Abstract
The IGF-IR is a multifunctional tyrosine kinase receptor involved in several biological processes including cell proliferation, differentiation, DNA repair, and cell survival. In the brain IGF-I plays a critical role during embryonic and early postnatal development. In the mature brain, IGF-I binding sites have been found in different regions of the brain, and multiple reports confirmed a strong neuroprotective action of the IGF-IR against different pro-apoptotic insults. When the IGF-IR signaling system is insufficiently deployed, either by low level of expression in elderly individuals, or by the inhibition associated with inflammatory cytokines, neuronal function and survival could be compromised. The examples of such CNS pathologies include HIV associated dementia, diabetic neuropathies, and Alzheimer’s disease. On the other hand, elevated expression activity of the IGF-IR may support uncontrolled cell proliferation and protection from apoptosis. Probably the best example of the IGF-IR involvement in brain tumors is medulloblastomas in which functional cooperation between viral oncoprotein, JC virus large T-antigen, and IGF-IR has been recently established. Therefore, better understanding of the beneficial and potentially harmful aspects of the IGF-IR can be critical for the development of new clinical regimens against neurodegenerative disorders and brain tumors.
Keywords: IGF-IR, Neuroprotection, Brain Tumors, Review
2. INTRODUCTION: IGF-IR SIGNALING SYSTEM
The insulin-like growth factor I receptor (IGF-IR) is a membrane associated multifunctional tyrosine kinase (TK), which plays a critical role in a number of basic biological events including cell proliferation, differentiation, DNA repair, and protection from apoptosis (1–5). The IGF-IR gene is located on chromosome 15q26 and encodes a single polypeptide of about 70% amino acid homology with insulin receptor (IR) (6). Following post-translational modifications, which include proteolitical cleavage of the pro-receptor and its glycosilation, the mature IGF-IR is a heterotetradimer consisting of two extracellular α subunits, which contain cysteine-reach ligand-binding pocket, and two β subunits with extracellular and transmembrane domains, and cytoplasmatic region containing tyrosine kinase domain and C-terminal domain (7) (Figure 1). There are three natural ligands, IGF-I, IGF-2 and insulin, which are capable of binding and activating the IGF-IR. IGF-I binds the receptor with the highest affinity (KD= 1nM); and the bindings of IGF-2 (KD= 15–20nM) and insulin (KD= 100nM) are much less effective (8, 9). IGF-I is a single chain polypeptide, structurally related to insulin, which is synthesized primarily by the liver in response to growth hormone (GH). IGF-I is produced also locally by a large number of tissues including the brain, in which GH –mediated control of IGF-I synthesis is much less evident (10). IGF-I blood levels range between 10 to 500 ng/ml, and more than 90% of this circulating IGF-I is associated with high affinity IGF binding proteins (IGFBP 1–6), which prolong IGF-I half-life by preventing its proteolysis, and modulate IGF-I availability for the receptors (11). The ligand binding leads to the clusterization and subsequent autophosphorilation of multiple IGF-IR molecules on tyrosine residues in the kinase domain, followed by the phosphorylation of juxtamembrane tyrosines and C-terminal serines (12). This initial activation is followed by the recruitment of multiple signaling molecules, such as insulin-receptor substrates (IRS-1 – IRS-4), Src homology domain C-terminal adaptor family members (Shc) (13), and 14-3-3 proteins (14), which link the activated IGF-IR with diverse intracellular pathways, leading to the activation of DNA replication, DNA repair, and the induction of multiple anti-apoptotic signals (1, 2, 15–17) (Figure 2). From IRS-1 that binds to Tyr 950 in the β-subunit of the IGF-IR, derive both the Ras-MAP and PI-3 kinase-Akt/PKB pathways (18–20). Another substrate of the IGF-IR has been identified in mice with a targeted disruption of the IRS-1 genes, and designated as IRS-2 (21). More recently, IRS-3 (22), and IRS-4 (23) have also been cloned providing an additional interface between the IGF-IR and intracellular signaling molecules. Besides IRS and Shc families, the IGF-IR has other direct substrates including, GRB10 (24), Crk (25), PI-3 kinase (26) Syp phosphatase (27), and C-terminal Src kinase (CSK) (28). Several laboratories have established that one of the most prominent pathways to protect cells from apoptosis via IGF-IR is the recruitment of PI-3 kinase, and subsequent activation of the serine/threonine kinase, Akt/PKB (29–32). What makes the IGF-IR different from other receptors is that at least three PI-3 kinase molecules can be recruited by one activated molecule of the IGF-IR. The PI-3 kinase binds directly to pY1316 residue of the C-terminal domain of the ligand activated IGF-IR (27), and two additional PI3 kinase molecules bind pY608 and pY939 of activated IRS-1 (33). Another unique property of the IGF-IR is that at least four signaling branches may account for IGF-I -mediated protection from apoptosis. Two of which depend on Akt activation, and result in the phosphorylation dependent inactivation of Bad (17, 34–36) and pro-caspase 9 (37). Two other pathways seem to be Akt independent, and involve either the activation of the Ras-MAP kinases pathway, which result in Raf phosphorylation and its translocation to mitochondria (17, 34–36), or NFκB–mediated activation of inhibitors of apoptosis (IAPs) (38). Because of these multiple growth promoting and pro-survival properties, the IGF-IR signaling system gained a lot of attention as a potential neurotrophic factor, and has been intensively studied in the peripheral and central nervous system disorders. Here we review recent literature on the role of IGF-IR signaling system in the CNS, and discuss how our continuously growing knowledge about this powerful receptor could contribute to the battle against neurodegenerative disorders and brain tumors.
