Abstract
Context
Polycystic ovary syndrome (PCOS) represents the commonest endocrine abnormality in women of reproductive age. The cause of PCOS remains largely unknown, but studies suggest an intrinsic ovarian abnormality.
Objective
To test our hypothesis that differences in granulosa cell proliferation and apoptosis may underlie abnormalities that affect follicular development.
Design
Granulosa cells were prepared from follicular fluid aspirated from 4-8 mm follicles of unstimulated ovaries during routine laparoscopy or laparotomy from women with anovulatory PCOS and those with regular ovulatory cycles.
Setting
University hospital.
Patients
14 women with anovulatory PCOS and 9 women with regular ovulatory cycles.
Main outcome measures
Immunocytochemistry on granulosa cells to investigate apoptotic and proliferation rates, together with real-time RT-PCR to analyze gene expression profiles of apoptotic regulators.
Results
Significantly lower apoptotic rates were found in granulosa cells from patients with PCOS compared to women with regular ovulatory cycles (P = 0.004). Lower apoptotic rates were associated with decreased levels of the apoptotic effector caspase 3 (P = 0.001) and increased levels of the anti-apoptotic survival factor cIAP-2 in the PCOS group that were coupled to higher proliferation rates (P = 0.032). Gene expression profiling confirmed the immunocytochemical findings.
Conclusions
Our findings indicate that there are significant differences in the rate of cell death and proliferation in granulosa cell populations in PCOS patients. These are associated with decreased expression of apoptotic effectors and increased expression of a cell survival factor. These results provide new insights that may be useful in developing specific therapeutic intervention strategies in PCOS.
Keywords: Polycystic ovary syndrome, granulosa cell, apoptosis, proliferation, cIAP2
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine abnormality in women of reproductive age, affecting 6.6-8 % of women in this age group (1). It is the main cause of anovulatory infertility and is characterized by chronic anovulation, hyperandrogenemia and polycystic ovaries on ultrasound scan (2).
Hughesdon (3) observed that PCOS ovaries contain twice the number of growing follicles at all stages of development. This suggests that all steps in the process of folliculogenesis may be disordered in PCOS, implying that there could be an intrinsic ovarian abnormality in PCOS. It remains unclear whether it is the resistance to atresia, or the arrest in the selection of follicles that leads to the accumulation of multiple small antral follicles in polycystic ovaries. Recent studies have documented the possibility that a pathway of cell death modulators may play a role in determining the fate of follicles in the ovary (4, 5). Apoptosis is the mechanism underlying follicular atresia and is fundamental to the cyclical growth and regression of follicles in the human ovary (6). In the early stages of follicle development (primordial, primary, and small preantral), atresia is initiated by oocyte apoptosis followed by death of the surrounding granulosa cells (7). In contrast, atresia of maturing (late preantral, antral) follicles is first demarcated by granulosa cell apoptosis (7). Throughout oocyte development, there is an interdependence between the oocyte and its surrounding granulosa cells, support of which is essential in providing the oocyte with nutrients and growth regulators. The oocyte in turn promotes growth and differentiation of the granulosa cells (8). There is evidence to suggest that dysfunction of these cells may contribute to the abnormal folliculogenesis observed in PCOS (9-11) although the underlying mechanism remains to be determined.
Caspases function at the penultimate stage of cell death (12) and Caspase-3 is functionally required for granulosa cell apoptosis during follicular atresia (13). The inhibitor of apoptosis proteins (IAPs) which include cIAP-1, cIAP-2, XIAP and Survivin, block apoptosis by inhibiting caspase-3 activity (14, 15). Studies have suggested that IAPs may be involved in the suppression of granulosa cell apoptosis in mammals (16, 17). Bcl-2 gene family members, including both pro (Bax, Bcl-XShort) and anti-apoptotic (Bcl-2, Bcl-XLong, Mcl-1) have also been proposed as cell death regulators in the ovary (18).
Previous studies at the cellular level, including those on gene expression profiling in PCOS have mostly been on archival tissue, whole ovarian specimens or on granulosa cells from patients undergoing in-vitro fertilization (IVF). The hormonal treatment used in IVF could affect granulosa cell survival and proliferation as well as gene expression profiles. Moreover, these cells may not behave in the same way as granulosa cells from normally developing follicles.
