3 (2.5) | −23.5 (2.9) | −15.3 (1.4) | −16.2 (2.1) | 15.3 (1.9) | 18 (2.7) |
| 16 | −23.4 (1.8) | −22.6 (2.4) | −16 (1.8) | −19.1 (2.1) | 16.2 (2.7) | 15.5 (3.5) |
| 20 | −18.9 (2.5) | −20.6 (1.9) | −14.3 (1.6) | −15.5 (1.6) | 11.4 (2.0) | 11.8 (2.8) |
| 24 | −21.3 (2.8) | −20.1 (2.3) | −17.4 (1.9) | −18.7 (1.8) | 20.3 (3.1) | 11.8 (2.7)* |
| 48 | −22.2 (2.8) | −16.4 (2.8) | −18 (2.1) | −14.4 (2.1) | 21.1 (2.4) | 14.8 (2.6) |
P < 0.05;
P < 0.01.
Side-effects
The most common side-effects reported were headache, dizziness and flushing. Overall, the incidence of side-effects was low, none was serious, and they did not cause any subject to withdraw from the study. Side-effects were most commonly reported when GTN was administered 1 h after sildenafil. At this time, dizziness was reported in 47%, flushing in 13% and headache in 13% in those taking sildenafil, compared with 20, 7 and 0%, respectively, in those taking placebo.
Study 2
Subjects
Of 23 subjects screened for the study, two were not suitable. Of the 21 recruited, one was withdrawn because of low baseline BP (90/58 mmHg) at his first visit. Analyses were performed using the data from the remaining 20 subjects. In one further subject, 4 h after sildenafil administration on visit 2, BP had decreased from 120/70 mmHg to 86/46 mmHg and, as a result, GTN was not administered. These data were included in the comparison of the effects of sildenafil and placebo on pre-GTN BP and HR, but the comparison of sildenafil with GTN and placebo with GTN at 4 h was analysed using the data from the remaining 19 subjects. GTN was administered to this subject at the 8-h time point on the same day and at all time points at the other three visits. One subject was taking regular isosorbide mononitrate and this was withdrawn 72 h prior to each visit. The baseline characteristics are given in Table 2. There was no difference between the two baseline measures, taken before sildenafil or placebo administration, of either BP or HR.
Table 2.
Study 2 subject baseline characteristics
| Mean (SEM) | |
|---|---|
| Age (years) | 66 (1.8) |
| Plasma glucose (mmol l−1) | 5.5 (0.1) |
| Systolic BP | 121 (2.4) |
| Diastolic BP | 68 (0.8) |
| Serum cholesterol: | |
| Total (mmol l−1) | 4.2 (0.1) |
| LDL (mmol l−1)* | 2.1 (0.1) |
| HDL (mmol l−1) | 1.1 (0.04) |
| Total:HDL ratio | 3.9 (0.2) |
| Triglyceride (mmol l−1)* | 2.4 (0.3) |
| Number (%) | |
|---|---|
| Current smokers | 7 (35) |
| Ex-smokers | 3 (15) |
| Previous percutaneous coronary intervention | 12 (60) |
| Previous coronary artery bypass graft | 2 (10) |
| Hypertension | 7 (35) |
| Current drugs: | |
| Aspirin | 19 (95) |
| Clopidogrel | 4 (20) |
| β-Adrenoreceptor antagonist | 19 (95) |
| Calcium channel blocker | 7 (35) |
| Isosorbide mononitrate | 1 (5) |
| Angiotensin converting enzyme inhibitor | 9 (45) |
| Angiotensin II receptor antagonist | 2 (10) |
| HMG-CoA reductase inhibitor | 19 (95) |
| Thiazide diuretic | 5 (25) |
Values from 18 subjects (in two subjects serum LDL concentration could not be calculated because serum triglyceride concentration was too high). BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A.
