Abstract
AIMS
Interindividual variability in efavirenz pharmacokinetics is not entirely explained by the well-recognized CYP2B6 516G→T single nucleotide polymorphism. The aim of this study was to determine whether polymorphisms in the CYP2A6 gene can be used to enhance the predictability of efavirenz concentrations in human immunodeficiency virus (HIV)-infected native African patients.
METHODS
Mid-dose efavirenz plasma concentrations were determined at 4 and 8 weeks following initiation of antiretroviral therapy in 65 HIV-infected Ghanaian patients. Selected CYP2B6 and CYP2A6 genotypes were determined by commercial 5′-nuclease assays. Relationships between averaged 4- and 8-week mid-dose efavirenz concentrations, demographic variables and genotypes were evaluated by univariate and multivariate statistical approaches including gene–gene interactions.
RESULTS
CYP2B6 c.516G→T, CYP2B6 c.983T→C, CYP2A6*9B and CYP2A6*17 allele frequencies were 45, 4, 5 and 12%, respectively. Rifampicin therapy, gender, age and body mass index had no significant influence on efavirenz mid-dose concentrations. Median efavirenz concentrations were more than five times higher (P < 0.001) in patients with CYP2B6 c.516TT genotype compared with GG and GT genotypes. Although none of the CYP2A6 genotypes was associated with altered efavirenz concentrations individually, CYP2A6*9B and/or CYP2A6*17 carriers showed a 1.8 times higher median efavirenz concentration (P= 0.017) compared with noncarriers. Multiple linear regression analysis indicated that the CYP2B6 c.516G→T polymorphism and CYP2A6 slow-metabolizing variants accounted for as much as 36 and 12% of the total variance in efavirenz concentrations, respectively.
CONCLUSIONS
Our findings support previous work showing efavirenz oxidation by CYP2A6, and suggest that both CYP2A6 and CYP2B6 genotyping may be useful for predicting efavirenz plasma concentrations.
Keywords: CYP2A6, CYP2B6, efavirenz concentration, rifampicin
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Cytochrome P450 (CYP) 2B6 polymorphisms, particularly c.516G→T, are strongly associated with plasma efavirenz concentrations, but do not entirely explain interindividual variability in efavirenz exposure.
In vitro data suggest that CYP2A6 is involved in the metabolism of efavirenz.
Rifampicin can induce the function and activity of the main metabolizing for efavirenz and causes small (22–26%) reductions in efavirenz area under the curve during co-administration, although with wide interindividual variability.
WHAT THIS STUDY ADDS
Identifies CYP2B6 516G→T polymorphism and carriers of CYP2A6*9B and/or *17 variants as independent predictors of efavirenz mid-dose concentration in human immunodeficiency virus-infected patients.
Factors such as concurrent therapy with rifampicin-containing tuberculosis regimen, gender and body mass index had no a significant influence on efavirenz mid-dose concentration.
Provides in vivo evidence that CYP2A6 is likely to be involved in the metabolism of efavirenz.
Introduction
Efavirenz is an essential component of the preferred non-nucleoside reverse transcriptase regimen for the initial treatment of human immunodeficiency virus (HIV) infection [1, 2]. Despite the potency and favourable tolerability of efavirenz-based regimens, some patients develop treatment-limiting toxicity or fail to achieve durable viral load suppression [3, 4]. Efavirenz plasma concentrations >4 µg/ml have been associated with an increased risk of adverse central nervous system effects, whereas concentrations <1 µg/ml have been associated with virological failure [5, 6]. However, other studies, including the large ‘double non-nucleoside (2NN)’ study, have failed to find any significant relationships between efavirenz plasma concentrations and virological failure [7, 8]. The fixed daily dose of 600 mg efavirenz for adults results in significant interindividual variability in plasma concentrations and clinical effects [5, 6, 9]. Consequently, it is of substantial clinical importance to identify factors that contribute to interindividual variability in efavirenz disposition, as efavirenz-based therapy is the preferred regimen in patients with tuberculosis (TB)/HIV co-infection receiving rifampicin-containing therapy in settings where rifabutin is not available [10, 11].
The main enzyme that metabolizes efavirenz is cytochrome P450 2B6 (CYP2B6) [12]. The CYP2B6 gene is highly polymorphic, and genotyping for functional single nucleotide polymorphisms (SNPs) has proven to be useful in the prediction of efavirenz pharmacokinetics [13, 14]. In particular, the CYP2B6 c.516G→T is a common polymorphism (21–38% allele frequency),[15] that has been consistently associated with reduced enzyme activity and higher efavirenz exposure in studies of different populations with varied racial and ethnic backgrounds [13, 15–18]. The more recently described CYP2B6 c.983T→C variant with up to 10% allele frequency is also associated with lower enzyme activity and higher efavirenz concentrations, but appears to be exclusively found in populations of African descent [14, 19, 20]. Other CYP2B6 polymorphisms that have been identified either have minimal impact on efavirenz metabolism, or are relatively rare (i.e. <5% allele frequency) [15].
Not all interindividual variability in efavirenz pharmacokinetics appears to be explained by CYP2B6 genetic variants [15], suggesting that there may be polymorphisms in other genes that influence efavirenz disposition. In addition to CYP2B6, several other CYPs, including CYP1A2, CYP2A6, CYP2C9, CYP3A4 and CYP3A5, may contribute to efavirenz metabolism [12, 16]. Several other studies have shown that polymorphisms in the CYP3A4 and CYP3A5 genes do not influence efavirenz plasma concentrations [21–23]. Polymorphisms in CYP1A2 and CYP2C9 either have no established association with phenotype [24], or, if associated, the variants are relatively rare in Black African populations [25]. However, several CYP2A6 genetic variants associated with reduced enzyme activity are relatively common in Black populations [26–28]. Specifically, the CYP2A6*9B allele with a mutation in the TATA box (T–48G) and CYP2A6*17 with the amino acid substitution (V365M) are associated with substantially decreased enzyme activity with reported allele frequencies of 5.7 and 9.4%, respectively, in Black populations [26–28]. The influence of these polymorphisms on efavirenz plasma concentrations in HIV-infected patients has yet to be reported. Consequently, in the present study we determined whether assaying for slow metabolizer CYP2A6 genetic variants can enhance the predictability of efavirenz plasma concentrations over that of CYP2B6 genotype alone in a cohort of HIV-infected Ghanaian patients receiving efavirenz-based therapy.