We aimed to study differences in apoptosis and cellular proliferation in granulosa cells from size-matched unstimulated ovarian follicles in anovulatory PCOS and patients with regular ovulatory cycles in order to investigate the mechanisms underlying follicular survival or atresia. We compared the number of apoptotic granulosa cells by TUNEL (Terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling) assay and activated caspase-3 expression, differences in gene expression of members of the IAP and Bcl-2 families by real-time quantitative polymerase chain reaction (PCR) and differences in cell proliferation by expression of the proliferation marker, Ki-67.
Patients and Methods
Patients
We recruited patients from Newham University Hospital, London, United Kingdom, between September 2003 and March 2005. The local research ethics committee approved the study and all women gave their written informed consent before participation.We recruited 14 women with PCOS, who were undergoing laparoscopic investigation for infertility or ovarian drilling for ovulation induction. The diagnosis of PCOS was established according to the revised Rotterdam ESHRE/ASRM criteria (2003) (19). Specifically, all women had oligomenorrhoea or amenorrhoea (eight or fewer spontaneous menses per year), clinical (hirsutism) and/or biochemical (elevated free androgen index) (20) evidence of hyperandrogenism and polycystic ovaries on ultrasound scan. Patients with hyperprolactinemia, thyroid disease, congenital adrenal hyperplasia, Cushing’s syndrome and androgen-secreting tumours were excluded. In the control group, we recruited 9 normally menstruating women, who were undergoing laparoscopic sterilisation, hysterectomy for benign conditions and diagnostic laparoscopy for pelvic pain. All patients in the control group had a normal pelvis apart from uterine fibroids. Only patients with a body mass index (BMI) of less than 30 were included in the study in order to avoid any potential influence of BMI on the study parameters. All patients underwent the procedure in the early follicular phase of the menstrual cycle as assessed by the last day of the menstrual cycle, their endocrine profile and by trans-vaginal ultra-sound scan. None of the patients in either group had taken any fertility drugs or hormonal medication for three months prior to sample collection. Endocrine parameters in control subjects were all within normal range. All patients underwent trans-vaginal ultrasonography one day prior to the procedure to assess the size and number of ovarian follicles. An independent clinician (MR), who was not involved in the rest of the project, performed all ultrasound scans. The ultrasound characteristics of the PCOS patients satisfied the revised Rotterdam ESHRE/ASRM criteria (2003) (19), namely, presence of 12 or more follicles measuring 2-9 mm in diameter, and/or increased ovarian volume (>10 ml) in one or both ovaries. Follicle diameter was estimated as the mean of 2 recorded diameters in the longitudinal and antero-posterior planes.
Blood samples for subsequent biochemical analyses were obtained just prior to the operation. Samples were analyzed for follicle-stimulating hormone (FSH), luteinising hormone (LH), progesterone, prolactin, thyroxine, thyroid stimulating hormone (TSH), testosterone, sex hormone-binding globulin (SHBG), androstenedione, 17-hydroxyprogesterone and dehydroepiandrosterone sulfate (DHEAS).
Clinical and endocrine parameters in normo-ovulatory controls and in patients with anovulatory PCOS are summarized in Table 1.
TABLE 1. Clinical and endocrine parameters in normo-ovulatory controls and in patients with anovulatory PCOS.
| Variable | PCOS (N = 14) | CONTROL (N = 9) |
|---|---|---|
|
| ||
| Age (years) | 26 (23- 32) | 32 (30-36) |
| BMI (kg/m2) | 27 (26 - 28) | 26 (26-28) |
| FSH (IU/L) | 5 (4-5.6) | 4.6 (4.1-6) |
| LH (IU/L) | 14.7 (12.3-17.8) | 4.9 (4.9-6.3) |
| Testosterone (ng/dl) | 80.8 (69.2-89.4) | 54.8 (43.3-57.7) |
| SHBG (nmol/liter) | 32.0 (24.3-37.9) | 42.2 (38.0-50.5) |
| Free Androgen Index a | 8.6 (7.1-10.7) | 4.0 (2.5-4.9) |
| DHEAS (μg/dl) | 217.4 (121.6-254.2) | 162.1 (95.8-221.1) |
| Androstenidione (ng/ml) | 3.1 (2.6-4.8) | 1.7 (1.3-3.1) |
| Progesterone (ng/ml) | 0.6 (0.5-0.9) | 0.4 (0.3-0.8) |
| Ultrasound parameters Number of follicles | 18 (16-28) | 5 (4-6) |
| Size of follicles (mm) | 4.9 (4.1-5.3) | 4.6 (4.1-5.1) |
Values are expressed as median and inter-quartile range in parentheses.