Effects of sildenafil alone
Compared with placebo, sildenafil alone reduced sitting systolic BP [mean (SEM) changes from baseline for sildenafil vs. placebo respectively: −10.3 mmHg (1.9) vs. 1.5 mmHg (3.1) at 1 h; −11.0 mmHg (3.2) vs. 1.2 mmHg (2.6) at 4 h; −8.5 mmHg (2.4) vs.−0.8 mmHg (2.4) at 6 h; −6.3 mmHg (3.0) vs. 3.3 mmHg (2.9) at 8 h; all P < 0.05] and sitting diastolic BP [−5.0 mmHg (1.5) vs.−1.5 mmHg (1.3) at 1 h; −6.2 mmHg (1.4) vs. 1.4 mmHg (1.4) at 4 h; −7.9 mmHg (1.0) vs.−4.1 mmHg (1.0) at 6 h; −4.5 mmHg (1.5) vs. 1.5 mmHg (1.5) at 8 h; P= NS at 1 h and < 0.01 at all other time points]. There was no effect of sildenafil alone on HR.
Effect of GTN with sildenafil
Changes from baseline
The effects of GTN with sildenafil and GTN with placebo on absolute sitting BP are shown in Figure 3 and on mean maximum reduction in sitting BP in Figure 4. Compared with the combination of GTN and placebo, the combination of GTN and sildenafil resulted in greater mean maximum reductions in systolic BP at 1, 4 and 8 h, and in diastolic BP at all time points. The mean (95% confidence interval) differences in maximum systolic BP reduction at 1, 4, 6 and 8 h were −16 mmHg (−12, −21), −12 mmHg (−4, −20), −6 mmHg (1, −12) and −9 mmHg (−3, −15). The equivalent differences for diastolic BP were −9 mmHg (−6, −11), −9 mmHg (−5, −12), −5 mmHg (−2, −8) and −7 mmHg (−4, −10). There was a greater effect on the maximum increase in HR with the combination of GTN and sildenafil than with the combination of GTN and placebo at 1 h [4.7 bpm (1.4) vs. 0.5 bpm (0.8), P < 0.01], but not at 4, 6 or 8 h. Table 3 shows the frequencies at which BP fell below various thresholds. The frequencies with which BP fell by different magnitudes are available as an online supplement (Table S1). There was also a greater reduction in standing systolic BP [−27 (3) vs.−5 (2), −17 (4) vs.−5 (4), −18 (3) vs.−2 (3) and −13 (3) vs.−4 mmHg (4) at 1, 4, 6 and 8 h, respectively] and diastolic BP [−12 (1) vs.−1 (1), −9 (2) vs.−1 (2), −8 (1) vs.−3 (2) and −8 (2) vs. 1 mmHg (2) at 1, 4, 6 and 8 h, respectively] with the combination of GTN and sildenafil than with the combination of GTN and placebo, all P < 0.05.
Figure 3.
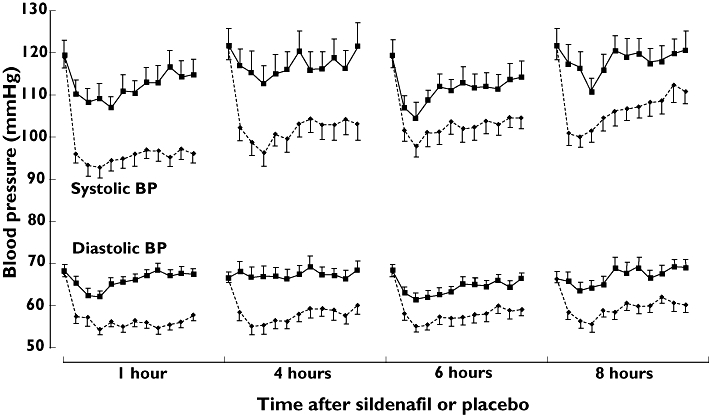
Mean effects on sitting blood pressure (BP) of glyceryl trinitrate (GTN) with sildenafil and with placebo in angina patients. Baseline values are those taken before sildenafil or placebo administration. For each time point, baseline values are followed by values measured every 3 min after GTN (pre-GTN values are not shown). Sildenafil (—♦—); Placebo ( )
)
Figure 4.