Methods
Study population and treatment regimens
Seventy-four HIV-infected patients with CD4 count ≤250 cells µl−1 were enrolled between January 2005 and December 2007 in a pilot trial of a simplified once-daily antiretroviral therapy. Thirty-four (46%) of the patients also had TB co-infection. Enrolled patients were naive to antiretroviral therapy, aged ≥18 years and had no other opportunistic conditions. The once-daily antiretroviral regimen consisted of didanosine-buffered tablets 400 mg (body weight >60 kg) or 300 mg (body weight <60 kg), lamivudine 300 mg, and efavirenz 600 mg. Adherence, assessed monthly by pill count and patient self-report, was found to be good in all patients except for one (not included in the final analysis), who had undetectable antiretroviral drug concentrations.
In the patients with TB, antiretroviral therapy was initiated 2–8 weeks after the start of rifampicin-containing TB therapy. Tuberculosis therapy consisted of daily self-administration of isoniazid 5 mg kg−1 (maximum 300 mg) daily, rifampicin 10 mg day−1 (maximum 750 mg) daily, pyrazinamide 20–25 mg kg−1 (maximum 2000 mg) daily and ethambutol 15–20 mg kg−1 (maximum 1600 mg) daily for 2 months in the induction phase. Patients enrolled before 1 July 2007 received daily isoniazid and ethambutol for 6 months in the continuation phase of therapy and those enrolled after that date received daily isoniazid and rifampicin for 4 months in accordance with the Ghana National TB treatment guidelines at the time of the study. Also, after 1 July 2007, all patients received a fixed dose combination tablet of isoniazid 75 mg, rifampicin 150 mg, pyrazinamide 400 mg and ethambutol 275 mg, isoniazid 75 mg and rifampicin 150 mg in the induction and continuation phases, respectively. The study was reviewed and approved by the Institutional Review Board of the Nogouchi Memorial Institute for Medical Research (Legon, Ghana). Informed written consent was obtained from all patients.
Study procedures
Clinical assessments including a medical history, physical examination and baseline CD4 cell counts and HIV RNA were performed at study entry and at scheduled follow-up visits. Antiretroviral therapy was self-administered at night and a mid-dose blood sampling was obtained at weeks 4 and 8 of therapy. Patients were asked to record the time of the evening dose before pharmacokinetic sampling, and samples were drawn at approximately 12 h after the efavirenz dose was taken. Plasma samples were stored at −70°C until testing. Mid-dose sampling (i.e. sampling done between doses, usually between 8–20 h postdose) is frequently used in clinical studies of efavirenz disposition for patient convenience since the drug is invariably taken at bedtime to minimize central nervous system side-effects during the day [5, 29]. We have recently reported that mid-dose efavirenz concentrations are highly correlated with efavirenz area under the curve (AUC) values when measured at steady state, which is consistent with the relatively prolonged half-life of this drug [18]. In HIV-infected patients, efavirenz steady-state concentrations are reached in 6–10 days [30]. Patients who had pharmacokinetic sampling performed during concurrent administration with rifampicin-containing TB therapy or for up to 1 week after stopping this therapy (two patients) were classified as receiving rifampicin-containing TB therapy. This was based on the known effects of rifampicin on increasing CYP activity, which are estimated to occur with 6 days of therapy initiation and to dissipate by 2 weeks after discontinuation [31].
Drug concentration assays
Efavirenz concentrations in plasma were measured using a validated high-performance liquid chromatography/ultraviolet method [32]. This method was validated over a range of 15–10 000 ng ml−1 and is accurate (90.4–110.5%) with intraday and interday precision of 2.3–8.3%. All analytical work was performed by the University of North Carolina Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Core, which is Clinical Laboratory Improvement Amendments certified and participates in quarterly national and international external proficiency testing [33, 34].
Genotyping and copy number assays
Subjects were genotyped for CYP2B6 c.516G→T (Q172H, rs3745274), c.983T→C (I328T, rs28399499), CYP2A6*9B, (g.1836G→T, rs8192726, which is in linkage disequilibrium with the *9B defining TATA box mutation g.-48T→G, rs28399433), and CYP2A6*17 (g.5065G→A, c.1093G→A, M365V, rs28399454) using the fluorometric 5′ nuclease genotyping assay (TaqMan Assays; Applied Biosystems, Foster City, CA, USA). CYP2A6 gene copy number variation was also performed using the gene copy number assay (TaqMan®; Applied Biosystems) according to the manufacturer's instructions. All reactions were performed in an ABI PRISM 7300 HT system (Applied Biosystems), and results were verified by two investigators (A.K., M.H.C.). We performed direct sequencing to confirm the genotyping results in one patient with CYP2B6 516TT genotype, who had a relatively low efavirenz concentration, and in all the six patients with CYP2B6 983TC genotype.
