Abstract
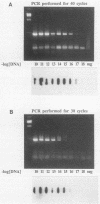
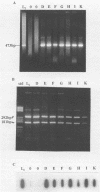
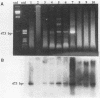
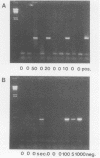
A polymerase chain reaction (PCR) assay was developed for detection of Chlamydia trachomatis DNA. From the published sequence of the common C. trachomatis plasmid, two primer sets were selected. Detection of amplified sequences was done by agarose gel electrophoresis of cleaved or uncleaved amplified sequences, Southern hybridization, or dot blot analysis. The PCR assay was optimized and, after 40 cycles of amplification with primer set II, demonstrated a sensitivity of 10(-17) g of DNA, which corresponds to the detection of one copy of the plasmid. Because of the high sensitivity, we developed a closed system in which airborne contamination was minimized. Analysis of 228 clinical samples tested by cell culture, IDEIA enzyme immunosorbent assay (Medico-Nobel, Boots-Celltech Ltd., Berkshire, United Kingdom), and PCR showed a sensitivity of 100%, a specificity of 93% when PCR was compared with cell culture, and a corrected specificity of 99% when PCR was compared with cell culture or IDEIA.
Full text
PDF


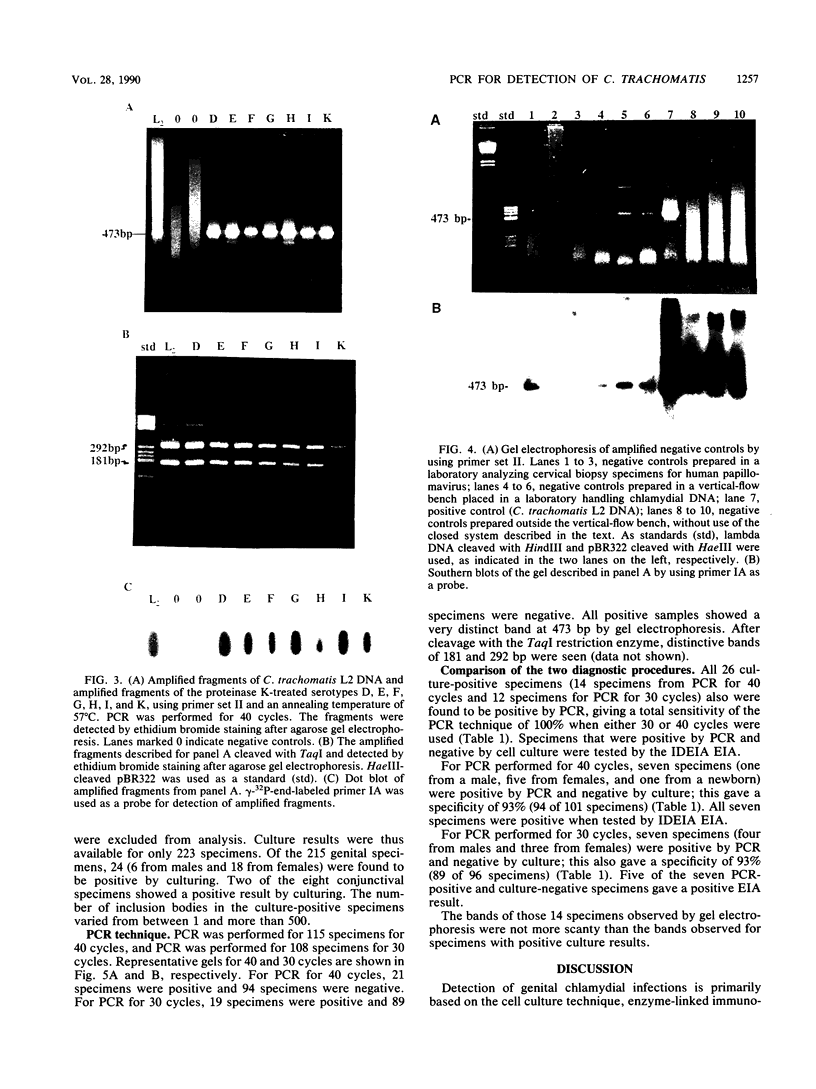
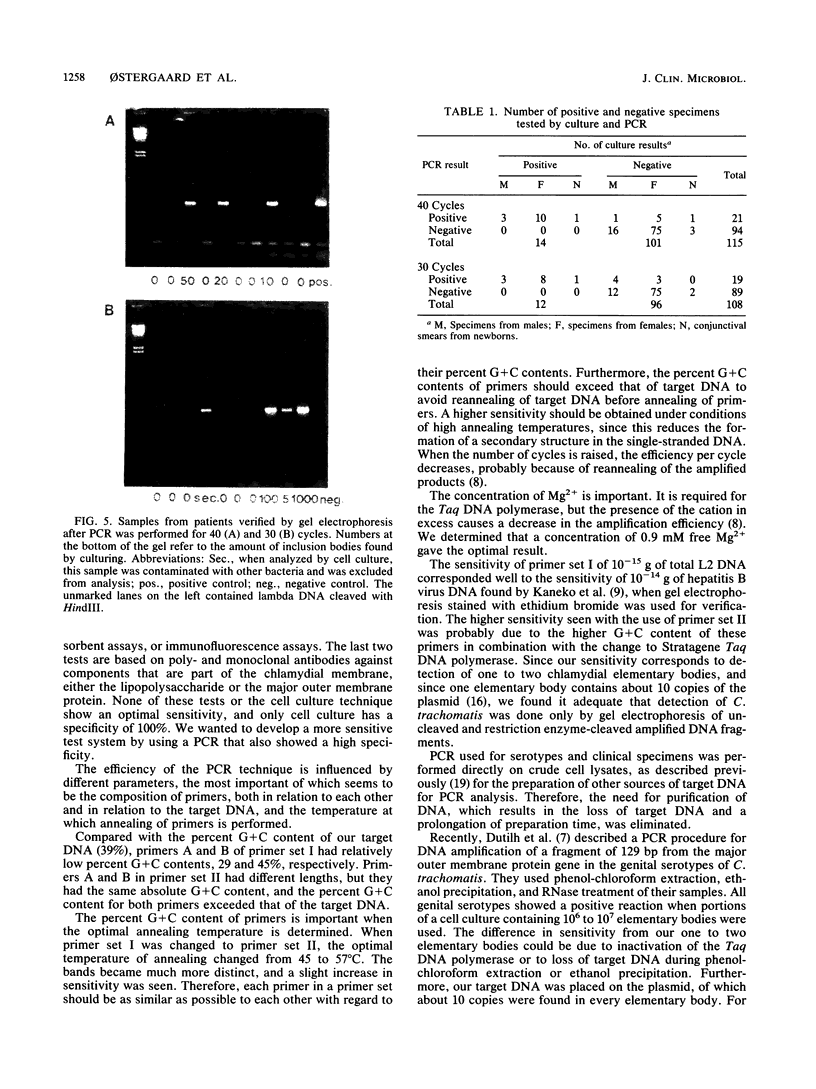


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkelund S., Lundemose A. G., Christiansen G. Chemical cross-linking of Chlamydia trachomatis. Infect Immun. 1988 Mar;56(3):654–659. doi: 10.1128/iai.56.3.654-659.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S. P., Zhang J. Z., Huang D. H., Wang Z. X., Kan Y. W. A simple approach to prenatal diagnosis of beta-thalassemia in a geographic area where multiple mutations occur. Blood. 1988 May;71(5):1357–1360. [PubMed] [Google Scholar]
- Dean D., Pant C. R., O'Hanley P. Improved sensitivity of a modified polymerase chain reaction amplified DNA probe in comparison with serial tissue culture passage for detection of Chlamydia trachomatis in conjunctival specimens from nepal. Diagn Microbiol Infect Dis. 1989 Mar-Apr;12(2):133–137. doi: 10.1016/0732-8893(89)90003-5. [DOI] [PubMed] [Google Scholar]