Figure 1.

Schematic illustration of the Insulin-like Growth Factor I Receptor (IGF-IR). The mature IGF-IR is a heterotetradimer consisting of two extracellular α subunits, which contain cysteine-reach ligand-binding pocket, and two β subunits with extracellular and transmembrane domains, and cytoplasmatic region containing tyrosine kinase domain and C-terminal domain. The positions of amino acid residues known to be involved in the process of IGF-IR activation are indicated on the left site, and the selected signaling molecules which bind directly to ligand activated IGF-IR are indicated on the right site of the molecule.
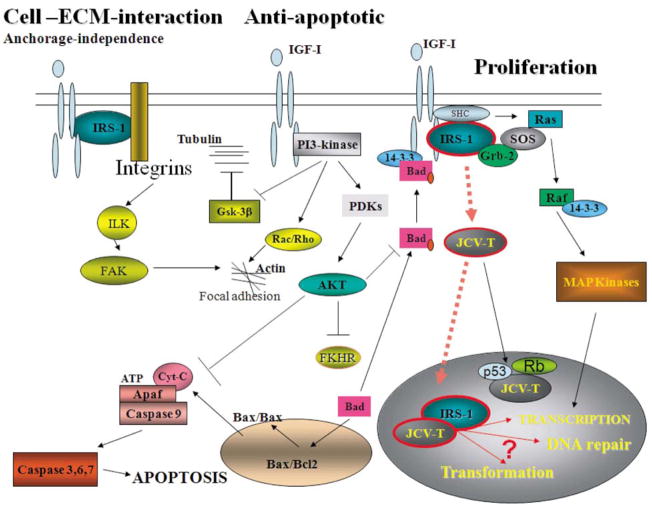
Figure 2.
Selected Signaling Pathways from the IGF-IR. The diagram illustrates some of the signaling connections between cellular proteins recruited by the activated IGF-IR, as well as the interaction with viral oncoprotein from human polyomavirus JC, JCV large T-antigen, which are potentially involved in signaling pathways supporting cell proliferation, protection from apoptosis, interaction with extracellular environment and growth in anchorage-independence. Abbreviations: IGF-I, insulin-like growth factor I; IGF-IR, receptor for IGF-I; IRS-1, insulin receptor substrate 1; PI-3 kinase, phosphatidylinositol kinase; Akt, protein kinase B –plays a multiple role in transducing anti-apoptotic signals; MAP kinases, mitogen activated protein kinases; Ras, Rac, and Rho, small G-proteins - involved in Raf recruitment to the membrane and cytoskeleton reorganization; SOS: son of sevenless - GDP/GTP exchange factor; Grb-2, growth factor receptor-bound protein-2; Raf, serine/threonine kinase – a direct activator of MAP kinases; JCV T-antigen: large T-antigen of human poliovirus JC early genome; PDKs, phosphoinositide-dependent kinase – a direct activators of Akt; FKHR, forkhead transcription factors; Bad, Bax, Bcl2, proteins involved in control of apoptotic process from mitochondria; Apaf: apoptosis protease activating factor – directly involved in caspase 9 activation; Cyt.C: cytochrome C; Rb: retinoblastoma protein; FAK, focal adhesion kinase; Gsk-3β, glucagon synthase kinase beta; ILK, integrin linked kinase.
3. IGF-IR IN THE BRAIN
In the brain IGF-IR and two of its ligands, IGF-I and IGF-2, are highly expressed during embryonic and early postnatal development, but decrease substantially during adolescence and adult life (39). In the mature brain, IGF-I binding sites and IGF-IR mRNA are still found in the choroid plexus, circumventricular areas, and the superior olivary nuclei (39). In the cerebellar cortex, the IGF-IR is expressed in granular and Purkinje neurons, where IGF-I is mitogenic in vitro, and protects neurons from low potassium induced apoptosis (39–41). Since apoptotic death of neuronal cells accompanies degenerative disorders of the CNS, it is not a surprise that the IGF-I signaling system became an important candidate for neuroprotection. Indeed, Gluckman et al. reported the protective effects of IGF-I in ischemic injuries of the CNS (42), and D’Mello et al. found that IGF-I inhibited low potassium-induced apoptosis of cerebellar granule neurons induced by low level of potassium (43). Dudek et al. showed that IGF-I –mediated protection from apoptosis is controlled by PI3-kinase activation of Akt, rather than PI3-kinase activation of p70SK6 kinase in primary cultures of cerebellar neurons (44). These early findings were followed by a myriad of reports providing overwhelming evidence for the role of IGF-I in neuroprotection. For instance, IGF-I protected neurons from oxidative stress (45), high glucose (46), glutamate-induced apoptosis (47), and from TNFα–mediated degeneration of neuronal processes (48). IGF-I has been also shown to protect hippocampal and cortical neurons from N-methyl-D-aspartate (NMDA) -induced and nitric oxide -induced apoptosis (49). A unique aspect of the IGF system in the brain is the presence of a truncated form of IGF-I (des-IGF-I), which lacks the first 3 amino acids. Probably the most relevant biologically feature of this truncation is that des-IGF-I does not bind IGF binding proteins, and therefore could be more active as a local auto- or para-crine regulator of cell proliferation and cell survival in normal and transformed brain cells (50).