Statistical analyses
Statistical analyses were performed using Sigmplot 11 software, which incorporates a comprehensive statistical analysis module (Systat, San Jose, CA, USA). Unless otherwise indicated, P < 0.05 was considered to be statistically significant. It was necessary to rank transform efavirenz concentration data to achieve data normality and equal variance. The relationship between efavirenz concentrations determined at 4 weeks and at 8 weeks of therapy was evaluated by Spearman rank order correlation analysis and compared by Mann–Whitney rank sum test. Univariate analyses of effects of patient demographics and genotypes on mean efavirenz mid-dose concentrations were assessed by Mann–Whitney rank sum test (gender, rifampin use, alcohol use, and for two genotype groups), analysis of variance (anova) (for three genotype groups) or by linear regression [for age, body weight and body mass index (BMI)]. Two-way anova, which incorporates an interaction model, was also used to investigate effects of CYP2B6 516G→T genotype and CYP2A6 variants. In instances where the anova (one-way or two-way) indicated a significant effect (P < 0.05), post hoc multiple pair-wise comparison testing was performed using the Student–Newman–Keuls method to identify groups that were significantly different from each other. Finally, multiple linear regression analysis was used to construct a predictive model using patient demographic factors and genotypes as independent variables and efavirenz concentrations as the dependent variable. Dichotomous variables, including gender, rifampin use, alcohol use, CYP2A6 variant, and CYP2B6 983T→C genotype were coded as 0 or 1, while age, body weight and BMI were included as continuous variables. Two variables were used to describe CYP2B6 516G→T genotype, coded as GG/GT = 0 and TT = 1 for the recessive genetic model and also as GG = 0 and GT/TT = 1 for the dominant genetic model. This also allowed for the additive genetic model if both CYP2B6 516G→T variables were found to be significant. Using an automated method implemented in Sigmaplot 11, a minimal multiple linear regression model was then identified by stepwise removal of nonsignificant variables until all remaining independent variables had coefficients with P-values < 0.05. The percentage of variance explained by the model was based on the coefficient of determination (R2). The contribution of each independent variable (expressed as a percentage) to the total explained variance was derived from the standardized regression coefficients, which had been normalized to the total explained variance.
Results
Study population
Seventy-four HIV-infected patients treated with efavirenz-containing highly active antiretroviral therapy were initially enrolled in the study. Of these, nine were excluded from the final genotype–phenotype analysis either because they had died before study completion (n= 5), had discontinued the study for other reasons prior to pharmacokinetic sampling (n= 2), or had undetectable concentrations of all antiretroviral drugs or incomplete data (n= 2).
Patient demographics and efavirenz plasma concentrations
Of the 65 patients included in final analysis, the mean (±SD) age was 38 ± 8 years, baseline body weight was 54 ± 12 kg, and BMI was 19.5 ± 4.1. Thirty-six patients (55%) were female, nine (14%) reported alcohol use, and 19 (29%) were receiving concurrent rifampicin-containing TB therapy at the time of efavirenz sampling. The mean time of pharmacokinetic sampling was 14.1 ± 1.1 h postdose, median 13.9 h (range 12–18 h) postdose.
As shown in Figure 1, efavirenz concentrations in plasma samples collected at 4 weeks and 8 weeks following initiation of antiretroviral therapy were well correlated (Spearman correlation coefficient Rs= 0.68; P < 0.001). Values were also equally clustered around the line of identity consistent with steady-state pharmacokinetics being reached by 4 weeks and maintained until the 8 weeks sampling time. The median [interquartile range (IQR)] mid-dose efavirenz plasma concentrations at 4 weeks and 8 weeks were 1763 (1251–2795) ng ml−1 and 1609 (1244–2465) ng ml−1, respectively, and were statistically indistinguishable (P= 0.40; Mann–Whitney rank sum test). Consequently, to simplify the presentation of results of further analyses, 4-week and 8-week values were averaged for each subject resulting in median (IQR) mid-dose efavirenz plasma concentrations of 1597 (1279–2803) ng ml−1 for all 65 subjects. This approach was also supported by preliminary analyses, which showed essentially identical results for the results reported below regardless of whether 4-week, 8-week, or averaged results were used.
Figure 1.
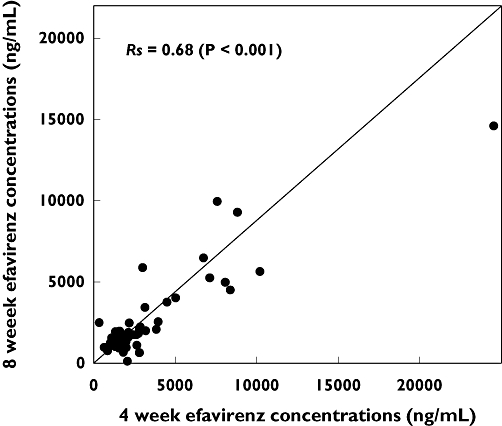
Correlation of mid-dose efavirenz concentrations in plasma from 65 human immunodeficiency virus-infected Ghanaian patients measured 4 weeks (abscissa) and 8 weeks (ordinate) after commencing treatment. Shown are the Spearman correlation coefficient (Rs) and associated P-value for the correlation analysis, and the line of identity representing perfect correlation
None of the patient demographic variables examined including age (P= 0.47; linear regression), BMI (P= 0.068; linear regression), gender (P= 0.98; Mann–Whitney rank sum test) or alcohol use (P= 0.42; Mann–Whitney rank sum test) was associated with altered mid-dose efavirenz concentrations (data not shown). Furthermore [median (IQR)] efavirenz concentrations in patients receiving rifampicin-containing TB therapy [1628 (1224–2315) ng ml−1] were not different from levels in patients that were not receiving this additional therapy [1582 (1360–3020) ng ml−1] (P= 0.86; Mann–Whitney rank sum test).
CYP2B6 and CYP2A6 polymorphisms
Variant allele frequencies for the 65 study patients were 45, 4, 5 and 12% for CYP2B6 c.516G→T, CYP2B6 c.983T→C, CYP2A6*9B and CYP2A6*17, respectively. The numbers of subjects with each individual genotype are given in Table 1. No subjects were identified who were homozygous variant for CYP2B6 c.983T→C. We also determined that all subjects had two copies of the CYP2A6 gene based on a CYP2A6 gene copy number variation assay.
Table 1.