Free Androgen Index was calculated according to the formula: [total testosterone (in nanomoles per liter) divided by SHBG (in nanomoles per liter)] × 100.
Conversion factor for SI units:
To convert values for testosterone to nanomoles per liter, multiply by 0.03467.
To convert values for DHEAS to μmol per liter, multiply by 0.02714.
To convert values for androstenidione to nanomoles per liter, multiply by 3.492.
To convert values for progesterone to nanomoles per liter, multiply by 3.18.
Follicular fluid collection and granulosa cell preparation
We collected follicular fluid from individual follicles at the time of laparoscopy, using a single lumen 17-gauge needle (Casmed, UK). Follicular fluid was selected from follicles 4-8mm in diameter, free from blood contamination. 3-4 follicles were aspirated from each patient. The size of the follicles in the PCOS group ranged from 4.9 (4.1-5.3) mm [median (inter-quartile range)] while the size of the follicles in the control group ranged from 4.6 (4.1-5.1) mm [P = 0.78]. Each sample was collected into a sterile tube without culture medium. From the control group, only size-matched follicles were taken. All samples were processed within an hour from collection to avoid post-aspiration cell death. The follicular fluid from all aspirated follicles from each patient was pooled in order to obtain enough granulosa cells for our assays. The follicular fluid was immediately centrifuged at 700 × g for 10 min. Red blood cells were removed with the help of a 50% Percoll gradient (Sigma Aldrich, Dorset, U.K). Dynabeads M-450 CD45 (Dynal Biotech, Oslo, Norway), which is a pan-leukocyte marker, were used to immunomagnetically deplete CD45 positive leucocytes from the granulosa cell suspension as per manufacturer’s instructions for negative cell selection. The number of granulosa cells in the pooled follicular fluid ranged from (1.5 ×104- 3 ×104) in each patient in the PCOS group (average of both ovaries) and the number of granulosa cells in the control group ranged from (1.6 × 104- 2.8 × 104). Granulosa cells were identified on cytospin preparations on the basis of their morphology - granulosa cells were small, round to cuboid cells with distinct nuclei - as well as by immunocytochemistry using rabbit polyclonal FSH-receptor antibody (Zymed, USA) as well as monoclonal inhibin antibody (Oxford-Bioinnovations, UK).
Identification of apoptotic nuclei by TUNEL assay
Apoptotic nuclei were identified using the DeadEnd™Colorimetric TUNEL System (Promega, Madison, USA) according to the manufacturer’s protocol. Endogenous peroxidases were blocked by immersing slides in 3% hydrogen peroxide. Negative controls were treated in the same manner except that the TdT labelling enzyme was omitted (21). A positive control was prepared by treating cells on a separate slide with DNAse 1. Apoptotic nuclei were visualized with diaminobenzidine (DAB) as chromogen substrate (Biogenex, San Ramon, CA), and counterstained with hematoxylin. Cells showing dark brown staining from the colorimetric reaction were considered positive for DNA fragmentation (22).
Immunocytochemistry
Immunocytochemistry was by standard techniques using cytospin preparations of granulosa cells, fixed in 4% neutral-buffered paraformaldehyde. Primary antibodies were: activated or cleaved Caspase-3 (rabbit monoclonal, 1:100 dilution, Cell Signaling Technology, Danvers, MA, USA), cIAP-2 (goat polyclonal, 1:100 dilution, R&D Systems, Minneapolis, USA) and Ki-67 (monoclonal mouse antibody, [MIB-1], 1:50 dilution, Dako, UK). Non-specific binding of antibodies was blocked with a 10% dilution of normal serum before application of the primary antibody. For negative controls, the primary antibody was replaced by normal serum to verify expression patterns. Detection used an appropriate biotinylated secondary antibody and the Vectastain Universal Elite ABC Kit (Vector Laboratories, Peterborough, UK). Slides were developed with DAB, counterstained with hematoxylin and visualized with a Leica light microscope (Leica Imaging Systems Ltd, Cambridge, UK).