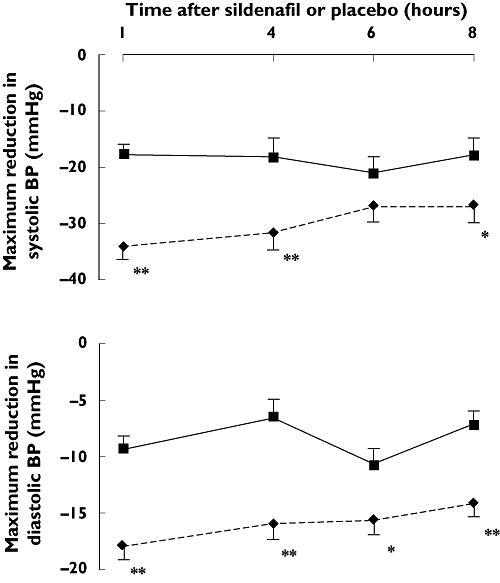
Mean maximum changes in sitting blood pressure (BP) with glyceryl trinitrate (GTN) given after sildenafil and after placebo in angina patients. Changes are from baseline recordings taken before sildenafil or placebo administration. *P < 0.05; **P < 0.01. Sildenafil (—♦—); Placebo ( )
)
Table 3.
Frequency with which blood pressure (BP) was reduced below different thresholds at each glyceryl trinitrate (GTN) challenge
| Systolic BP < 100 mmHg | Systolic BP < 90 mmHg | Systolic BP < 80 mmHg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 4 h | 6 h | 8 h | 1 h | 4 h | 6 h | 8 h | 1 h | 4 h | 6 h | 8 h | |
| Sildenafil | 19 (95) | 15 (79) | 15 (75) | 12 (60) | 12 (60) | 8 (42) | 10 (50) | 8 (40) | 7 (35) | 4 (21) | 2 (10) | 2 (10) |
| Placebo | 11 (55) | 9 (45) | 12 (60) | 7 (35) | 2 (10) | 2 (10) | 1 (5) | 3 (15) | 0 (0) | 1 (5) | 1 (5) | 0 (0) |
| Diastolic BP < 60 mmHg | Diastolic BP < 50 mmHg | Diastolic BP < 40 mmHg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 4 h | 6 h | 8 h | 1 h | 4 h | 6 h | 8 h | 1 h | 4 h | 6 h | 8 h | |
| Sildenafil | 19 (95) | 18 (95) | 17 (85) | 17 (85) | 11 (55) | 9 (47) | 6 (30) | 6 (30) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Placebo | 9 (45) | 11 (55) | 13 (65) | 11 (55) | 1 (5) | 1 (5) | 1 (5) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Numbers (percentages) of subjects whose sitting BP fell to below different thresholds at some point in the 33-min monitoring period after each GTN challenge.
Changes from pre-GTN values
The absolute changes in sitting BP from pre-GTN values are shown in Figure 5 and the maximum changes from pre-GTN values are shown in Figure 6. At 1 h, the change in diastolic BP from the pre-GTN value was greater with sildenafil than with placebo [mean change in AUC −222 AU (88) vs.−42 AU (110), P= 0.0004]. At other time points for diastolic BP [−118 (46) vs.−38 (47), −84 (42) vs.−18 (40) and −98 (53) vs.−38 AU (49) for 4, 6 and 8 h, respectively] and at all time points for both systolic BP [−420 (88) vs.−304 (110), −314 (111) vs.−242 (82), −259 (71) vs.−254 (69) and −287 (58) vs.−243 AU (131) for 1, 4, 6 and 8 h, respectively] and HR [49 (43) vs. 28 (31), 79 (24) vs. 45 (21), 72 (33) vs. 55 (31) and 101 (27) vs. 80 AU (29)] there were no statistically significant differences between sildenafil and placebo in the change from pre-GTN values. The frequencies with which BP fell by different magnitudes from pre-GTN values are available as an online supplement (Table S2). Sildenafil did not affect the time to the maximum effect on BP of GTN at any time point.
