Herbal drugs may be useful in the control of pain, especially when associated with rheumatic diseases, inflammatory conditions, headache and dysmenorrhoea. These remedies usually exert their activity on mild–moderate pain, with a delayed onset of the analgesic effect; herbal drugs might also be useful in pain prevention (e.g. in headaches), but they are not usually very effective in the management of acute pain. Among most studied analgesic herbs are Devil's Claw (Harpagophytum procumbens), Feverfew (Tanecetum parthenium) and Chaste tree (Vitex agnus castus). The product PC.28 PLUS, containing a combination of these three herbs, was marketed by the Italian company Cosval (Arese, Italy) with the indication ‘For headache, painful joints and painful menstrual cycle’; the label on the drug's box also stated ‘Natural innovative products’. Declared components of each tablet were: T. parthenium 0.5% in parthenolides 120 mg (total parthenolides per tablet 0.06 mg), V. agnus castus 0.5% in agnucastosides 80 mg (total agnucastosides per tablet 0.04 mg), and H. procumbens 20 mg (no reference to harpagosides). The product also contained magnesium, vitamin E and vitamin B6.
Several informative reports were received by the pharmacovigilance and phytovigilance system of the Florence University Medical School and the Centre for Natural Medicine of Local Health Service of Empoli (ASL 11, Florence, Italy) by patients who attributed a very high effectiveness to the product in the treatment of headache and menstrual pain. One or two tablets (even sublingually) at pain onset were rapidly effective, often with complete suppression of pain.
Since high effectiveness and very rapid onset appeared suspicious in view of the extremely low doses of active compounds reported by the producer, we decided to analyse the product to assess the possible presence of synthetic drugs.
Tablets were analysed by means of a high-performance liquid chromatography–mass spectrometry system (Varian 500-MS LC Ion Trap; Varian, Inc., Palo Alto, CA, USA). A peak compatible with the anti-inflammatory drug nimesulide was identified in the output (Figure 1a) and the characterization of the drug was confirmed by fragmentation analysis (Figure 1b). The concentration of nimesulide was 50 mg per 1000 mg of product (about 20.5 mg per tablet). Since the dosage of PC.28 PLUS suggested by the producer was one to two tablets per day, a daily dose was therefore 20–41 mg nimesulide. Some patients reported taking up to four to five tablets per day, since the product was considered natural and safe. It should be noted that nimesulide is the most used anti-inflammatory/analgesic drug in Italy, and can be dispensed only after a medical prescription. Nimesulide was recently reviewed by the Italian Ministry of Health following several reports of lethal and life-threatening side-effects [1]. The finding of nimesulide contamination was further confirmed by analyses conducted in a different laboratory on tablets from different batches. The producer subsequently decided to recall PC.28 PLUS, stating that an internal analysis had shown contamination with nimesulide ‘in traces’, probably due to the presence of the drug in one of the herbs’ stocks; the Italian Ministry of Health also ordered product withdrawal from the market.
Figure 1.
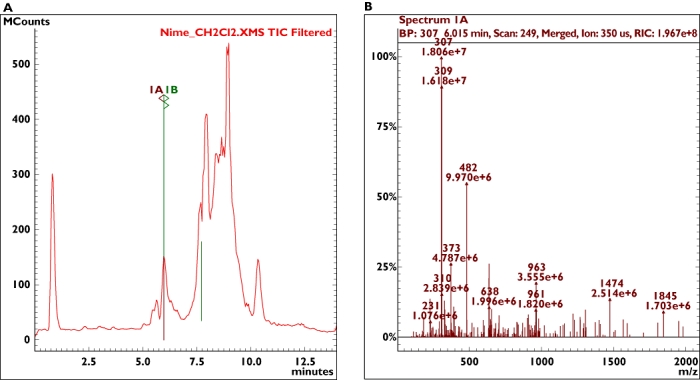
HPLC/MS analysis of the product. A peak compatible with the anti-inflammatory drug nimesulide was identified in the output (a) and the characterization of the drug was confirmed by fragmentation analysis (b)
The three natural products contained in PC.28 PLUS may have some analgesic/anti-inflammatory effects [2–6], but the concentrations in the tablets were extremely low.
In conclusion, pharmacovigilance and phytovigilance deal with the identification of ‘signal alarms’. These usually derive from spontaneous reports of adverse drug reactions or lack of efficacy of certain drugs. In the field of herbal medicine, it appears that the report of an unusually high therapeutic effect might also be an important signal alarm. We therefore encourage clinicians to report to pharmacovigilance systems adverse reactions to herbal drugs, but also unexpected high efficacy or any therapeutic effect not in keeping with the pharmacodynamics and pharmacokinetics of herbal drugs active compounds.
Competing interests
None declared.
REFERENCES
- 1.Leone R, Sottosanti L, Luisa IM, Santuccio C, Conforti A, Sabatini V, Moretti U, Venegoni M. Drug-related deaths: an analysis of the Italian spontaneous reporting database. Drug Saf. 2008;31:703–13. doi: 10.2165/00002018-200831080-00007. [DOI] [PubMed] [Google Scholar]
- 2.Henneicke-von Zepelin HH. Feverfew for migraine prophylaxis. Headache. 2006;46:531. doi: 10.1111/j.1526-4610.2006.00391_5.x. [DOI] [PubMed] [Google Scholar]
- 3.Wuttke W, Jarry H, Christoffel V, Spengler B, Seidlova-Wuttke D. Chaste tree (Vitex agnus-castus) – pharmacology and clinical indications. Phytomedicine. 2003;10:348–57. doi: 10.1078/094471103322004866. [DOI] [PubMed] [Google Scholar]
- 4.Gagnier JJ, van Tulder MW, Berman B, Bombardier C. Herbal medicine for low back pain: a Cochrane review. Spine. 2007;32:82–92. doi: 10.1097/01.brs.0000249525.70011.fe. [DOI] [PubMed] [Google Scholar]
- 5.Loew D, Mollerfeld J, Schrodter A, Puttkammer S, Kaszkin M. Investigations on the pharmacokinetic properties of Harpagophytum extracts and their effects on eicosanoid biosynthesis in vitro and ex vivo. Clin Pharmacol Ther. 2001;69:356–64. doi: 10.1067/mcp.2001.115445. [DOI] [PubMed] [Google Scholar]
- 6.Diener HC, Pfaffenrath V, Schnitker J, Friede M, Henneicke-von Zepelin HH. Efficacy and safety of 6.25 mg t.i.d. feverfew CO2-extract (MIG-99) in migraine prevention – a randomized, double-blind, multicentre, placebo-controlled study. Cephalalgia. 2005;25:1031–41. doi: 10.1111/j.1468-2982.2005.00950.x. [DOI] [PubMed] [Google Scholar]


