Abstract
AIMS
To investigate the effect of age and gender on the tolerability, safety and pharmacokinetics (PK) of tomopenem (RO4908463/CS-023), a novel carbapenem antibiotic, and its major metabolite.
METHODS
Forty-two subjects were assigned to one of the following three groups: young men, elderly men and elderly women. The PK, safety and tolerability of an intravenous infusion of 1500 mg tomopenem and its resultant major metabolite (open beta-lactam ring) were assessed.
RESULTS
Minor differences in exposure of both tomopenem and the major metabolite were seen. The area under the curve (AUC) of tomopenem was 22% higher in elderly men compared with young men, and 19% higher in elderly women relative to the elderly men. Total clearance of tomopenem decreased with decreasing creatinine clearance. In the two male groups, renal clearance values of tomopenem were similar (3.52 and 3.67 l h−1) and higher than in the elderly female group (2.83 l h−1). The mean half-lives ranged from 2.03 (healthy young men) to 2.41 h (elderly men). The difference in AUC of tomopenem can be explained by differences in the mean creatinine clearances of 116 (young men), 101 (elderly men) and 84.7 (elderly women) ml min−1 1.73 m−2, respectively.
CONCLUSIONS
While some PK parameters were statistically different among the three groups, the differences were mostly minor and unlikely to be clinically meaningful. The difference in the PK can be largely attributed to the difference in creatinine clearance of these groups.
Keywords: age, carbapenem, gender, pharmacokinetics, RO4908463, tomopenem
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT.
This compound belongs to a well-described class of antibacterials, the carbapenems, which are characterized by excellent bactericidal activity.
This compound is unique in that it has activity against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa.
The pharmacokinetics (PK) of all molecules in this class is characterized by short plasma half-lives and renal elimination as the major source of clearance.
Age- and gender-related effects on renal and hepatic function have been shown to necessitate adjustments in dosing regimens to ensure safe and efficacious exposures to drugs.
WHAT THIS STUDY ADDS
The extent of changes in PK of tomopenem in the elderly and women is minor when compared with healthy male volunteers, so dose adjustments are not necessary.
Instead of age or gender, creatinine clearance can be used to explain the slight effect of these variables on PK.
Introduction
Tomopenem is a novel 1β-methyl carbapenem. It has demonstrated potent broad-spectrum in vitro bactericidal activity against beta-lactam-susceptible and -resistant target species [1], specifically against clinically significant drug-resistant pathogens including methicillin-resistant Staphylococcus aureus, ceftazidime-resistant Pseudomonas aeruginosa and expanded spectrum cephalosporin-resistant Enterobacteriaceae[2, 3].
Compared with imipenem and meropenem, tomopenem has longer half-life (1.7 h), lower clearance (8.1 l h−1) and similar distribution volume (17 l) [4]. It is primarily (59%) eliminated unchanged in the urine. Renal blood flow, glomerular filtration rate (GFR) and active renal tubular secretory processes all decline with age [5] and drugs that are eliminated primarily by glomerular filtration show a decrease in elimination clearance and an increase in exposure with age [6]. In addition, assessment of gender effect on pharmacokinetic parameters is relevant in the context of gender differences in GFR.
Material and methods
Subjects
A total of 42 subjects were divided among three treatment groups according to age and gender criteria as follows: young men aged 18–40 years; elderly men ≥65 years old, and elderly females ≥65 years old. Fourteen subjects were randomized to each group, 12 receiving active drug and two placebo. All subjects gave informed consent and were willing to comply with study procedures.
Study design
This was a single-centre, randomized, double-blind, third-party unblinded, placebo-controlled study, conducted at the Institut de Pharmacologie Clinique Roche (Strasbourg, France). The pharmacokinetics of tomopenem and its major metabolite were assessed after a single dose of 1500 mg of tomopenem given as a 60-min intravenous infusion.
The study was approved by the local Institutional Review Board and conducted according to the principles outlined in the ‘Guideline for Good Clinical Practice’ International Conference on Harmonisation Tripartite Guideline (January 1997), which has its basis in the principles of the ‘Declaration of Helsinki’ (1996) and the principles of ‘Good Clinical Practice’ as outlined in the current version of 21 Code of Federal Regulations, subchapter D, parts 312, 50 and 56.
Bioanalytical methods
The concentrations of tomopenem and its major metabolite, RO4957463, in plasma and urine (pre-buffered with 0.5 mol l−1N-morpholino-propanesulfonic acid) were determined using a selective, accurate, reproducible and validated bioanalytical method. The analytes were extracted by protein precipitation. Reconstituted samples were analysed by liquid chromatography–mass spectrometry–mass spectrometry. The lower limit of quantification for the parent drug and its metabolite were 0.2 µg ml−1 in plasma and 2 µg ml−1, respectively.