Slides were analysed systematically at a magnification of ×200, × 400 and ×1000 images. Digital images were captured with Leica DC 200 software. Slides were anonymised and coded until they had been examined. Two investigators (MD and LG) examined the slides. The average of 2 readings was taken. At least 200 cells were counted per slide.
RNA Isolation and Real Time PCR Analyses
RNA was isolated from granulosa cells using the Pico Pure RNA isolation kit (Arcturus,USA) in accordance with the manufacturer’s instructions. A DNAase treatment to remove genomic DNA was carried out with the RNAse-Free DNAse Set (Quiagen,UK). cDNA was synthesized with AMV Reverse Transcriptase (Promega, Madison, USA) according to the manufacturer’s protocol. RNA was isolated from the granulosa cells as soon as possible after harvesting and stored. RT reactions to generate cDNA were performed on all the samples simultaneously.
To quantify the mRNA expression levels of the IAP and Bcl-2 families in the granulosa cells, real-time RT PCR was performed on a continuous fluorescence DNA Engine Opticon™ 2 system (MG Research) using DyNamo HS SYBR Green qPCR kit (Finnzymes, GRI, UK). The IAP family included cIAP-1,cIAP-2, XIAP and Survivin. The Bcl-2 family members included both up regulators (Bax) and down regulators (Bcl-XLong, Mcl-1) of apoptosis. To ensure absence of genomic DNA, a step omitting reverse transcriptase during cDNA synthesis was carried out. Melting curve analysis and gel electrophoresis were used to control the specificity and quality of the PCR reactions. ‘Primer-only’ controls were included to ensure the absence of primer-dimers. ß-actin was used as a reference standard for all analyses to control for the amount of sample material. PCR reactions were carried out in triplicate for each sample and the average of the three readings was taken. Fold-induction values (x) were calculated using the following formula: x=2-∆∆C t, where Ct represents the mean threshold cycle of all replicate analyses of a given gene and ∆Ct represents the difference between the Ct values of the gene in question (target) and the Ct value of the reference gene (ß-actin) (23). (∆∆Ct is the difference between ∆Ct values of the samples for each target and the mean ∆Ct of the calibrator, ß-actin). The primer sequences are represented in Table 2.
TABLE 2. Primer Sequences.
| Primers | Sequence (5′-3′) |
|---|---|
| cIAP-1 | Forward: CAGCCTGAGCAGCTTGCAA Reverse: CAAGCCACCATCACAACAAAA |
| cIAP-2 | Forward: TCCGTCAAGTTCAAGCCAGTT Reverse: TCTCCTGGGCTGTCTGATGTG |
| Xiap | Forward: AGTGGTAGTCCTGTTTCAGCATCA Reverse: CCGCACGGTATCTCCTTCA |
| Survivin | Forward: CCCATAGAGGAACATAAAAAGCATTC Reverse: TCAAAAATTCACCAAGGGTTAATTCT |
| Bcl-2 | Forward: GGCAATGTGACTTTTTCCAA Reverse: GGCTGATATTCTGCAACACTG |
| Bax | Forward: CCAGCTGCCTTGGACTGT Reverse: ACCCCCTCAAGACCACTCTT |
| Bcl-XLong | Forward: GTAAACTGGGGTCGCATTGT Reverse: TGGATCCAAGGCTCTAGGTG |
| Mcl-1 L | Forward: ATCTCTCGGTACCTTCGGGAGC Reverse: GCTGAAAACATGGATCATCACTCG |
| ß-actin | Forward:TCACCCACACTGTGCCCATCTACGA Reverse:CAGCGGAACCGCTCATTGCCAATGG |
Statistical Analysis
As the data was not normally distributed, the non-parametric Mann Whitney U test was used to analyze data using the SPSS software Version14 for Windows. A value of P < 0.05 was considered statistically significant.
Results
Granulosa cells from PCOS patients have lower apoptotic rates
We tested the hypothesis that defects in apoptosis in granulosa cells from PCOS patients may be associated with, and partly be responsible, for the development of the syndrome. We used a colorimetric TUNEL assay to determine the granulosa cell apoptotic rate in PCOS compared to cells isolated from normal women. We found that significantly fewer apoptotic nuclei were present in the PCOS group as compared to the normal control group (P = 0.004). Typical immunocytochemical staining of TUNEL-positive granulosa cells is shown in Figure 1A, and quantification of the results depicted in Figure 1B. No cell nuclei were stained in the negative controls. TUNEL positive cells showed the characteristic appearance of apoptosis including chromatin condensation and shrinkage of cytoplasm (24).
