Abstract
AIM: To examine the portal hemodynamics of gastric fundal varices (GV) without gastro-renal shunt (GRS), and to retrospectively investigate the effects of various kinds of treatment on eradication.
METHODS: Ninety-four liver cirrhosis patients at high-risk of GV were treated in our hospital and enrolled in this study. We retrospectively examined their characteristics, liver function, and portal hemodynamics of GV. We performed balloon-occluded retrograde transvenous obliteration (BRTO) at first. If it was not technically possible to perform BRTO, endoscopic injection sclerotherapy using α-cyanoacrylate glue (CA) or percutaneous transhepatic obliteration (PTO) was performed.
RESULTS: Among the 94 patients, a GRS was present in 79 (84.0%), and absent in the remaining 15 (16.0%). The subphrenic vein was connected to the inferior vena cava as the drainage vein in 13 (86.7%) out of the 15 cases without GRS. We performed BRTO in 6 patients, CA in 4 patients and PTO in 5 patients. The eradication rate was 100% for each procedure, but the rate of early recurrence within 6 mo was 16.7% for BRTO, 50.0% for CA and 40.0% for PTO, respectively.
CONCLUSION: We should examine the hemodynamics before treatment of GV irrespective of the existence of GRS. If this hemodynamic examination reveals that the drainage vein connects directly to the inferior vena cava in GV without GRS, BRTO may be an effective treatment for GV with GRS.
Keywords: Gastric fundal varices, Gastro-renal shunt, Balloon-occluded retrograde transvenous obliteration
INTRODUCTION
Rupture of esophagogastric varices is one of the most severe complications of portal hypertension in patients with liver cirrhosis. Gastric varices bleed less frequently than esophageal varices, but bleeding from gastric fundal varices (GV) tends to be more severe and is associated with a high mortality rate[1,2]. Effective management of such bleeding is essential. Endoscopic injection sclerotherapy and endoscopic variceal ligation are currently the mainstay of treatment for esophageal varices. Most cases of GV cannot be treated effectively with endoscopic injection sclerotherapy alone[3–5] because of the rapid blood flow within their vessels with a large diameter. Endoscopic injection of n-butyl-2-cyanoacrylate or isobutyl-2-cyanoacrylate is used as the standard first-line treatment for bleeding GV[6] in the Western countries. However, the reported severe complications related to embolization of other organs have raised concerns for the safety of injection of tissue adhesive agents[7–11]. Transjugular intrahepatic portosystemic shunt (TIPS) placement, one of the interventional radiological techniques, is currently the second-line treatment for bleeding esophagogastric varices[6,12] and can effectively control GV bleeding[13–15]. However, it was reported that this treatment is associated with a lower success rate for GV than for esophageal varices[16–18].
Recently, some newer interventional radiological techniques have been used to treat GV with good results. Balloon-occluded retrograde transvenous obliteration (BRTO) is a very useful treatment for GV in terms of efficacy, safety, and degree of invasiveness[19–29], and the recurrence rate of GV has been also reported to be 0%-10%[23–29] after BRTO, which shows excellent long-term results. Prophylactic treatment with BRTO can effectively prevent GV rupture, and improve patient survival[28]. Ninoi et al[29] showed that GV bleeding is better controlled with BRTO than with TIPS. Therefore, not only radiologists but also hepatologists and gastroenterologists in Japan have considered BRTO the standard first-line treatment for GV in place of endoscopic variceal obturation therapy or TIPS. However, it is generally considered that BRTO cannot be used in the treatment of GV without GRS because BRTO is performed through GRS, which exists in almost 85% of GV[7]. It is very crucial to investigate whether BRTO can be feasible for GV without GRS and the effects of BRTO compared with other methods.
The portal hemodynamics of GV without GRS is not well known, and there is no well-established treatment for the eradication of these GV. This study was to examine the portal hemodynamics of GV without GRS compared with those with GRS, and to retrospectively investigate the effects of BRTO compared with some kinds of treatment on the eradication of these varices.