Pharmacokinetic analysis
Pharmacokinetic parameters for tomopenem and its major metabolite were estimated by the noncompartmental method using WinNonlin®. The actual sampling times were used to calculate individual pharmacokinetic parameters (Table 1).
Table 1.
Estimated geometric group means of key pharmacokinetic parameters with 95% confidence intervals
| Analyte | Group | AUC∞ | Cmax | T1/2 | CLR |
|---|---|---|---|---|---|
| [h µg−1 ml−1] | [µg ml−1] | (h) | [ml h−1] | ||
| Tomopenem | Young men | 208 (184, 236) | 88.3 (78.4, 99.3) | 2.03 (1.87–2.22) | 3.52 (2.83, 4.38) |
| Elderly men | 254 (224, 287) | 88.5 (78.6, 99.6) | 2.41 (2.22–2.64) | 3.67 (2.95, 4.57) | |
| Elderly women | 301 (267, 340) | 110 (97.9, 124.1) | 2.27 (2.13–2.44) | 2.83 (2.28, 3.52) | |
| RO4957463 | Young men | 21.0 (18.0, 24.6) | 5.28 (4.35, 6.40) | 2.53 (2.19–3.04) | 9.84 (8.09, 11.973) |
| Elderly men | 29.3 (25.1, 34.3) | 4.83 (3.98, 5.86) | 4.22 (3.60–5.10) | 8.63 (7.10, 10.50) | |
| Elderly women | 35.8 (30.7, 41.9) | 6.26 (5.16, 7.59) | 3.89 (3.39–4.60) | 5.87 (4.83, 7,14) |
Creatinine clearance was estimated in two ways. For enrolment purposes, the CLcr was estimated using the Cockcroft–Gault equation [7]:
where CLcr (ml min−1) is the creatinine clearance, BW (kg) is the actual body weight, Scr (mg dl−1) is the serum creatinine concentration. For data analysis, creatinine clearance was calculated using the following formula:
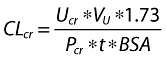 |
where Ucr is the concentration of creatinine in urine (mg dl−1), VU is the urine volume (ml), t is the collection interval in minutes, Pcr is the plasma creatinine concentration (mg dl−1), BSA is the estimated body surface area (m2) [8] and 1.73 is the normal BSA.
Statistical analysis
The primary pharmacokinetic parameters (AUC∞, Cmax and CLR) and population means for the three treatment groups were estimated together with 95% confidence intervals (CI). The calculation for difference was based on a one-way anova using the logarithms of the observed parameter values. The relative ratios of AUC∞ between the three populations were estimated (with 95% CI) using treatment contrasts of the anova.
Results
Forty-two subjects completed the study. The subjects were mostly White, aged between 23 and 77 years. Tomopenem was generally well tolerated. No clinically significant changes were observed in the laboratory test values, ECGs or vital signs. Overall, the incidence of adverse events (AEs) was low. The same number of AEs was observed in young men and elderly men (three AEs each) and no AE was observed in elderly women. The AEs related to study medication in young men were: pruritis, diarrhoea and headache. In elderly men, there was one instance each of epistaxis, paraesthesia and herpes simplex. All AEs were mild in intensity, apart from one unrelated event of moderate toothache in a placebo subject.
Plasma pharmacokinetics
The principal pharmacokinetic parameters after a single dose of 1500 mg are summarized in Table 1. The mean half-lives ranged from 2.03 (young men) to 2.41 h (elderly men). Total and creatinine clearance were lower in the elderly women (4.98 l h−1) relative to young men (7.25 l h−1). Total clearance was strongly correlated with creatinine clearance (Figure 1a). AUC∞ increased across the groups from young men (208 µg h−1 ml−1) to elderly women (301 µg h−1 ml−1). Cmax values were the highest in elderly women.
Figure 1.
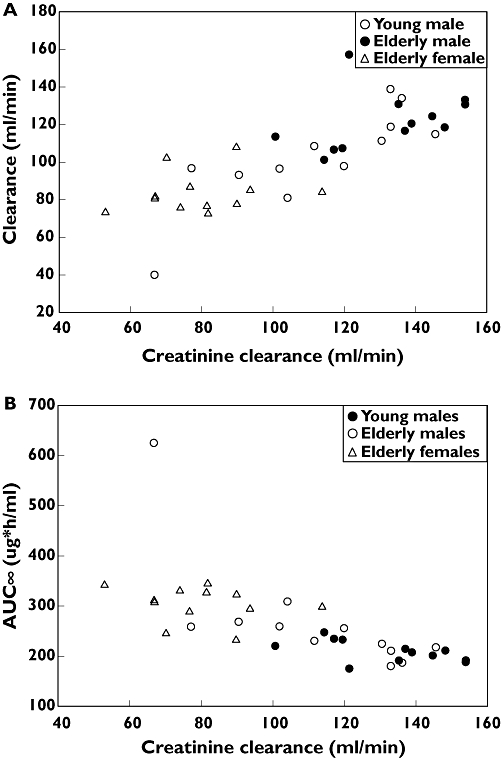
Relation between creatinine clearance and total clearance and AUC and creatinine clearance of tomopenem following a single intravenous dose of 1500 mg of tomopenem (n= 12)
The values for AUC0-∞ of the metabolite as a percentage of the exposure to the parent were similar across all groups, ranging from 10.1 to 11.7% of the parent drug (Table 1). Young male subjects showed the lowest mean AUC value and the older female subjects the highest. Cmax values in the elderly women were higher by approximately 19% and 30% vs. young and elderly men, respectively. Statistically significant differences in the primary pharmacokinetic parameters were observed among the groups.