Figure 5.
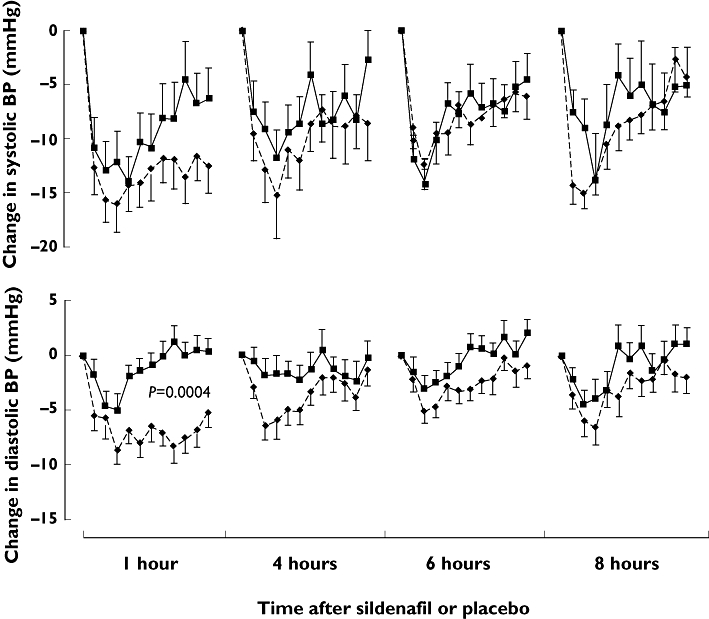
Mean changes in sitting blood pressure (BP) with glyceryl trinitrate (GTN) given after sildenafil and placebo in angina patients. Changes are from pre-GTN recordings. AUCs were compared statistically between sildenafil and placebo (the only significant difference being in the effect on diastolic BP at 1 h). Sildenafil (—♦—); Placebo ( )
)
Figure 6.
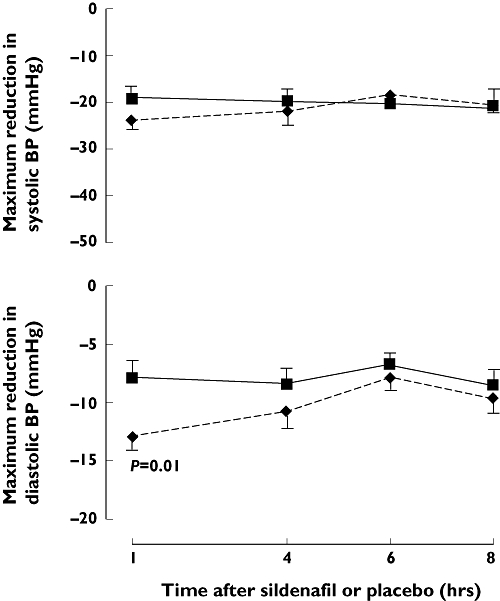
Mean maximum changes in sitting blood pressure (BP) with glyceryl trinitrate (GTN) given after sildenafil and placebo in angina patients. Changes are from pre-GTN recordings. The only significant comparison between sildenafil and placebo was with diastolic BP at 1 h). Sildenafil (—♦—); Placebo ( )
)
Side-effects
Hypotensive symptoms (of feeling dizzy or light headed) following GTN occurred in eight subjects during the sildenafil phase but in none of the subjects during the placebo phase. Of the eight subjects who experienced hypotensive symptoms with sildenafil, six experienced them at the 1 h GTN challenge (three of whom needed to be laid supine with the foot of the bed elevated) and three experienced them at the 4-h GTN challenge (two of whom needed to be laid supine with the foot of the bed elevated). No subjects experienced hypotensive symptoms at the 6- and 8-h challenges. Hypotensive symptoms were not reported by any subject outside the monitoring periods following each GTN administration. Headache was experienced by seven subjects after sildenafil (in four instances temporally related to GTN administration) and three subjects after placebo (in one instance temporally related to GTN administration and in a further instance a pre-existing headache was worsened with GTN). Indigestion/heartburn, stuffy nose and facial flushing were each experienced by two subjects following sildenafil but no subjects after placebo. One subject experienced a subconjunctival haemorrhage following sildenafil. This did not affect vision and resolved over several weeks, as would be expected. One subject experienced an episode of sickness, facial pain and headache that did not start until 1 h following discharge (about 8 h after sildenafil).