MATERIALS AND METHODS
Ninety-four liver cirrhosis patients at high-risk of GV were treated in our hospital between February 1996 and June 2002 and enrolled in this study. All patients had GV with acute bleeding or were at high-risk of GV. GV were evaluated based on esophagogastroduodenoscopy (EGD) criteria according to the system adopted in Japan[30,31]. In brief, the location of gastric varices (Lg) was classified as being adjacent to the cardiac ring (Lg-c), separated from the cardiac ring (Lg-f), or continuing from the Lg-f to the gastric fundus (Lg-cf). Varices were further classified as straight and small caliber varices (F1), beaded varices (F2), or tumor-shaped varices (F3). We categorized GV as a high risk according to the criteria reported by Kim et al[32], who found that a diameter of at least 5 mm and the presence of red spots are independent factors that predict a high-risk of variceal bleeding. All patients were evaluated for variceal hemodynamics with color Doppler endoscopic ultrasonography (CD-EUS) and multidimensional computed tomography (MDCT) or CD-EUS and magnetic resonance angiography (MRA). Of the 94 patients, 23 were urgent or elective cases with episodes of variceal bleeding, and 71 were prophylactic cases. We retrospectively examined their characteristics, liver function, and portal hemodynamics of GV. CD-EUS (FG-32UA, Pentax Co., Ltd., EUB-555 US scanner, Hitachi Co., Ltd., Tokyo, Japan) was performed in all patients to measure the diameters of variceal vessels as well as blood flow velocity before treatment. Blood flow was calculated from blood vessel diameter and blood flow velocity. All patients underwent MDCT or MRA to detect the supply and drainage vein and to determine whether they had a gastro-renal shunt. The patients underwent repeated CD-EUS within 1 wk after treatment to assess the sclerosing effect. The efficiency of each treatment was confirmed by the rate of disappearance of varices. EGD was done every 3-6 mo after treatment to evaluate the status of GV. The recurrence of GV was defined as detection of appearance of high-risk signs (as described above) or variceal bleeding. The follow-up period for recurrence was calculated as days from the date of treatment until the first date when EGD revealed recurrence.
Regarding the selection of the method for treatment, BRTO is the first-line treatment for GV with GRS in our hospital because it is generally considered the first-line treatment in Japan[19–29]. Therefore, in cases where it was possible, we performed BRTO even for varices without GRS. If it was not technically possible to perform BRTO, endoscopic injection sclerotherapy using α-cyanoacrylate glue (CA) or percutaneous transhepatic obliteration (PTO) was used.
BRTO procedure
BRTO is a method for treating GV by injection of a sclerosant after the main drainage vein of GV is blocked angiographically to stagnate blood flow. In general, a balloon catheter is inserted via the right femoral vein and wedged into the left adrenal vein. After the balloon is inflated, the gastro-renal shunt is visualized with the contrast agent iopamidol to judge whether shunt occlusion has been achieved. In case of GV without GRS, if the drainage vein is connected directly to the inferior vena cava, BRTO could be performed after an occlusive balloon catheter is placed in the drainage vein through the right femoral