Urine pharmacokinetics
Renal clearance of the parent drug was 47–59% of creatinine clearance in all subjects. The elderly women had approximately 40% and 31% lower renal clearance than young men and elderly men, respectively. The amount of tomopenem excreted was similar in all groups, accounting for 49–62% of the dose. The amount of RO4957463 excreted was similar for all groups as well, reflecting 14–16% of the parent dose. Evaluation of the relationship between creatinine clearance and total clearance was done graphically and is shown in Figure 1A. Similarly, an exploration of the relationship of creatinine clearance and area under the curve (AUC) is shown in Figure 1B.
Discussion
This single-centre, randomized, double-blind, third-party unblinded, placebo-controlled study investigated the effects of age and gender on the pharmacokinetics of tomopenem and its major metabolite RO4957463 in healthy volunteers. The drug was well tolerated by all subjects and no differences in AEs were noted among the three groups. Comparison of the AUC values indicated a slight difference in exposure of both parent and metabolite. Statistical analysis of the two male groups confirmed 22% higher AUC in elderly men vs. young men, and a 19% higher AUC in elderly women relative to elderly men. Similarly, a difference in AUC is noted for the metabolite. No statistically significant difference existed in the Cmax of either the parent or metabolite across the three groups.
The mean total clearance and volume of distribution were similar across the groups. Renal clearance of the parent and metabolite were similar in the two male groups; however, they were lower in elderly women relative to both male groups. Given the relationship between the creatinine clearance and total clearance (Figure 1A) of tomopenem, women tended to have lower creatinine clearance, and lower total clearance. This is consistent with the higher plasma AUC seen in the female group. In addition, the difference between the two male groups (22%) can probably be attributed to the lower creatinine clearance in elderly men (mean CLCR= 113 ml min−1) relative to the young men (mean CLCR= 132 ml min−1). Thus, there is no gender-specific effect, and the apparent age effect can be attributed to differences in renal function rather than age itself.
Competing interests
SL, TG and EL own shares in Hoffmann-La Roche, Inc.
Hoffmann-La Roche, Inc. provided financial support for the conduct of this study. The authors would like to gratefully acknowledge the contribution of the following colleagues at Daiichi-Sankyo: Drs Kazutaka Yoshihara, Naotoshi Yamamura and Tetsufumi Koga. Part of this work was presented at the 2007 AAPS Annual Meeting and Exposition in San Diego, CA, USA.
REFERENCES
- 1.Ohya S, Fukuoka T, Kawada H, Kubota M, Kitayama A, Abe T, Kuwahara S. 40th Intersci Conf Antimicrob Agents Chemother, abstr F-1232 R-115685, a novel parenteral carbapenem: in vivo antibacterial activity. Abstr 40th Intersci Conf Antimicrob Agents Chemother, abstr F-1232. 2000;40:205. [Google Scholar]
- 2.Noel AR, Bowker KE, MacGowan AP. The RO4908463 (CS-023) T > MIC antibacterial effect relationship for MRSA established in an in vitro pharmacokinetic model of infection. Abstr 46th Intersci Conf Antimicrob Agents Chemother abstr A-638. 2006;46 [Google Scholar]
- 3.Thomson KS, Moland ES. CS-023 (R-115685), a novel carbapenem with enhanced in vitro activity against oxacillin-resistant staphylococci and Pseudomonas aeruginosa. J Antimicrob Chemother. 2004;54:557–62. doi: 10.1093/jac/dkh328. [DOI] [PubMed] [Google Scholar]
- 4.Mouton JW, Touw DJ, Horrevorts AM, Vinks AATMM. Comparative pharmacokinetics of the carbapenems: clinical implications. Clin Pharmacokinet. 2000;39:185–201. doi: 10.2165/00003088-200039030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Muhlberg W, Platt D. Age-dependent changes of the kidneys: pharmacological implications. Gerontology. 1999;45:243–53. doi: 10.1159/000022097. [DOI] [PubMed] [Google Scholar]
- 6.Mayersohn M. Pharmacokinetics in the elderly. Environ Health Perspect. 1994;102(Suppl.)(11):119–24. doi: 10.1289/ehp.94102s11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 8.Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54:225–35. [PubMed] [Google Scholar]