Impact of CYP2B6 and CYP2A6 polymorphisms on mid-dose efavirenz concentrations* in 65 human immunodeficiency virus-infected patients
| Variable | n (%) | Median (IQR) efavirenz mid-dose concentration* | P-value |
|---|---|---|---|
| CYP2B6 c.516G→T | <0.001 | ||
| GG | 22 (30%) | 1 282 (1089–1901)† | |
| GT | 38 (51%) | 1 560 (1379–2205)† | |
| TT | 14 (19%) | 8 320 (6085–10 673)† | |
| CYP2B6 c.983T→C | 0.88 | ||
| TT | 59 (91%) | 1 567 (1274–2649) | |
| TC | 6 (9%) | 1 832 (1334–3102) | |
| CYP2A6*9B (g.1836G→T) | 0.07 | ||
| TT | 59 (91%) | 1 562 (1265–2356) | |
| TG | 5 (8%) | 3 102 (1568–4712) | |
| GG | 1 (1%) | 10 726 | |
| CYP2A6*17 (c.1093G→A) | 0.15 | ||
| GG | 51 (78%) | 1 557 (1274–2334) | |
| GA | 12 (19%) | 1 994 (1325–4063) | |
| AA | 2 (3%) | 6 374 (4220–8529) | |
| CYP2A6*9B or *17 | 0.017 | ||
| Reference | 47 (72%) | 1 516 (1242–2084) | |
| Variant carrier | 18 (28%) | 2 694 (1597–5943) |
Values are the average of efavirenz concentrations determined at 4 and 8 weeks in each patient; note that four patients had missing efavirenz concentration data at week 4, and seven patients had missing concentration data at week 8.
P < 0.001 for 516TT vs. GT, P < 0.001 for 516TT vs. GG, P= 0.027 for 516GG vs. GT groups determined by Student–Newman–Keuls test.
CYP2B6 and CYP2A6 genotype effects on efavirenz exposure
Univariate analyses
Figure 2 shows the distribution of efavirenz plasma levels stratified by CYP2B6 and CYP2A6 genotypes, while the results of univariate statistical analyses of these genotypes are given in Table 1. As expected, CYP2B6 516G→T genotype was significantly associated with decreased efavirenz concentration (P < 0.001, anova). Median efavirenz concentrations for CYP2B6 516TT genotype was at least five times higher than both the CYP2B6 516GT (P < 0.001) and 516GG (P < 0.001) genotype groups. The median efavirenz concentration for CYP2B6 516GT genotype was about 1.2 times higher than values for the 516GG genotype (P > 0.027). None of the other individual genotypes evaluated, including CYP2B6 c.983T→C (P= 0.61), CYP2A6*9B (P= 0.07) and CYP2A6*17 (P= 0.15), was individually associated with altered efavirenz concentrations. However, when both CYP2A6 slow metabolizer variants were considered together, the median efavirenz concentrations among CYP2A6 variant carriers (*9 or *17) was 1.8 times higher than among individuals without either of these variants (P= 0.017).
Figure 2.
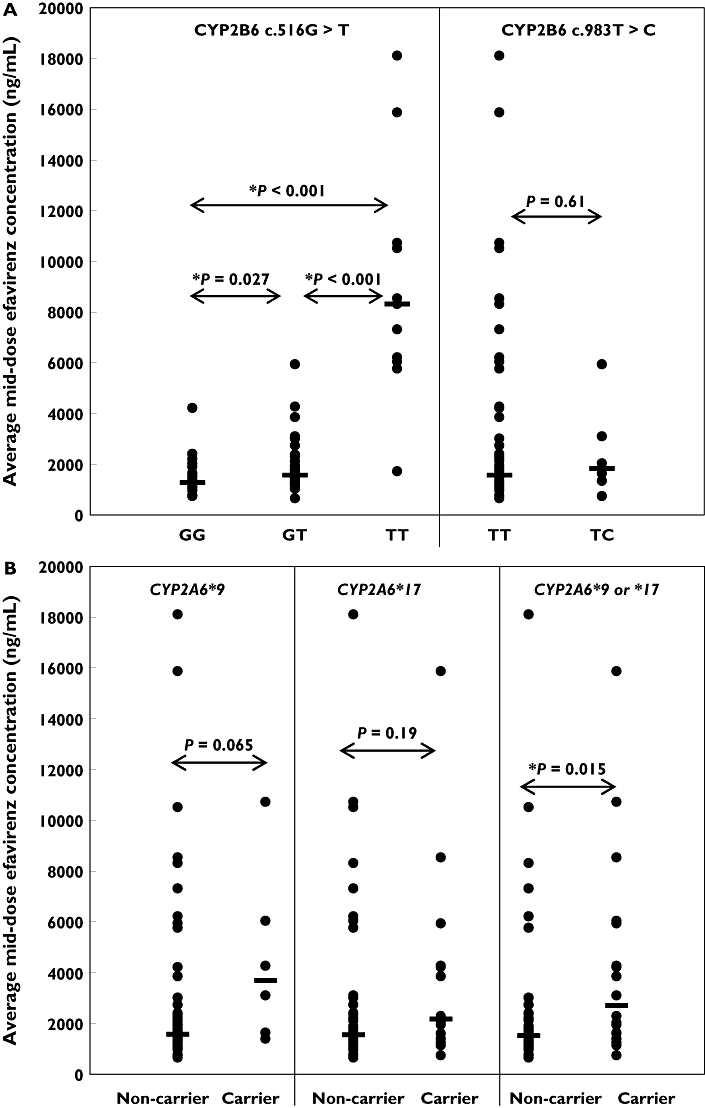
Influence of CYP2B6 c.516G→T and CYP2B6 c.983T→C polymorphisms (A) and of CYP2A6*9 and CYP2A6*17 polymorphisms (B) on efavirenz mid-dose concentrations (average of 4-week and 8-week samples) in human immunodeficiency virus (HIV) and HIV/tuberculosis co-infected patients in Ghana. Differences between groups were examined by Mann–Whitney rank sum test (two genotype groups) or by anova (three genotype groups) with post hoc Student–Newman–Keuls pair-wise multiple comparisons testing
Bivariate analysis
Two-way anova was then used to control for the CYP2B6 516G→T genotype effect and evaluate whether there was any additional influence from the CYP2A6 slow metabolizer variants, including evaluation of possible gene–gene interactions. As shown in Figure 3, CYP2A6 slow metabolizer variants were independently associated with higher efavirenz concentrations regardless of CYP2B6 516G→T genotype (P < 0.001 for CYP2B6 516G→T genotype; P= 0.028 for CYP2A6 variants; P= 0.55 for interaction). Post hoc analysis of the CYP2A6 variant effect within each of the CYP2B6 516G→T genotype groups using the Student–Newman–Keuls method showed that patients with CYP2B6 516GT genotype who also had CYP2A6 variants had significantly higher median efavirenz concentration than noncarriers (2694 vs. 1448; P= 0.01) (Figure 3). Trends for higher median efavirenz concentrations in CYP2A6 variant carriers compared with noncarriers were also observed in the two other CYP2B6 516G→T genotype groups, although the differences did not achieve statistical significance (P > 0.05).