vein (Figure 1A and B). If retention of contrast agent is insufficient in GV because of other collateral veins, metallic coils are placed in the collateral veins to reduce the blood flow. In our study, after venography showed sufficient retention of contrast agent in GV, 5% solution of ethanolamine oleate with iopamidol (EOI) was continuously injected through the catheter in the drainage vein until the varices were sufficiently filled with the sclerosant. Thereafter, the catheter was left in place for 24 h to allow sclerosis to occur within the gastric fundal varices.
Figure 1.
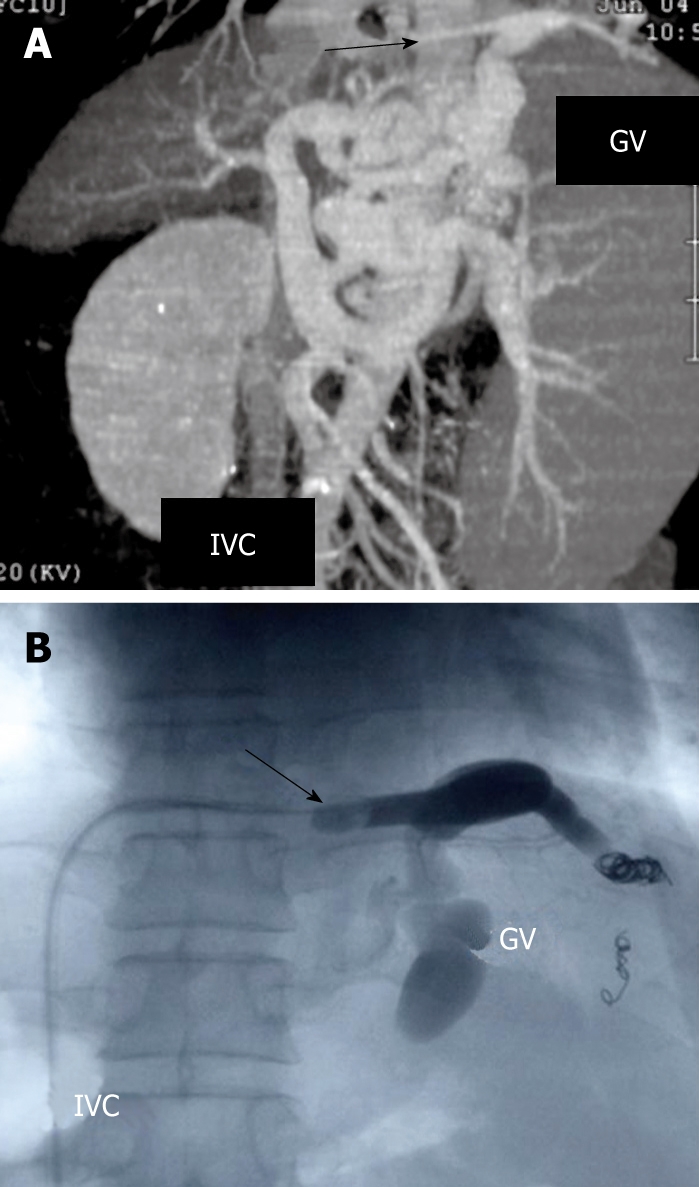
Multidimensional computed tomography (MDCT) revealing the varices supplied by the left gastric vein and drained into the subphrenic vein (arrow) which is connected to the inferior vena cava (IVC) (A), and fluoroscopic image of BRTO showing placement of the occlusive balloon catheter in the subphrenic vein (arrow) (B). After the blood vessels from the GV except for the main drainage vein were blocked with coils, 5% solution of ethanolamine oleate with iopamidol (EOI) was injected into the varices.
Modified PTO procedure with injection of sclerosant
The modified PTO using sclerosant and metallic coils[29,33] can embolize GV more selectively than original PTO[34]. In our study, percutaneous transhepatic portography was performed, and the supply and drainage veins of the GV were identified on portography. After metallic coils were placed in the supply vein to reduce blood flow in the GV, EOI was injected through the catheter until the gastric fundal varices were sufficiently filled with the sclerosant.
CA procedure
CA is a method for treating GV by injection of a sclerosant endoscopically. In our study, a sclerotherapy injector, with a 20 gauge needle, was used for variceal injection. The GV were endoscopically punctured and injected with 2.5 mL of a lipiodol-α-cyanoacrylate mixture (64% α-cyanoacrylate) in one shot, and injection was stopped when the varices were filled sufficiently after several injections.
Statistical analysis
Values are presented as mean ± SD, or as percentages. The Mann-Whitney U test was used to assess differences in age, sex, form, location, diameter of variceal vessels, blood flow velocity, blood flow volume, and liver function tests. Categorical variables were analyzed by the chi square test, with Yates’ correction for continuity where appropriate, or by the Fisher’s exact test. The Kaplan-Meier method was used to calculate the recurrence rate for GV. Differences between the groups were compared by means of the log-rank test. Statistical software (SPSS 10.0J, SPSS Japan Inc., Tokyo, Japan) was used in statistical analysis. P < 0.05 was considered statistically significant.
RESULTS
Ninety-four patients (60 men, 34 women; mean age 62.3 ± 9.7 years, range 33-80 years) underwent hemodynamic evaluation of GV with CD-EUS and MDCT or CD-EUS and MRA. Among the 94 patients, hepatic function was classified as class A in 53, class B in 36 and class C in 5 by the Child-Pugh classification. The location of GV was at Lg-f in 16 patients and at Lg-cf in 78 patients. The form of gastric varices was F2 in 32 patients and F3 in 62 patients. There were 14 patients who had red color spots on the GV. Among the 94 patients, a GRS was present in 79 (84.0%), and absent in the remaining 15 (16.0%) (Table 1).
Table 1.