Discussion
In healthy men the hypotensive interaction between sildenafil and sublingual GTN was confined to within 4 h of sildenafil administration. In angina patients, the clinically relevant population, there was a greater mean maximum reduction in BP with the combination of sildenafil and sublingual GTN than with the combination of placebo and sublingual GTN for at least 8 h after sildenafil administration, but the magnitude of this difference was greater at 1 and 4 h than at 6 and 8 h after sildenafil.
In the angina patients, sildenafil alone reduced BP, raising the possibility that at least some of the interaction on BP may be the result of the additive effects of sildenafil and GTN. In this respect, analysis of the effects of GTN from pre-GTN values provides some insight into the nature of the interaction (at each time point). At 6 and 8 h the acute change in systolic and diastolic BPs from pre-GTN values was no different between sildenafil and placebo phases, suggesting that the overall greater reduction in BP observed with sildenafil and GTN compared with placebo and GTN was, at least on average, no more than additive. The trend at 4 h for a greater reduction in diastolic BP with GTN following sildenafil than following placebo suggested that the interaction might be more than additive at this time. At 1 h the reduction in BP, especially diastolic BP, with GTN was clearly greater following sildenafil than following placebo, suggesting synergistic interaction between the two drugs. However, it should be noted that the study design does not allow for precise determination of the nature of the interaction in terms of additive or synergistic effects. Thus, the effects of sildenafil on BP were only measured at 1, 4, 6 and 8 h (pre-GTN values). Although the peak plasma concentrations of sildenafil generally occur 1 h after oral administration, it is possible that its effect on BP continued to increase after 1 h, potentially accounting for at least some of the difference observed between sildenafil and placebo phases in the change in diastolic BP following GTN at this time. To address this, it would be necessary also to determine the effects of sildenafil with placebo GTN and placebo sildenafil with placebo GTN. However, these additional data would not have altered the conclusion that the interaction between sildenafil and GTN on BP is, on average, no more than additive from 6 h after sildenafil. It should also be noted that assessment of changes in BP from pre-GTN values was a secondary analysis of the data. It remains possible that a larger study would suggest a degree of synergism at other time points.
The discussion of whether the interaction is additive or synergistic at different time points has important clinical relevance. When treating a patient with acute angina who has recently taken sildenafil, the likely effect of GTN on BP will be the major factor in determining whether it might be safe to administer GTN. Although the BP at presentation will probably be lower than the patient's usual BP, due to the effect of sildenafil alone, if the expected further reduction in BP with GTN is likely to be no greater than would be expected if sildenafil had not been taken, then cautious use of GTN could be carefully considered. In contrast, if the interaction between sildenafil and GTN were synergistic then the potential for severe hypotension would mean that GTN should not be administered.
In contrast to the angina patients, in healthy men sildenafil alone reduced BP at 1 h, but had no effect on BP at subsequent time points. Given that the interaction between sildenafil and GTN in angina patients was generally no more than additive at 6 and 8 h, it is likely that the difference between the groups in the effect on BP of sildenafil alone largely explains the different effects on mean maximum reductions in BP between the studies. Other than the presence of coronary artery disease, different ages of the participants and the use of a variety of vasoactive medicines, including β-adrenoreceptor antagonists, calcium channel blockers and inhibitors of the renin–angiotensin system, by the angina patients may explain the different responses to sildenafil observed between the two groups.