- Demmler G. J., Buffone G. J., Schimbor C. M., May R. A. Detection of cytomegalovirus in urine from newborns by using polymerase chain reaction DNA amplification. J Infect Dis. 1988 Dec;158(6):1177–1184. doi: 10.1093/infdis/158.6.1177. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Huang W. M., Woo S. L. Screening for phenylketonuria mutations by DNA amplification with the polymerase chain reaction. Lancet. 1988 Mar 5;1(8584):497–499. doi: 10.1016/s0140-6736(88)91295-0. [DOI] [PubMed] [Google Scholar]
- Duggan D. B., Ehrlich G. D., Davey F. P., Kwok S., Sninsky J., Goldberg J., Baltrucki L., Poiesz B. J. HTLV-I-induced lymphoma mimicking Hodgkin's disease. Diagnosis by polymerase chain reaction amplification of specific HTLV-I sequences in tumor DNA. Blood. 1988 Apr;71(4):1027–1032. [PubMed] [Google Scholar]
- Dutilh B., Bébéar C., Rodriguez P., Vekris A., Bonnet J., Garret M. Specific amplification of a DNA sequence common to all Chlamydia trachomatis serovars using the polymerase chain reaction. Res Microbiol. 1989 Jan;140(1):7–16. doi: 10.1016/0923-2508(89)90053-3. [DOI] [PubMed] [Google Scholar]
- Guatelli J. C., Gingeras T. R., Richman D. D. Nucleic acid amplification in vitro: detection of sequences with low copy numbers and application to diagnosis of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 1989 Apr;2(2):217–226. doi: 10.1128/cmr.2.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S., Miller R. H., Feinstone S. M., Unoura M., Kobayashi K., Hattori N., Purcell R. H. Detection of serum hepatitis B virus DNA in patients with chronic hepatitis using the polymerase chain reaction assay. Proc Natl Acad Sci U S A. 1989 Jan;86(1):312–316. doi: 10.1073/pnas.86.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Chang K. S., Cabanillas F., Freireich E. J., Trujillo J. M., Stass S. A. Detection of minimal residual cells carrying the t(14;18) by DNA sequence amplification. Science. 1987 Jul 10;237(4811):175–178. doi: 10.1126/science.3110950. [DOI] [PubMed] [Google Scholar]