Clinical characteristics of patients and profile of varices in GRS (+) and GRS (-) groups n (%)
| GRS (+) | GRS (-) | P | |
| (n = 79) | (n = 15) | value | |
| Age: yr (range) | 61.9 ± 10 (42-80) | 61.5 ± 10 (44-76) | NS |
| Gender (Male:Female) | 1:0.58 | 1:0.5 | NS |
| Child-Pugh classification | |||
| A | 47 (59.5) | 6 (40) | NS |
| B | 28 (35.4) | 8 (53.3) | NS |
| C | 4 (5.1) | 1 (6.7) | NS |
| Endoscopic featres of GV | |||
| Form | |||
| F2 | 27 (34.1) | 5 (33.3) | NS |
| F3 | 52 (65.8) | 10 (66.7) | NS |
| Location | |||
| Lgf | 12 (15.2) | 4 (26.7) | NS |
| Lgcf | 67 (84.8) | 11 (73.3) | NS |
| Presence of red color spots | 12 (15.2) | 2 (13.3) | NS |
| History of GV bleeding | 20 (25.3) | 3 (20) | NS |
| Finding of CD-EUS | |||
| Blood vessel diameter (mm) | 9.5 ± 3.4 | 6.7 ± 2.8a | 0.009 |
| Blood flow velocity (cm/s) | 16.5 ± 4.3 | 14.5 ± 3.5 | NS |
| Blood flow volume (cm3/s) | 50.8 ± 45.0 | 26.1 ± 25.7a | 0.043 |
NS: Not significant. GV: Gastric fundal varices; GRS: Gastro-renal shunt; CD-EUS: Color doppler endoscopic ultrasonography. Values for age and findings of CD- EUS are the median.
P < 0.05 vs GRS (+) group.
Regarding the hemodynamics of GV without GRS, there were a number of different supply veins, including the left gastric vein (21.7%), the short gastric vein (18.8%), and the retrogastric vein (59.4%). However, among the 15 cases without GRS, the subphrenic vein was connected to the inferior vena cava as the drainage vein in 13 cases (86.7%) out of the 15 cases without GRS (Table 2). Between the GRS (+) and GRS (-) groups, there were no significant differences in age, sex, form and location of the varices, presence of red-colored spots, velocity of blood flow in the GV, or in Child-Pugh classification. The diameter of varices and blood flow volume were higher in the GRS (+) group than in the GRS (-) group (P = 0.009, P = 0.043) (Table 1). Regarding the treatment of GV without GRS, we performed BRTO in 6 patients, CA in 4 patients and PTO in 5 patients, without severe complications (Table 2). The GV eradication rate in the GRS (-) group for each procedure was 100%.
Table 2.
Gastric fundal varices without gastro-renal shunt
| Age | Sex | Form | Diameter (mm) | Verocity (cm/s) | Supplying vein | Drainage vein | Treatment |
| 75 | M | LgcfF2 | 6.8 | 9.3 | Rgv, Sgv | Spv | CA |
| 45 | M | LgcF2 | 8.8 | 15.0 | Lgv, Rgv | Spv | CA |
| 69 | F | LgcfF3 | 12.0 | 20.1 | Lgv | Pe | CA |
| 52 | M | LgcfF2 | 6.1 | 14.3 | Lgv, Sgv | Spv | CA |
| 67 | M | LgfF3 | 4.5 | 15.0 | Lgv, Rgv | Spv, Pcv | PTO |
| 66 | M | LgcfF3 | 4.7 | 18.5 | Lgv, Rgv, Sgv | Pcv | PTO |
| 64 | M | LgcF3 | 4.8 | 18.1 | Rgv | Spv | PTO |
| 65 | M | LgcfF3 | 7.2 | 10.5 | Rgv | Spv | PTO |
| 76 | F | LgcF2 | 3.8 | 12.0 | Lgv | Spv, Pev | PTO |
| 44 | F | LgcfF3 | 8.0 | 13.0 | Rgv, Lgv | Spv, Pcv | BRTO |
| 66 | F | LgfF3 | 11.0 | 17.7 | Rgv | Spv | BRTO |
| 54 | M | LgfF3 | 10.0 | 17.7 | Lgv | Spv | BRTO |
| 52 | M | LgfF3 | 6.0 | 15.6 | Lgv, Rgv, Sgv | Spv, Pcv | BRTO |
| 64 | M | LgcfF3 | 4.4 | 12.4 | Sgv | Spv | BRTO |
| 63 | M | LgcF2 | 2.0 | 8.9 | Lgv | Spv | BRTO |
CA: Endoscopic injection sclerotherapy using α-cyanoacrylate glue; Rgv: Retro gastric vein; Sgv: Short gastric vein; Lgv: Left gastric vein; Spv: Subphrenic vein; Pev: Para esophageal vein; Pcv: Peri-cardial vein; BRTO: Balloon occluded retrograde transvenous obliteration; PTO: Percutaneous transhepatic obliteration.
The rate of early recurrence, within 6 mo, was 16.7% for BRTO, 50.0% for CA and 40.0% for PTO, respectively, in the GRS (-) group (Table 3). When the main drainage vein was found to be connected to the inferior vena cava, BRTO was the most effective treatment even for GV without GRS. No severe complication occurred in each method except for slight abdominal discomfort or hematuria. Most of the patients treated with BRTO or PTO revealed hematuria caused by sclerosant, but it was recovered when haptoglobin was used without renal damage (haptoglobin was used prophylactically in all cases treated with BRTO or PTO).