Figure 3.
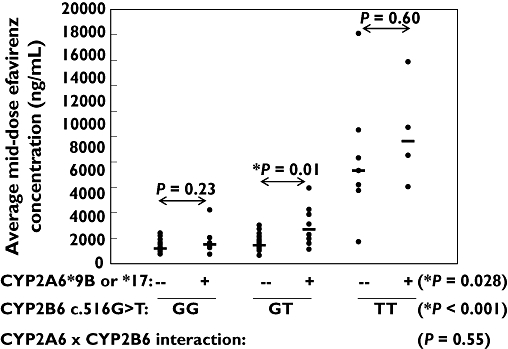
Influence of CYP2A6 slow metabolizer genotypes (*9B and/or *17) and CYP2B6 c.516G→T genotypes on efavirenz mid-dose concentrations (average of 4-week and 8-week samples). Shown are the P-values from the two-way anova (including analysis of gene–gene interactions) and post hoc multiple pair-wise comparisons (Student–Newman–Keuls test) within genotype groups
Of note in this analysis were three patients (indicated in Figure 3) that did not have the slow metabolizer CYP2B6 516TT genotype and yet had relatively high efavirenz concentrations (>4000 ng ml−1). Of these, one individual (patient 159 with CYP2B6 516GG genotype) was homozygous for the CYP2A6*17 variant, another individual (patient 158 with CYP2B6 516GT genotype) carried both the CYP2A6*9B and *17 alleles, and the other individual (patient 13 with CYP2B6 516GT genotype) was heterozygous for both the CYP2A6*9 and CYP2B6 983T→C variants.
Multiple linear regression analysis
Stepwise backward multiple linear regression analysis was then performed to identify the minimum set of independent variables that are predictive of efavirenz plasma concentrations, and also determine the relative contribution of these variables to overall variability in efavirenz concentrations. Variables entered into the initial model included sex, age, weight, BMI, alcohol use, rifampin treatment, CYP2B6 516G→T genotypes, 983T→C genotype, as well as CYP2A6 slow metabolizer variant carrier status. Only three independent variables remained in the final model with P < 0.05, including CYP2B6 516TT genotype (standardized regression coefficient = 0.51; P < 0.001), CYP2B6 516GG genotype (standardized regression coefficient = 0.24; P= 0.017), and CYP2A6 variants (standardized regression coefficient = 0.25; P= 0.008). The coefficient of determination (R2) for the regression was 0.48, indicating that 48% of the total variance in efavirenz concentrations was explained by the model. A comparison of the standardized regression coefficients indicated that CYP2B6 516TT, CYP2B6 516GG and CYP2A6 variant genotypes accounted for 24, 12 and 12% (respectively) of the total variance in efavirenz plasma concentrations. Inclusion of both CYP2B6 516TT and CYP2B6 516GG genotypes in the final model is consistent with an additive genetic model with all CYP2B6 c.516G→T genotypes accounting for 36% of the total variance. We found no significant association (P > 0.05) between efavirenz plasma concentrations and patient age, gender, weight, BMI, alcohol use, or rifampicin-containing TB therapy.
Discussion
In this study, we have identified CYP2A6 slow metabolizer variants (*9B and/or *17) as independent predictors of efavirenz plasma concentration beyond that provided by the well-known CYP2B6 516G→T polymorphism. This finding suggests that CYP2A6 genotyping may be useful in enhancing the accuracy of prediction of efavirenz concentrations in HIV-infected patients over that of CYP2B6 516G→T alone. Our published data have been confirmed by a recent report (unpublished conference abstract) demonstrating an association between slow metabolizer CYP2A6 alleles and increased efavirenz concentrations in 169 patients in the Swiss HIV Cohort [35]. However, in that study the effect of the CYP2A6*7, *9 and/or *17 alleles was observed only in patients with CYP2B6 slow metabolizer genotypes [35], which contrasts with our study, which showed an effect regardless of CYP2B6 genotype as reflected by a lack of significant gene–gene interaction in the two-way anova analysis. However, we did observe the greatest CYP2A6 variant effect in patients with CYP2B6 516GT genotype (see Figure 2). The reasons for the differences between these two studies are not immediately apparent but could be related to differences in populations, as our study enrolled only native Africans, whereas the other study was primarily of Whites. There were also differences in use of concurrent medications, as 19 of our patients with HIV/TB co-infection also received anti-TB drugs, which may have altered CYP gene expression.
In vitro studies have demonstrated that recombinant CYP2A6 is capable of hydroxylating efavirenz at both the 8- and 14- positions, although the activity of CYP2A6 compared with CYP2B6 in these reactions is admittedly low [12]. Furthermore, the rate of efavirenz 7-hydroxylation measured in a human liver bank was most highly correlated with CYP2A6 immunoquantified protein and coumarin hydroxylation, a CYP2A6-selective enzyme activity [16]. Unfortunately, as yet there are no reports of the quantitative contribution of CYP2A6 to efavirenz metabolism in human liver, such as through use of isoform-specific CYP2A6 chemical or antibody inhibitors. Nevertheless, the results of the present study showing higher efavirenz concentrations in patients with CYP2A6 slow metabolizer alleles support a role for CYP2A6 in efavirenz metabolism in patients.