Figure 1.
Panel A TUNEL staining identifies apoptotic granulosa cells isolated from PCOS patients (original magnification × 400).
Panel B Quantification of TUNEL staining. Box and whisker plots depicting the percentage of TUNEL-positive cells in anovulatory women with PCOS and normo-ovulatory women. Solid lines inside boxes depict the median value, whereas the upper and lower limits of the boxes and whiskers indicate the 75th, 25th, 95th and 5th percentiles respectively. PCOS: Mean 2.6; SD 2.2; Median 2.3, Normal Group: Mean 6.2; SD 2.9; Median 5.7; P=0.004. Black filled arrows represent the TUNEL positive granulosa cells and the white unfilled arrow represents the TUNEL negative granulosa cell.
PCOS: (n = 14 subjects), Control: (n = 9 subjects).
Expression of apoptotic regulators in granulosa cells
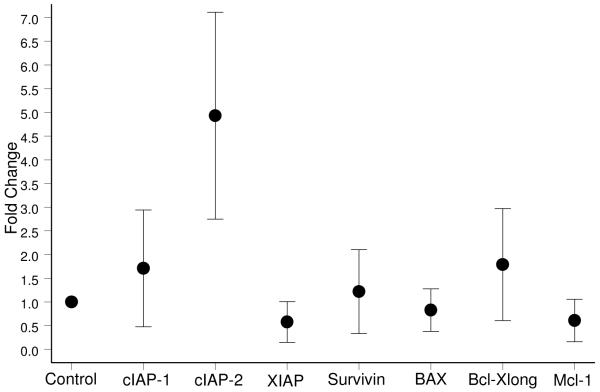
Having noted a significantly lower rate of apoptosis in granulosa cells from PCOS patients, we were interested to investigate the mechanism underlying this defect. We first analyzed the gene expression profiles of apoptotic inducers and inhibitors in mRNA isolated from the granulosa cells using quantitative PCR. Among the genes investigated were the IAP family of proteins, since these act as survival factors by inhibiting the activity of caspases, including executioner caspases such as caspase-3. The fold change in the expression of the target gene normalized to ß-actin in the PCOS group and relative to its expression in the control group was analyzed for each sample. Melting curve analysis confirmed the specificity of the PCR reactions (data not shown). We found that the level of expression of cIAP-2 was almost 5-fold higher in the PCOS group as compared to the normal group (Figure 2). In addition, the expression of a number of other apoptotic regulators was also examined. The level of Survivin and Bax gene expression was very similar to that of controls. Slight increases in cIAP-1 and Bcl-XL as well as small decreases in XIAP, Mcl-1L and Bax gene expression were noted, however none of these changes reached statistical significance in our studies (Figure 2). Further work on larger sample populations will be needed to confirm whether any change in expression of these genes plays a role in the development of the disease.
Figure 2.
Graphs representing the fold change in gene expression patterns of cIAP-2, cIAP-1, XIAP, Survivin, Bax, Bcl-XL, and Mcl-1 in anovulatory PCOS compared with normo-ovulatory individuals. Expression levels are given as fold change compared to normo-ovulatory samples. In the case of cIAP-2, the graph indicates a near 5-fold increase in PCOS over the normal group. The dark circles represent the fold change values and the error bars represent the 95% confidence intervals.
PCOS: (n = 14 subjects), Control: (n = 9 subjects).