Table 3.
Treatment methods and recurrence rate of varices in GRS (-) group n (%)
| GRS (-) | n= 15 | P value |
| Treatment | ||
| BRTO | 6 (40) | NS |
| PTO | 5 (33) | NS |
| CA | 4 (27) | NS |
| Early recurrence rate within 6 mo | ||
| BRTO | 1 (16.7) | |
| PTO | 2 (40) | |
| CA | 2 (50) | |
BRTO: Balloon-occluded retrograde transvenous obliteration; PTO: Percutaneous transhepatic obliteration; CA: Endoscopic injection sclerotherapy using α-cyanoacrylate glue; GRS: Gastro-renal shunt.
DISCUSSION
The present study is the first clinical study examining the portal hemodynamics of GV without GRS compared with those with GRS and the effects of some kinds of treatment for high-risk GV without GRS. The hemodynamic patterns in patients with GV are distinctly different from those in patients with esophageal varices[6,35]. Solitary GV are more frequently supplied by the short and posterior gastric veins, and drain to the inferior vena cava through GRS. However, this shunt is absent in around 15% of patients with GV[25]. The present study revealed that in 13 (87%) of 15 cases without GRS, the subphrenic vein was connected to the inferior vena cava as a drainage vein. Although the varices in the GRS (-) group had thinner veins and a lower blood-flow volume than those in the GRS (+) group, the hemodynamics in the GRS (-) group was more complicated than that in the GRS (+) group, suggesting that it is necessary to examine hemodynamics before treatment, so that the most suitable treatment can be selected.
There is no established treatment for the eradication of GV in these patients without GRS. Our results show that the effect of BRTO on GV without GRS was excellent. The eradication rate of GV without GRS for each procedure (BRTO, PTO, and CA) was high, but BRTO was superior to CA and PTO in early recurrence rate within 6 mo (16.7% for BRTO, 50.0% for CA and 40.0% for PTO). In this respect, no randomized controlled trials comparing BRTO with CA or PTO are available. Regarding the long-term efficacy of CA, GV rebleeding occurs in 23%-50% of patients with most of them occurring in the first year[6]. In our hospital, the recurrence rates at 1, 3, and 5 years of GV with GRS are 2.7%, 2.7%, and 2.7%, respectively, for BRTO in 78 patients[27], 4%, 41.5%, and 53.2%, respectively, for CA in 38 patients, and 28.6%, 42.9%, and 61.9%, respectively, for PTO in 13 patients (data not shown). These results suggest that recurrence rates of GV after BRTO are lower than those of GV after other procedures, showing the excellent long-term effect of BRTO. We speculate that the reason why CA and PTO are associated with a higher recurrence rate than BRTO may be due to insufficient injection of the occlusive substances into the gastric varices and the drainage vein with the PTO and CA procedures, leading to recanalization of the GV. The results of this study suggest that BRTO is an effective treatment for GV without GRS as well as for GV with GRS.
When examination of the hemodynamics reveals that the main drainage vein is connected directly to the inferior vena cava, BRTO is indicated for GV not only with GRS but also without GRS if a catheter can be inserted into the main drainage vein. The purpose of this method is to inject the sclerosant from the drainage vein, so the efficacy of treatment may depend on the sufficient retention of the sclerosant rather than on the approach route. Therefore there is no difference in efficacy depending on the presence or absence of GRS. The most important factor for performing BRTO is the presence of the major shunt directly connected to the inferior vena cava. If the drainage vein is too thin to insert the catheter or not connected to the inferior vena cava, it is impossible to perform BRTO.