We also examined the effect of the CYP2B6 983TC genotype on efavirenz exposure but found no significant relationship, possibly because of the small size of our population, which contained no homozygous variant individuals. Recent clinical studies suggest that high efavirenz concentrations are more likely to occur in patients who are CYP2B6 983T→C homozygous variant [14] or in patients who are heterozygous for CYP2B6 983T→C and are heterozygous for CYP2B6 516G→T [15]. The CYP2B6 983T→C polymorphism results in a I328T amino acid substitution and has been considered a null allele since recombinant expression in COS-1 cells results in complete lack of CYP2B6 protein and activity [19]. However, CYP2B6 983T→C has also been reported to occur as an allele (CYP2B6*16) with CYP2B6 c.785A→G (results in amino acid substitution K262R), and expression of CYP2B6*16 cDNA constructs with both these mutations results in somewhat decreased (but not absent) enzyme protein and activity [36].
Our data are consistent with previous reports of a high frequency of CYP2B6 516G→T variant alleles in native Africans [18, 37], but a somewhat lower frequency of CYP2B6 983T→C genetic variation [20]. The frequency of the CYP2A6*17 variant allele of 11% in our population was similar to that among Black subjects recruited in the USA and Asia [26, 28]. CYP2A6*9B variation in our population was also similar to that reported among healthy Ghanaian subjects [27]. Overall, nearly one-third of our patients had CYP2A6*9B and/or CYP2A6*17 variants, and if the association between CYP2A6 genetic variation and efavirenz disposition is confirmed in other studies, genotyping for CYP2A6 variation would help improve pharmacokinetic prediction afforded by CYP2B6 516G→T SNP screening alone.
As a CYP2B6 enzyme inducer, rifampicin co-administration would be expected to enhance efavirenz clearance and reduce plasma concentrations. However, in this study we found no difference in efavirenz concentrations between patients receiving rifampicin-containing TB therapy and those not receiving this therapy. These data are reassuring, as HIV/TB co-infection is common in sub-Saharan Africa, and efavirenz-based antiretroviral therapy is often needed in patients receiving rifampicin-containing TB treatment. It is important to recognize that this was not a crossover study and so results could have been confounded by population differences. However, available published studies in which efavirenz concentrations were evaluated with and without rifampicin-containing treatment at different times in the same patients have also reported no significant differences in efavirenz concentrations [38, 39]. In the South African study, the geometric mean efavirenz trough concentration on rifampicin was 1730 ng ml−1 (range 345–27 179) and 1377 ng ml−1 (range 572–3975) off rifampicin (P= 0.55) [38]. The study by López-Cortés et al., in which they evaluated the effect of rifampicin on efavirenz pharmacokinetic parameters in a subgroup, also did not reach statistical significance (P > 0.05) [39]. Although the mean efavirenz AUC declined by 22% during co-administration with rifampicin (P > 0.05) in the aforementioned study, the change in efavirenz AUC ranged from a decrease of 65% to an increase of 37%, suggesting wide interpatient variability in the rifampicin effect [39]. Since efavirenz itself induces CYP2B6 (i.e. autoinduction), it is possible that rifampicin cannot increase CYP2B6 expression beyond that resulting from chronic efavirenz exposure. However, it is also important to point out that other drugs were administered to our patients for TB therapy in addition to rifampicin that may have confounded any effect of rifampicin. For example, it is also possible that the use of isoniazid with rifampicin in the TB co-infected patients could have minimized the induction effect of rifampicin on CYP2B6, as isoniazid has been shown to be an inhibitor of cytochrome P450 in vitro[40–42]
We recognize that our study has several limitations. First, the size of the study population was small, as there were six or fewer patients each with CYP2B6 983T→C or CYP2A6*9B variants, limiting our ability to detect small treatment and genotype effects. Second, we were unable to assess efavirenz concentrations before or after rifampicin treatment to exclude the effect of differences in population on the analysis. Third, patients self-administered the evening dose and so the exact timing of the mid-dose pharmacokinetic sample (relative to dose) could have differed between patients.
Despite the above limitations, our findings demonstrate that in addition to the strong predictive value of CYP2B6 516G→T polymorphism, CYP2A6 genetic variation is an independent determinant of efavirenz plasma concentrations. If confirmed in larger studies, CYP2A6 genotyping could be a useful adjunct to CYP2B6 genotyping for the prediction of efavirenz concentrations in HIV-infected patients.
Competing interests
A.K. has previously received a research grant not related to this study and has been on the speaker's bureau of Bristol Myers-Squibb. M.L., N.L.R., M.H.C. and K.W.S. report no conflicts of interest.