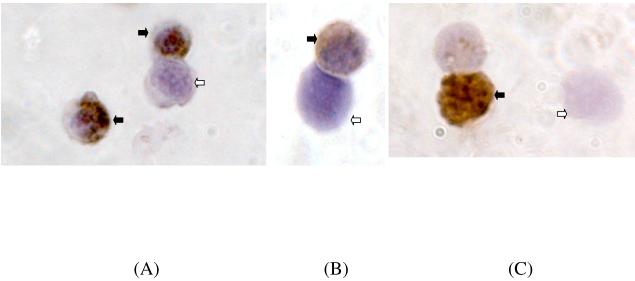
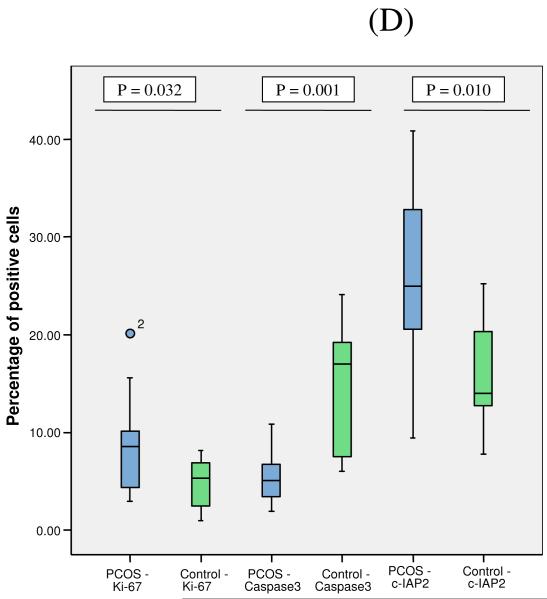
We followed up the gene expression profiling with an immunocytochemical analysis to determine whether the changes in gene expression levels were manifested by changes in protein expression. The percentage of cells showing immunoreactivity for cleaved activated caspase-3 was significantly higher in the normal group as compared to the PCOS group, P =0.001 (Figure 3A). As expected, immunoreactivity for caspase-3 was noted mainly in the cytoplasm of the granulosa cells. The number of granulosa cells expressing active caspase-3 was higher than the number of TUNEL-positive cells in both PCOS and normal groups, consistent with activation of caspase-3 preceding DNA fragmentation during atresia of granulosa cells.
Figure 3.
Immunocytochemical staining for the following proteins (×1000 original magnification): active caspase 3 (Panel A), cIAP-2 (Panel B) and the proliferation marker Ki-67 (Panel C). Black arrows represent the immunopositive granulosa cells and the white unfilled arrows represent the immunonegative granulosa cells.
Panel D: Quantification of the number of positively staining cells is shown in the graph. Box and whiskers plot depicting the percentage of positively staining cells. For description of the box-plots, please refer to Figure 1. For caspase-3 positive cells: PCOS: Mean 5.1; SD 2.5; Median 5.1; Normal Group: Mean 14.7; SD 7.1; Median 17.0; P=0.001. For cIAP-2: PCOS: Mean 26.0; SD 9.1; Median 25.0; Normal Group: Mean 15.7; SD 5.9; Median 14.0; P=0.010. For Ki-67: PCOS: Mean 9.0; SD 5.0; Median 8.6; Normal Group: Mean 4.9; SD 2.6; Median 5.3; P=0.032.
PCOS: (n = 14 subjects), Control: (n = 9 subjects).
In agreement with the gene expression data, the percentage of granulosa cells showing immunopositivity for cIAP-2 antibody was significantly higher in the PCOS group as compared to the normal group, P=0.010 (Figure 3B). We next asked whether the lower apoptotic rates in PCOS were accompanied by an increase in the number of actively proliferating cells using a well-recognized indicator, the nuclear staining of Ki-67. The percentage of Ki-67 positive granulosa cell nuclei was found to be significantly higher in the PCOS group, P=0.032 (Figure 3C). Quantification of the number of positively staining caspase 3, cIAP-2 and Ki-67 cells from PCOS and normal controls is shown in Figure 3D. Since the changes in gene expression of cIAP-1, XIAP, Survivin, Bax and Mcl 1-L were not significant in our PCR studies, immunocytochemistry of protein levels in PCOS cells were not pursued further.
Discussion
Our findings indicate that there are significant differences in the rate of apoptosis and proliferation in granulosa cells in PCOS patients. We have for the first time demonstrated that activated caspase-3, which is functionally required for the execution of cell death, is significantly lower in granulosa cells of anovulatory PCOS follicles than in normal ovulatory follicles. Our study also identified for the first time differences in the expression of survival proteins of the IAP and Bcl-2 families in PCOS, which might account for the disordered folliculogenesis in PCOS.
Our findings are consistent with and advance the findings of Almahbobi et al.(25) who demonstrated that most of the granulosa cells of polycystic ovaries are non-apoptotic and express more functional FSH receptors than granulosa cells of normal follicles. Whereas they noted that granulosa cells from normal ovaries had the same proportion of apoptotic cells as in ovulatory PCO, we in fact observed that there were fewer number of apoptotic granulosa cells in anovulatory PCOS than in normal ovarian follicles.