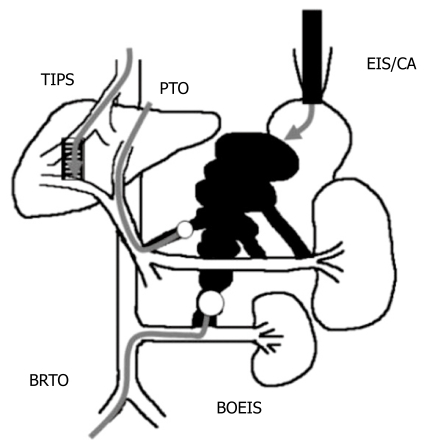
Various techniques are now available for the treatment of GV (Figure 2). PTO is used for the treatment of ruptured varices, but the use of this method has decreased following the development of endoscopic treatment. The modified PTO using sclerosant and metallic coils[29,33] can embolize GV more selectively than the original PTO[34]. When the drainage vein is not connected to the inferior vena cava, the modified PTO might be a good option for the treatment of GV without GRS, and some good results have been reported[29,33]. However, this method is not used outside Japan as BRTO. TIPS placement is currently the second-line treatment for bleeding esophagogastric varices[6,12] and can effectively control GV bleeding[13–15]. However, it was reported that its rate (25%-63%) is lower for GV than for esophageal varices[14–16]. Because patients with GV are reported to have a lower portocaval pressure gradient[35], they have extensive spontaneous portosystemic shunts and respond poorly to TIPS. Ninoi et al[29] reported that transcatheter sclerotherapy using BRTO and modified PTO can control GV bleeding better than TIPS[29]. However, larger prospective studies need to be performed. We reported that balloon-occluded endoscopic injection sclerotherapy (BOEIS) has an advantage in that it appears to be applicable to patients with GV without GRS[36]. However, BOEIS needs both interventional and endoscopic methods, and is more invasive than other methods. BOEIS is limited only to cases for which other methods are not suitable.
Figure 2.
Treatment of gastric fundal varices with balloon-occluded retrograde transvenous obliteration (BRTO), percutaneous transhepatic obliteration (PTO), balloon-occluded endoscopic injection sclerotherapy (BO-EIS), endoscopic injection sclerotherapy using α-cyanoacryrate glue (CA), transjugular intrahepatic portosystemic shunt (TIPS).
In conclusion, we should examine the hemodynamics before treatment of GV irrespective of the existence of GRS. If this hemodynamic examination reveals that the drainage vein is connected directly to the inferior vena cava in GV without GRS, BRTO might be an effective treatment for GV with GRS as well.
COMMENTS
Background
Recently, a newer interventional radiological technique has been used to treat gastric fundal varices (GV) with good results. Balloon-occluded retrograde transvenous obliteration (BRTO) is a very useful treatment for GV in terms of efficacy, safety, and degree of invasiveness.
Research frontiers
The present study is the first clinical study examining the portal hemodynamics of GV without gastro-renal shunt (GRS) compared to those with GRS and the effects of some kinds of treatment for high-risk GV without GRS.
Innovations and breakthroughs
BRTO is a very useful treatment for GV. However, it is generally considered that BRTO cannot be used in the treatment of GV without GRS. The results of this study suggest that BRTO may be an effective treatment for GV without GRS as well as for GV with GRS.
Applications
When hemodynamic examination reveals that the drainage vein is connected directly to the inferior vena cava in GV without GRS, BRTO might be an effective treatment for GV with GRS as well.
Terminology
BRTO: A method for treating GV by injecting a sclerosant after the main draining vein of GV is blocked angiographically to stagnate for blood flow.
Peer review
In this study, the authors described the hemodynamics and response to different treatments modalities for gastric varices without GRS. The authors support the use of BTRO as the first line treatment for patients in which the drainage vein is connected directly to the inferior vena cava.
Peer reviewers: Juan G Abraldes, MD, Hepatic Hemodynamic Laboratory, Liver Unit. Hospital Clinic. University of Barcelona, Hepatic Hemodynamic Lab. Liver Unit, Hospital Clinic, Villarroel 170, Barcelona 08036, Spain; Juan Carlos Garcia-Pag¨¢n, MD, Liver Unit Hospital Clinic, Villaroel 170, Barcelona 08036, Spain; Kazuma Fujimoto, Professor, Department of Internal Medicine, Saga Medical School, Nabeshima, Saga 849-8501, Japan
S- Editor Zhu LH L- Editor Wang XL E- Editor Ma WH