The authors thank the study participants, the Medical Officers (Ernest Kenu, Fafa Xexemeku, Joseph Oliver-Commey, Vincent Boima) at the Fever's Unit, staff of Clinical Virology (Makafui Seshi, Anna Aba Hayford and Isaac Boamah), the Study Coordinators (Adjoa Obo-Akwa and Esther Manche) as well as the study nurse (Janet May Ayi) for all their valuable assistance in recruitment, evaluation of patients as well as obtaining and handling the samples. We also appreciate the help of Jacklyn Kurpewski in handing the pharmacokinetic samples. This research was supported in part by a K23 developmental award (NIH K23 AI071760) to A.K. and a ACRiA grant from Doris Duke Foundation to M.L. The University of North Carolina at Chapel Hill, Center for AIDS research #9P30 AI50410, Clinical Pharmacology and Analytical Chemistry Laboratory (CPACL) performed the efavirenz concentrations. M.H.C. was supported by grant R01GM061834 from the National Institute of General Medical Sciences (NIGMS), National Institutes of Health (Bethesda, MD, USA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
REFERENCES
- 1.US Department of Health and Human Services (DHHS) Panel on Antiretroviral Guideline for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [last accessed 20 October 2008]. 29 January 2008. Available at http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 2.Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 3.Arribas JR, Pozniak AL, Gallant JE, Dejesus E, Gazzard B, Campo RE, Chen SS, McColl D, Holmes CB, Enejosa J, Toole JJ, Cheng AK. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr. 2008;47:74–8. doi: 10.1097/QAI.0b013e31815acab8. [DOI] [PubMed] [Google Scholar]
- 4.Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, Lu B, McColl D, Chuck S, Enejosa J, Toole JJ, Cheng AK. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 5.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–5. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 6.Stahle L, Moberg L, Svensson JO, Sonnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit. 2004;26:267–70. doi: 10.1097/00007691-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Leth FV, Kappelhoff BS, Johnson D, Losso MH, Boron-Kaczmarska A, Saag MS, Livrozet J-M, Hall DB, Leith J, Huitema ADR, Wit FW, Beijnen JH, Lange JMA. Pharmacokinetic parameters of nevirapine and efavirenz in relation to antiretroviral efficacy. AIDS Res Hum Retroviruses. 2006;22:232–9. doi: 10.1089/aid.2006.22.232. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Cortes LF, Ruiz-Valderas R, Ruiz-Morales J, Leon E, de Campos AV, Marin-Niebla A, Marquez-Solero M, Lozano F, Valiente R. Efavirenz trough levels are not associated with virological failure throughout therapy with 800 mg daily and a rifampicin-containing antituberculosis regimen. J Antimicrob Chemother. 2006;58:1017–23. doi: 10.1093/jac/dkl357. [DOI] [PubMed] [Google Scholar]
- 9.Csajka C, Marzolini C, Fattinger K, Decosterd LA, Fellay J, Telenti A, Biollaz J, Buclin T. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 2003;73:20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Managing drug interactions in the treatment of HIV-related tuberculosis. 2007. [last accessed 20 October 2008]. [online]. Available at http://www.cdc.gov/tb/TB_HIV_Drugs/PDF/tbhiv.pdf.
- 11.Pozniak AL, Miller RF, Lipman MC, Freedman AR, Ormerod LP, Johnson MA, Lucas SB. BHIVA treatment guidelines for tuberculosis (TB)/HIV infection 2005. HIV Med. 2005;6(Suppl. 2):62–83. doi: 10.1111/j.1468-1293.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 12.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchiya K, Gatanaga H, Tachikawa N, Teruya K, Kikuchi Y, Yoshino M, Kuwahara T, Shirasaka T, Kimura S, Oka S. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun. 2004;319:1322–6. doi: 10.1016/j.bbrc.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 14.Wyen C, Hendra H, Vogel M, Hoffmann C, Knechten H, Brockmeyer NH, Bogner JR, Rockstroh J, Esser S, Jaeger H, Harrer T, Mauss S, van Lunzen J, Skoetz N, Jetter A, Groneuer C, Fatkenheuer G, Khoo SH, Egan D, Back DJ, Owen A. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemother. 2008;61:914–8. doi: 10.1093/jac/dkn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotger M, Tegude H, Colombo S, Cavassini M, Furrer H, Decosterd L, Blievernicht J, Saussele T, Gunthard HF, Schwab M, Eichelbaum M, Telenti A, Zanger UM. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin Pharmacol Ther. 2007;51:557–66. doi: 10.1038/sj.clpt.6100072. [DOI] [PubMed] [Google Scholar]
- 16.Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, Flockhart DA, Zanger UM. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics. 2007;8:547–58. doi: 10.2217/14622416.8.6.547. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann MH, Blievernicht JK, Klein K, Saussele T, Schaeffeler E, Schwab M, Zanger UM. Aberrant splicing caused by single nucleotide polymorphism c.516G>T [Q172H], a marker of CYP2B6*6, is responsible for decreased expression and activity of CYP2B6 in liver. J Pharmacol Exp Ther. 2008;325:284–92. doi: 10.1124/jpet.107.133306. [DOI] [PubMed] [Google Scholar]
- 18.Kwara A, Lartey M, Sagoe KW, Xexemeku F, Kenu E, Oliver-Commey J, Boima V, Sagoe A, Boamah I, Greenblatt DJ, Court MH. Pharmacokinetics of efavirenz when co-administered with rifampicin in TB/HIV co-infected patients: pharmacogenetic effect of CYP2B6 variation. J Clin Pharmacol. 2008;48:1032–40. doi: 10.1177/0091270008321790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein K, Lang T, Saussele T, Barbosa-Sicard E, Schunck WH, Eichelbaum M, Schwah M, Zanger UM. Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet Genomics. 2005;15:861–73. doi: 10.1097/01213011-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Mehlotra RK, Bockarie MJ, Zimmerman PA. CYP2B6 983T>C polymorphism is prevalent in West Africa but absent in Papua New Guinea: implications for HIV/AIDS treatment. Br J Clin Pharmacol. 2007;64:391–5. doi: 10.1111/j.1365-2125.2007.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas DW, Ribaudo H, Motsinger A, Schackman B, Gulick R, Acosta E, Schwab M, Schaeffeler E, Morse G, Robbins G. Pharamcogenetics of plasma drug exposure and treatment outcomes with efavirenz-containing regimens: an ACTG study. In 15th Conference on Retroviruses and Opportunistic Infections. 2008. [last accessed 20 October 2008]. Boston, MA, Available at http://www.retroconference.org/2008/PDFs/759.pdf.