Probing the underlying mechanism for the observed apoptotic defect revealed changes in both protein and mRNA levels for important apoptotic regulators, in particular the IAP family member, cIAP-2. Our results also suggested that there may be small differences in the expression of other Bcl-2 family members, which have been proposed to play an important role in granulosa cell apoptosis (18). We observed that the expression of Bcl-XLong, an anti-apoptotic factor, was increased nearly 2-fold while Bax, which is pro-apoptotic, was marginally down-regulated in the PCOS group. However, more extensive studies would be needed to substantiate these findings. Interestingly however, Bax-deficient mice were noted to have abnormal follicles with an excessive number of granulosa cells (26).
To investigate whether proliferation rates were altered in PCOS granulosa cells we also examined the expression of the cell proliferation marker, Ki-67, as the granulosa cells in PCOS have often been deemed to be atretic. We showed that the expression of Ki-67 was significantly higher in the PCOS group, indicating a significant imbalance between apoptotic and proliferation rates in PCOS patients.
The differences in apoptosis and cellular proliferation observed in our study may be due to the high concentrations of androgens that are characteristically found in PCOS, including this study (see Table 1). Supporting this idea, androgen treatment of adult Rhesus monkeys was shown to stimulate growth and decrease apoptosis of small antral follicles (27). Moreover, granulosa cell proliferation, as determined by immunodetection of the Ki-67 antigen, was significantly increased in androgen treated monkeys (27).
Folliculogenesis may also be affected by a premature response of PCO follicles to LH compared to normal ovaries. This could partly be due to amplification of LH action on granulosa cells of the developing follicle by hyperinsulinemia (10). The appearance of LH receptors on follicles of a smaller size in polycystic as compared to follicles from regularly cycling women could contribute to the arrest of follicle development (11). High LH levels could also cause the decreased apoptosis seen in granulosa cells in PCOS, as it has previously been reported in other systems that LH interferes with Fas-induced apoptosis in human ovarian surface epithelial cancer lines (28). Eventually, the inhibition of granulosa cell apoptosis in PCOS may be overcome by other death signaling pathways.
The decrease in apoptosis associated with lower expression of activated caspase-3 noted in PCOS granulosa cells may be due to an overall up-regulation of the survival proteins of both IAP and Bcl-2 families in PCOS. This does not exclude the possibility that there could be other factors involved which may act in concert with these survival proteins to promote cell survival and proliferation in PCOS.
Using micro-array analysis of whole ovarian samples, Diao et al. (29) also noted that anti-apoptotic factors were overexpressed in PCOS. This is in contrast to the study by Jansen et al. (30) who reported an increase in the expression of apoptotic factors in PCOS ovaries. However, it is worth noting that both studies used archival or whole ovarian tissue. Moreover, all the PCOS patients in the latter study had undergone ovulation induction that may have resulted in altered gene expression profiles. Our study is consistent with the findings of others but now pinpoints the defect in apoptosis to the granulosa cell population.
In summary, our findings indicate that there are fundamental and significant differences in the rate of cell death and proliferation in granulosa cells in PCOS. This study provides new insights into the molecular mechanisms underlying the aberrant folliculogenesis in PCOS. Future studies focusing on key regulators described here could further unravel the complexities of this syndrome and help in developing novel strategies for therapeutic intervention.
Acknowledgements
We thank T Young (ScHARR, University of Sheffield, United Kingdom) for statistical assistance. We also thank all the subjects who participated in this study.
This study was supported by a research grant from Queen Mary, University of London, United Kingdom.