References
- 1.Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343–1349. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- 2.Smith JL, Graham DY. Variceal hemorrhage: a critical evaluation of survival analysis. Gastroenterology. 1982;82:968–973. [PubMed] [Google Scholar]
- 3.Sarin SK, Sachdev G, Nanda R, Misra SP, Broor SL. Endoscopic sclerotherapy in the treatment of gastric varices. Br J Surg. 1988;75:747–750. doi: 10.1002/bjs.1800750809. [DOI] [PubMed] [Google Scholar]
- 4.Trudeau W, Prindiville T. Endoscopic injection sclerosis in bleeding gastric varices. Gastrointest Endosc. 1986;32:264–268. doi: 10.1016/s0016-5107(86)71843-9. [DOI] [PubMed] [Google Scholar]
- 5.Sarin SK. Long-term follow-up of gastric variceal sclerotherapy: an eleven-year experience. Gastrointest Endosc. 1997;46:8–14. doi: 10.1016/s0016-5107(97)70202-5. [DOI] [PubMed] [Google Scholar]
- 6.Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126:1175–1189. doi: 10.1053/j.gastro.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 7.Thakeb F, Salama Z, Salama H, Abdel Raouf T, Abdel Kader S, Abdel Hamid H. The value of combined use of N-butyl-2-cyanoacrylate and ethanolamine oleate in the management of bleeding esophagogastric varices. Endoscopy. 1995;27:358–364. doi: 10.1055/s-2007-1005714. [DOI] [PubMed] [Google Scholar]
- 8.See A, Florent C, Lamy P, Levy VG, Bouvry M. Cerebrova-scular accidents after endoscopic obturation of esophageal varices with isobutyl-2-cyanoacrylate in 2 patients. Gastroenterol Clin Biol. 1986;10:604–607. [PubMed] [Google Scholar]
- 9.Roesch W, Rexroth G. Pulmonary, cerebral and coronary emboli during bucrylate injection of bleeding fundic varices. Endoscopy. 1998;30:S89–S90. doi: 10.1055/s-2007-1001406. [DOI] [PubMed] [Google Scholar]
- 10.Cheng PN, Sheu BS, Chen CY, Chang TT, Lin XZ. Splenic infarction after histoacryl injection for bleeding gastric varices. Gastrointest Endosc. 1998;48:426–427. doi: 10.1016/s0016-5107(98)70018-5. [DOI] [PubMed] [Google Scholar]
- 11.Hwang SS, Kim HH, Park SH, Kim SE, Jung JI, Ahn BY, Kim SH, Chung SK, Park YH, Choi KH. N-butyl-2-cyanoacrylate pulmonary embolism after endoscopic injection sclerotherapy for gastric variceal bleeding. J Comput Assist Tomogr. 2001;25:16–22. doi: 10.1097/00004728-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Stanley AJ, Hayes PC. Portal hypertension and variceal haemorrhage. Lancet. 1997;350:1235–1239. doi: 10.1016/S0140-6736(97)06283-1. [DOI] [PubMed] [Google Scholar]
- 13.Barange K, Peron JM, Imani K, Otal P, Payen JL, Rousseau H, Pascal JP, Joffre F, Vinel JP. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology. 1999;30:1139–1143. doi: 10.1002/hep.510300523. [DOI] [PubMed] [Google Scholar]
- 14.Rees CJ, Nylander DL, Thompson NP, Rose JD, Record CO, Hudson M. Do gastric and oesophageal varices bleed at different portal pressures and is TIPS an effective treatment? Liver. 2000;20:253–256. doi: 10.1034/j.1600-0676.2000.020003253.x. [DOI] [PubMed] [Google Scholar]
- 15.Stanley AJ, Jalan R, Ireland HM, Redhead DN, Bouchier IA, Hayes PC. A comparison between gastric and oesophageal variceal haemorrhage treated with transjugular intrahepatic portosystemic stent shunt (TIPSS) Aliment Pharmacol Ther. 1997;11:171–176. doi: 10.1046/j.1365-2036.1997.106277000.x. [DOI] [PubMed] [Google Scholar]
- 16.Sanyal AJ, Freedman AM, Luketic VA, Purdum PP 3rd, Shiffman ML, DeMeo J, Cole PE, Tisnado J. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology. 1997;112:889–898. doi: 10.1053/gast.1997.v112.pm9041251. [DOI] [PubMed] [Google Scholar]
- 17.Somberg KA. TIPS: safe, effective, better? Am J Gastroenterol. 1997;92:1412–1416. [PubMed] [Google Scholar]
- 18.Urata J, Yamashita Y, Hatanaka Y, Sumi S, Matsuno Y, Tsuchigame T, Takahashi M. Transjugular intrahepatic portosystemic shunt: initial clinical experience and three-year follow-up. Radiat Med. 1997;15:341–351. [PubMed] [Google Scholar]
- 19.Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996;11:51–58. doi: 10.1111/j.1440-1746.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 20.Saeki H, Hashizume M, Ohta M, Kishihara F, Kawanaka H, Sugimachi K. The treatment of gastric varices by a balloon-occluded retrograde transvenous obliteration; a transjugular venous approach. Hepatogastroenterology. 1996;43:571–574. [PubMed] [Google Scholar]