- 22.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, Clifford DB, Hulgan T, Marzolini C, Acosta EP. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–400. [PubMed] [Google Scholar]
- 23.Haas DW, Smeaton LM, Shafer RW, Robbins GK, Morse GD, Labbe L, Wilkinson GR, Clifford DB, D'Aquila RT, De Gruttola V, Pollard RB, Merigan TC, Hirsch MS, George AL, Jr, Donahue JP, Kim RB. Pharmacogenetics of long-term responses to antiretroviral regimens containing Efavirenz and/or Nelfinavir: an Adult Aids Clinical Trials Group Study. J Infect Dis. 2005;192:1931–42. doi: 10.1086/497610. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Z, Dragin N, Jorge-Nebert LF, Martin MV, Guengerich FP, Aklillu E, Ingelman-Sundberg M, Hammons GJ, Lyn-Cook BD, Kadlubar FK, Saldana SN, Sorter M, Vinks AA, Nassr N, von Richter O, Jin L, Nebert DW. Search for an association between the human CYP1A2 genotype and CYP1A2 metabolic phenotype. Pharmacogenet Genomics. 2006;16:359–67. doi: 10.1097/01.fpc.0000204994.99429.46. [DOI] [PubMed] [Google Scholar]
- 25.Allabi AC, Gala JL, Horsmans Y, Babaoglu MO, Bozkurt A, Heusterspreute M, Yasar U. Functional impact of CYP2C95, CYP2C96, CYP2C98, and CYP2C911 in vivo among black Africans. Clin Pharmacol Ther. 2004;76:113–8. doi: 10.1016/j.clpt.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Fukami T, Nakajima M, Yoshida R, Tsuchiya Y, Fujiki Y, Katoh M, McLeod HL, Yokoi T. A novel polymorphism of human CYP2A6 gene CYP2A6*17 has an amino acid substitution (V365M) that decreases enzymatic activity in vitro and in vivo. Clin Pharmacol Ther. 2004;76:519–27. doi: 10.1016/j.clpt.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Gyamfi MA, Fujieda M, Kiyotani K, Yamazaki H, Kamataki T. High prevalence of cytochrome P450 2A6*1A alleles in a black African population of Ghana. Eur J Clin Pharmacol. 2005;60:855–7. doi: 10.1007/s00228-004-0854-9. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima M, Fukami T, Yamanaka H, Higashi E, Sakai H, Yoshida R, Kwon JT, McLeod HL, Yokoi T. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther. 2006;80:282–97. doi: 10.1016/j.clpt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Manosuthi W, Sungkanuparph S, Thakkinstian A, Vibhagool A, Kiertiburanakul S, Rattanasiri S, Prasithsirikul W, Sankote J, Mahanontharit A, Ruxrungtham K. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. AIDS. 2005;19:1481–6. doi: 10.1097/01.aids.0000183630.27665.30. [DOI] [PubMed] [Google Scholar]
- 30.Bristol-Myers Squibb Company. Sustiva® (Efavirenz) Capsules and Tablets Package Insert. Princeton, NJ: Bristol-Myers Squibb Company; 2008. [Google Scholar]
- 31.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 32.Rezk NL, Crutchley RD, Yeh RF, Kashuba AD. Full validation of an analytical method for the HIV-protease inhibitor atazanavir in combination with 8 other antiretroviral agents and its applicability to therapeutic drug monitoring. Ther Drug Monit. 2006;28:517–25. doi: 10.1097/00007691-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Droste JA, Aarnoutse RE, Koopmans PP, Hekster YA, Burger DM. Evaluation of antiretroviral drug measurements by an interlaboratory quality control program. J Acquir Immune Defic Syndr. 2003;32:287–91. doi: 10.1097/00126334-200303010-00007. [DOI] [PubMed] [Google Scholar]
- 34.Holland DT, DiFrancesco R, Connor JD, Morse GD. Quality assurance program for pharmacokinetic assay of antiretrovirals: ACTG proficiency testing for pediatric and adult pharmacology support laboratories, 2003 to 2004: a requirement for therapeutic drug monitoring. Ther Drug Monit. 2006;28:367–74. doi: 10.1097/01.ftd.0000211817.58052.b8. [DOI] [PubMed] [Google Scholar]
- 35.Lulio J, Rotger M, Lubomirov R, Decosterd L, Eap CB, Telenti A. Genetic variation in accessory metabolic pathways is associated with extreme efavirenz exposure in individuals with impaired CYP2B6 function. [last accessed 20 October 2008]. abstract 133]. 15th Conference on Retroviruses and opportunistic Infections. 3–6 February 2008. Boston, MA, 2008. Available at http://www.retroconference.org/2008/Abstracts/32236.htm.
- 36.Wang J, Sonnerborg A, Rane A, Josephson F, Lundgren S, Stahle L, Ingelman-Sundberg M. Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet Genomics. 2006;16:191–8. doi: 10.1097/01.fpc.0000189797.03845.90. [DOI] [PubMed] [Google Scholar]
- 37.Nyakutira C, Roshammar D, Chigutsa E, Chonzi P, Ashton M, Nhachi C, Masimirembwa C. High prevalence of the CYP2B6 516G→T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur J Clin Pharmacol. 2008;64:357–65. doi: 10.1007/s00228-007-0412-3. [DOI] [PubMed] [Google Scholar]
- 38.Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother. 2006;58:1299–302. doi: 10.1093/jac/dkl399. [DOI] [PubMed] [Google Scholar]
- 39.López-Cortés LF, Ruiz-Valderas R, Viciana P, Alarcón-González A, Gómez-Mateos J, León-Jimenez E, Sarasa-Nacenta M, López-Pua Y, Pachón J. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41:681–90. doi: 10.2165/00003088-200241090-00004. [DOI] [PubMed] [Google Scholar]
- 40.Desta Z, Soukhova NV, Flockhart DA. Inhibition of cytochrome P450 (CYP450) isoforms by isoniazid: potent inhibition of CYP2C19 and CYP3A. Antimicrob Agents Chemother. 2001;45:382–92. doi: 10.1128/AAC.45.2.382-392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura Y, Kurata N, Sakurai E, Yasuhara H. Inhibitory effect of antituberculosis drugs on human cytochrome P450-mediated activities. J Pharmacol Sci. 2004;96:293–300. doi: 10.1254/jphs.fp0040296. [DOI] [PubMed] [Google Scholar]
- 42.Wen X, Wang JS, Neuvonen PJ, Backman JT. Isoniazid is a mechanism-based inhibitor of cytochrome P450 1A2, 2A6, 2C19 and 3A4 isoforms in human liver microsomes. Eur J Clin Pharmacol. 2002;57:799–804. doi: 10.1007/s00228-001-0396-3. [DOI] [PubMed] [Google Scholar]