Consistent with the effects on BP, in the angina patients symptoms suggestive of hypotension did not occur at 6 and 8 h after sildenafil. Of the three subjects who experienced hypotensive symptoms at 4 h, only one had also experienced them at 1 h, suggesting variability between individuals in the time to peak interaction rather than some individuals being susceptible to the interaction for prolonged periods.
Is it safe to administer sublingual GTN from 6 h after sildenafil in clinical practice? The lack of any obvious synergistic effect on BP from 6 h may suggest that, in patients with relatively well-preserved BP despite prior sildenafil administration, GTN may not be hazardous. However, we cannot exclude the possibility of a synergistic effect from 6 h, at least in some patients. Moreover, even after 6 h there is a greater average reduction in BP when GTN is given after sildenafil and there is also a higher risk of BP being reduced to a potentially hazardous level, e.g. 90/50 mmHg. A larger scale safety study would be required to quantify precisely the frequency with which significant hypotension occurs from 6 h after sildenafil. Nevertheless, these data are relatively reassuring and, when otherwise clinically strongly indicated, would support the cautious use of GTN in subjects hospitalized with angina who have taken sildenafil at least 6 h previously and who are haemodynamically stable.
Limitations
Patients presenting to hospital with angina are generally kept supine or semi-recumbent. In this study we measured BP in the sitting and standing positions, with the intention of maximizing the observed interaction. If anything, it is likely that lesser reductions in BP would occur in the supine position, so the conclusion that the interaction between sildenafil and GTN is, on average, no more than additive from 6 h after sildenafil remains valid to the clinical situation.
In both studies there were similar effects on standing BP as on sitting BP. However, so as not to disturb sitting measures, standing BP was not assessed until at least 30 min after GTN administration. Moreover, in the angina patients standing BP was not measured before each GTN challenge, again to avoid affecting sitting measures. Therefore, it is not possible to assess formally whether the interaction between the two drugs was additive or synergistic, although the greater magnitude of the interaction at 1 h might suggest a degree of synergism at this point. Although the effects on standing BP may be of academic interest, they are not of direct clinical relevance because patients are not kept standing once hospitalized with angina.
When subjects experienced symptoms of hypotension it was often necessary to lay them supine and elevate the foot of the bed. Although this is clearly clinically appropriate, it has the potential to minimize the observed effect on BP – if subjects had remained sitting it is likely that their BP would have remained lower than in the supine position and may even have fallen further than the minimum BP that was actually recorded. However, this would not materially affect the main conclusion of the study, as the differences between sildenafil and placebo would only be greater at 1 and 4 h, whereas there would have been no effect on the data at 6 and 8 h because it was not necessary to lay any subjects supine at these time points.
Hepatic metabolism of sildenafil is reduced, and its plasma concentration increased, by a number of drugs, including cimetidine [12], macrolide antibiotics [13] and antifungal agents [14]. Therefore, concomitant administration of these drugs could prolong the time after taking sildenafil that individuals would be susceptible to a significant interaction on BP with GTN.
Subjects received two doses of GTN at each visit, either at 4 and 8 h or at 1 and 6 h after sildenafil or placebo. During the placebo phase of the study the effects of GTN on BP were no less at 8 and 6 h than at 4 and 1 h, indicating that no significant nitrate tolerance occurred following the first dose of GTN on each day.
We measured plasma concentrations of sildenafil in study 1 (data not presented) but were unable to do this for study 2. These would have provided evidence of ingestion, although drug administration was supervised and witnessed, and allowed us to investigate the relationship between plasma concentration and effect on BP. Our study was performed in men only, because the majority of sildenafil use is in men with erectile dysfunction. The time course of the interaction in women would need to be studied separately.