- 21.Chikamori F, Shibuya S, Takase Y, Ozaki A, Fukao K. Transjugular retrograde obliteration for gastric varices. Abdom Imaging. 1996;21:299–303. doi: 10.1007/s002619900068. [DOI] [PubMed] [Google Scholar]
- 22.Koito K, Namieno T, Nagakawa T, Morita K. Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol. 1996;167:1317–1320. doi: 10.2214/ajr.167.5.8911204. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12:327–336. doi: 10.1016/s1051-0443(07)61912-5. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto A, Hamamoto N, Nomura T, Hongou Y, Arisaka Y, Morikawa H, Hirata I, Katsu K. Balloon-occluded retrograde transvenous obliteration of high risk gastric fundal varices. Am J Gastroenterol. 1999;94:643–649. doi: 10.1111/j.1572-0241.1999.00928.x. [DOI] [PubMed] [Google Scholar]
- 25.Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Eight years of experience with transjugular retrograde obliteration for gastric varices with gastrorenal shunts. Surgery. 2001;129:414–420. doi: 10.1067/msy.2001.112000. [DOI] [PubMed] [Google Scholar]
- 26.Kitamoto M, Imamura M, Kamada K, Aikata H, Kawakami Y, Matsumoto A, Kurihara Y, Kono H, Shirakawa H, Nakanishi T, et al. Balloon-occluded retrograde transvenous obliteration of gastric fundal varices with hemorrhage. AJR Am J Roentgenol. 2002;178:1167–1174. doi: 10.2214/ajr.178.5.1781167. [DOI] [PubMed] [Google Scholar]
- 27.Ninoi T, Nishida N, Kaminou T, Sakai Y, Kitayama T, Hamuro M, Yamada R, Nakamura K, Arakawa T, Inoue Y. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005;184:1340–1346. doi: 10.2214/ajr.184.4.01841340. [DOI] [PubMed] [Google Scholar]
- 28.Takuma Y, Nouso K, Makino Y, Saito S, Shiratori Y. Prophylactic balloon-occluded retrograde transvenous obliteration for gastric varices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2005;3:1245–1252. doi: 10.1016/s1542-3565(05)00744-5. [DOI] [PubMed] [Google Scholar]
- 29.Ninoi T, Nakamura K, Kaminou T, Nishida N, Sakai Y, Kitayama T, Hamuro M, Yamada R, Arakawa T, Inoue Y. TIPS versus transcatheter sclerotherapy for gastric varices. AJR Am J Roentgenol. 2004;183:369–376. doi: 10.2214/ajr.183.2.1830369. [DOI] [PubMed] [Google Scholar]
- 30.Hashizume M, Kitano S, Yamaga H, Koyanagi N, Sugimachi K. Endoscopic classification of gastric varices. Gastrointest Endosc. 1990;36:276–280. doi: 10.1016/s0016-5107(90)71023-1. [DOI] [PubMed] [Google Scholar]
- 31.Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (1991). Japanese Society for Portal Hypertension. World J Surg. 1995;19:420–422; discussion 423. doi: 10.1007/BF00299178. [DOI] [PubMed] [Google Scholar]
- 32.Kim T, Shijo H, Kokawa H, Tokumitsu H, Kubara K, Ota K, Akiyoshi N, Iida T, Yokoyama M, Okumura M. Risk factors for hemorrhage from gastric fundal varices. Hepatology. 1997;25:307–312. doi: 10.1053/jhep.1997.v25.pm0009021939. [DOI] [PubMed] [Google Scholar]
- 33.Chikamori F, Kuniyoshi N, Kawashima T, Shibuya S, Takase Y. Percutaneous transhepatic obliteration for isolated gastric varices with gastropericardiac shunt: case report. Abdom Imaging. 2006;31:249–252. doi: 10.1007/s00261-005-0372-y. [DOI] [PubMed] [Google Scholar]
- 34.Lunderquist A, Vang J. Transhepatic catheterization and obliteration of the coronary vein in patients with portal hypertension and esophageal varices. N Engl J Med. 1974;291:646–649. doi: 10.1056/NEJM197409262911303. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K, Kimura K, Matsutani S, Ohto M, Okuda K. Portal hemodynamics in patients with gastric varices. A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology. 1988;95:434–440. doi: 10.1016/0016-5085(88)90501-x. [DOI] [PubMed] [Google Scholar]
- 36.Shiba M, Higuchi K, Nakamura K, Itani A, Kuga T, Okazaki H, Fujiwara Y, Arakawa T. Efficacy and safety of balloon-occluded endoscopic injection sclerotherapy as a prophylactic treatment for high-risk gastric fundal varices: a prospective, randomized, comparative clinical trial. Gastrointest Endosc. 2002;56:522–528. doi: 10.1067/mge.2002.127410. [DOI] [PubMed] [Google Scholar]