Footnotes
Disclosure information: The authors have nothing to disclose.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 3.Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. 1982;37:59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Johnson AL, Bridgham JT, Witty JP, Tilly JL. Susceptibility of avian ovarian granulosa cells to apoptosis is dependent upon stage of follicle development and is related to endogenous levels of bcl-xlong gene expression. Endocrinology. 1996;137:2059–2066. doi: 10.1210/endo.137.5.8612548. [DOI] [PubMed] [Google Scholar]
- 5.Kugu K, Ratts VS, Piquette GN, Tilly KI, Tao XJ, Martimbeau S, Aberdeen GW, Krajewski S, Reed JC, Pepe GJ, Albrecht ED, Tilly JL. Analysis of apoptosis and expression of bcl-2 gene family members in the human and baboon ovary. Cell Death Differ. 1998;5:67–76. doi: 10.1038/sj.cdd.4400316. [DOI] [PubMed] [Google Scholar]
- 6.Tilly JL. Apoptosis and ovarian function. Rev Reprod. 1996;1:162–172. doi: 10.1530/ror.0.0010162. [DOI] [PubMed] [Google Scholar]
- 7.Morita Y, Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol. 1999;213:1–17. doi: 10.1006/dbio.1999.9344. [DOI] [PubMed] [Google Scholar]
- 8.Buccione R, Schroeder AC, Eppig JJ. Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol Reprod. 1990;43:543–547. doi: 10.1095/biolreprod43.4.543. [DOI] [PubMed] [Google Scholar]
- 9.Erickson GF, Magoffin DA, Garzo VG, Cheung AP, Chang RJ. Granulosa cells of polycystic ovaries: are they normal or abnormal? Hum Reprod. 1992;7:293–299. doi: 10.1093/oxfordjournals.humrep.a137638. [DOI] [PubMed] [Google Scholar]
- 10.Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83:3984–3991. doi: 10.1210/jcem.83.11.5232. [DOI] [PubMed] [Google Scholar]
- 11.Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86:1318–1323. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- 12.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 13.Matikainen T, Perez GI, Zheng TS, Kluzak TR, Rueda BR, Flavell RA, Tilly JL. Caspase-3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology. 2001;142:2468–2480. doi: 10.1210/endo.142.6.8078. [DOI] [PubMed] [Google Scholar]
- 14.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. Embo J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 16.Li J, Kim JM, Liston P, Li M, Miyazaki T, Mackenzie AE, Korneluk RG, Tsang BK. Expression of inhibitor of apoptosis proteins (IAPs) in rat granulosa cells during ovarian follicular development and atresia. Endocrinology. 1998;139:1321–1328. doi: 10.1210/endo.139.3.5850. [DOI] [PubMed] [Google Scholar]
- 17.Johnson AL, Langer JS, Bridgham JT. Survivin as a cell cycle-related and antiapoptotic protein in granulosa cells. Endocrinology. 2002;143:3405–3413. doi: 10.1210/en.2002-220107. [DOI] [PubMed] [Google Scholar]
- 18.Tilly JL, Tilly KI, Kenton ML, Johnson AL. Expression of members of the bcl-2 gene family in the immature rat ovary: equine chorionic gonadotropin-mediated inhibition of granulosa cell apoptosis is associated with decreased bax and constitutive bcl-2 and bcl-xlong messenger ribonucleic acid levels. Endocrinology. 1995;136:232–241. doi: 10.1210/endo.136.1.7828536. [DOI] [PubMed] [Google Scholar]
- 19.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 21.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huppertz B, Frank HG, Kaufmann P. The apoptosis cascade--morphological and immunohistochemical methods for its visualization. Anat Embryol (Berl) 1999;200:1–18. doi: 10.1007/s004290050254. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almahbobi G, Anderiesz C, Hutchinson P, McFarlane JR, Wood C, Trounson AO. Functional integrity of granulosa cells from polycystic ovaries. Clin Endocrinol (Oxf) 1996;44:571–580. doi: 10.1046/j.1365-2265.1996.724545.x. [DOI] [PubMed] [Google Scholar]
- 26.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 27.Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slot KA, de Boer-Brouwer M, Houweling M, Vaandrager AB, Dorrington JH, Teerds KJ. Luteinizing hormone inhibits Fas-induced apoptosis in ovarian surface epithelial cell lines. J Endocrinol. 2006;188:227–239. doi: 10.1677/joe.1.06087. [DOI] [PubMed] [Google Scholar]
- 29.Diao FY, Xu M, Hu Y, Li J, Xu Z, Lin M, Wang L, Zhou Y, Zhou Z, Liu J, Sha J. The molecular characteristics of polycystic ovary syndrome (PCOS) ovary defined by human ovary cDNA microarray. J Mol Endocrinol. 2004;33:59–72. doi: 10.1677/jme.0.0330059. [DOI] [PubMed] [Google Scholar]
- 30.Jansen E, Laven JS, Dommerholt HB, Polman J, van Rijt C, van den Hurk C, Westland J, Mosselman S, Fauser BC. Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol. 2004;18:3050–3063. doi: 10.1210/me.2004-0074. [DOI] [PubMed] [Google Scholar]