Conclusion
We have investigated the time course of the interaction on BP between oral sildenafil 100 mg and sublingual GTN 400 µg in both healthy men and men with stable angina. In healthy men, the interaction is confined to within 4 h of sildenafil administration. In men with stable angina there is an interaction for at least 8 h after sildenafil administration, but this is, on average, no more than additive from 6 h after sildenafil administration.
Competing interests
The study was funded through an educational grant from Pfizer UK Ltd. J.J.O. was supported by an unrestricted Junior Cardiovascular Research Fellowship from Pfizer UK Ltd.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1
Numbers (percentages) of subjects whose sitting blood pressure (BP) fell by more than various thresholds at some point in the 33-min monitoring period after each glyceryl trinitrate (GTN) challenge. Baseline values are those taken before sildenafil or placebo administration
Table S2
Numbers (percentages) of subjects whose sitting blood pressure (BP) fell, from pre-GTN values, by more than various thresholds at some point in the 33-min monitoring period after each glyceryl trinitrate (GTN) challenge
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author of the article.
REFERENCES
- 1.Stone JR, Marletta MA. Heme stoichiometry of heterodimeric soluble guanylate cyclase. Biochemistry. 1995;34:14668–74. doi: 10.1021/bi00045a007. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci. 2000;113:1671–6. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- 3.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–91. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 4.Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96:257–80. doi: 10.1111/j.1464-410X.2005.05614.x. [DOI] [PubMed] [Google Scholar]
- 5.Oliver JJ, Melville VP, Webb DJ. Effect of regular phosphodiesterase type 5 inhibition in hypertension. Hypertension. 2006;48:622–7. doi: 10.1161/01.HYP.0000239816.13007.c9. [DOI] [PubMed] [Google Scholar]
- 6.Parker JD, Parker JO. Nitrate therapy for stable angina pectoris. N Engl J Med. 1998;338:520–31. doi: 10.1056/NEJM199802193380807. [DOI] [PubMed] [Google Scholar]
- 7.Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure-lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol. 1999;83:21C–28C. doi: 10.1016/s0002-9149(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 8.Webb DJ, Muirhead GJ, Wulff M, Sutton JA, Levi R, Dinsmore WW. Sildenafil citrate potentiates the hypotensive effects of nitric oxide donor drugs in male patients with stable angina. J Am Coll Cardiol. 2000;36:25–31. doi: 10.1016/s0735-1097(00)00705-1. [DOI] [PubMed] [Google Scholar]
- 9.Cheitlin MD, Hutter AM, Jr, Brindis RG, Ganz P, Kaul S, Russell RO, Jr, Zusman RM. ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;33:273–82. doi: 10.1016/s0735-1097(98)00656-1. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien E, Mee F, Atkins N, Thomas M. Evaluation of three devices for self-measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM-705CP, Philips HP5332, and Nissei DS-175. Blood Press Monit. 1996;1:55–61. [PubMed] [Google Scholar]
- 11.Chang JJ, Rabinowitz D, Shea S. Sources of variability in blood pressure measurement using the Dinamap PRO 100 automated oscillometric device. Am J Epidemiol. 2003;158:1218–26. doi: 10.1093/aje/kwg274. [DOI] [PubMed] [Google Scholar]
- 12.Wilner K, Laboy L, LeBel M. The effects of cimetidine and antacid on the pharmacokinetic profile of sildenafil citrate in healthy male volunteers. Br J Clin Pharmacol. 2002;53:31S–36S. doi: 10.1046/j.0306-5251.2001.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muirhead GJ, Faulkner S, Harness JA, Taubel J. The effects of steady-state erythromycin and azithromycin on the pharmacokinetics of sildenafil in healthy volunteers. Br J Clin Pharmacol. 2002;53:37S–43S. doi: 10.1046/j.0306-5251.2001.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warrington JS, von Moltke LL, Shader RI, Greenblatt DJ. In vitro biotransformation of sildenafil (Viagra) in the male rat: the role of CYP2C11. Drug Metab Dispos. 2002;30:655–7. doi: 10.1124/dmd.30.6.655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