- McClenaghan M., Herring A. J., Aitken I. D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984 Aug;45(2):384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Olive D. M. Detection of enterotoxigenic Escherichia coli after polymerase chain reaction amplification with a thermostable DNA polymerase. J Clin Microbiol. 1989 Feb;27(2):261–265. doi: 10.1128/jcm.27.2.261-265.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L., Lundemose A. G., Birkelund S., Christiansen G. Age and sex correlation of Chlamydia trachomatis infections evaluated by the culture technique and by an enzyme immunosorbent assay, IDEIA. Eur J Obstet Gynecol Reprod Biol. 1990 Mar;34(3):273–281. doi: 10.1016/0028-2243(90)90081-b. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Palmer L., Falkow S. A common plasmid of Chlamydia trachomatis. Plasmid. 1986 Jul;16(1):52–62. doi: 10.1016/0147-619x(86)90079-x. [DOI] [PubMed] [Google Scholar]
- Ripa K. T., Mårdh P. A. Cultivation of Chlamydia trachomatis in cycloheximide-treated mccoy cells. J Clin Microbiol. 1977 Oct;6(4):328–331. doi: 10.1128/jcm.6.4.328-331.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D., Martin W. J., Appleman M. D., Causey D. M., Leedom J. M., Arnheim N. Detection of cytomegalovirus DNA in peripheral blood of patients infected with human immunodeficiency virus. J Infect Dis. 1988 Dec;158(6):1185–1192. doi: 10.1093/infdis/158.6.1185. [DOI] [PubMed] [Google Scholar]
- Shyamala V., Ames G. F. Amplification of bacterial genomic DNA by the polymerase chain reaction and direct sequencing after asymmetric amplification: application to the study of periplasmic permeases. J Bacteriol. 1989 Mar;171(3):1602–1608. doi: 10.1128/jb.171.3.1602-1608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriprakash K. S., Macavoy E. S. Characterization and sequence of a plasmid from the trachoma biovar of Chlamydia trachomatis. Plasmid. 1987 Nov;18(3):205–214. doi: 10.1016/0147-619x(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]