Different transgenic animal models overexpressing IGF-I confirmed the importance of the IGF system in CNS. It has been reported for instance that transgenic mice overexpressing the growth hormone grew to larger size than control mice (51), a phenomenon which could be associated with the fact that growth hormone induces expression of IGF-I in the liver, which is the major source of plasma IGF-I. Mathews and coworkers detected that transgenic mice overexpressing IGF-I have an increased brain weight, slightly larger than the increase in total body weight (52). In a transgenic mice, in which IGF-I construct was engineered to be expressed in the adult heart, the overproduction of IGF-I was sufficient to cause an 85% increase in IGF-I plasma levels and a significant increase in brain weight (53).
In contrast, transgenic mice with targeted disruption of the IGF-IR gene (IGF-IR−/−) have reduced brain size and altered brain structures, including reduced myelination due to decreased proliferation and maturation of oligodendrocytes, which associates with the attenuation of axonal growth, and a marked decrease in the density of neural cells mostly observed in the spinal cord and brainstem (54). Our recent immunohistochemical analysis of the IGF-IR−/− embryos indicated significantly higher levels of apoptotic cells detected in the brain and in the dorsal root ganglia, which was accompanied by much lower levels of anti-apoptotic protein, Survivin (Figure 3). In addition to the lower levels of Survivin, the brain and dorsal root ganglia of the knockout mice were smaller and poorly differentiated (Figure 3). This lower degree of differentiation in the IGF-IR−/− embryos correlated with less abundant expression of the neuronal marker, class III β-tubulin, and higher levels of expression of the early marker of neural progenitors, nestin, in comparison to bigger and better differentiated brains from age-matching non-transgenic littermates (Figure 3).
Figure 3.
Immunohistochemical characterization of the IGF-IR knockout embryos. The knockout embryos are smaller in size and volume and show decrease expression of Survivin particularly in the brain, spinal cord and dorsal root ganglia -DRG- (montages). At higher magnification, both the brain and DRG show lower levels of Survivin which correlated with increased number of apoptotic cells. In the knockout mice, a significant number of cells undergoing apoptosis is detected in both the brain and DRG, compared with the wild type mice in which no apoptotic cells were detected by TUNEL assay. In addition to the lower levels of Survivin and its expected effect on cell survival, the brain and DRG of knockout mice is smaller and poorly differentiated. This degree of differentiation correlates with lower levels of Class III β-Tubulin and higher levels of the earlier marker, Nestin, compared to brains of the wild type mice, expressing higher levels of βIII-Tubulin and less nestin, suggesting a higher degree of differentiation.
In a different transgenic model, gene ablation of IGF-I has revealed deficits in neuronal and oligodendrocytic populations in olfactory bulb, dentate gyrus and striatum, and in the cochlear ganglion neurons (55). These major anatomical differences were likely caused by lower rate of cell proliferation, compromised cells survival, and less effective neuronal differentiation caused by the absence of IGF-I during embryonic and postnatal development. In addition to these gross structural brain abnormalities, the IGF-I null mice displayed also changes in metabolic brain function, such as reduced glucose uptake, which is the major source of energy for neurons (56).
Several studies provided evidence that IGF-I and IGF-2 are able to increase the number of neural cells, including neurons, astrocytes, and oligodendrocytes, in vivo and in vitro, mostly by increasing proliferation of their precursors (57–60). IGF-I actions on the cell cycle progression of neural progenitors seem to depend on signaling cooperation with other growth factors. For instance, IGF-I by itself had only minimal effects on DNA replication of oligodendrocyte progenitors; however, in synergy with FGF-2, IGF-I strongly activated G1 – S phase progression and cell proliferation (61). In addition to its mitogenic properties, IGF-I can also direct differentiation process towards neuronal and oligodendrocytic lineages. It has been reported for instance that during development IGF-I promoted differentiation of neural progenitors towards neurons (62), and affected the fate of multipotent adult neural progenitors by directing their differentiation towards oligodendrocytes (63). In other reports, neural progenitors from embryonic and adult mouse striatum, which normally proliferate in response to epidermal growth factor (EGF) (64), differentiated preferentially into the neurons following IGF-I stimulation in a dose dependent manner. Further experiments demonstrated that the observed increase in the number of neurons produced by the IGF-I was not mediated by an increase of cell survival or cell proliferation, but rather depended upon induction of the differentiation program (57, 62).
Taken together, presented results from neuronal cell cultures, clinical samples and from transgenic animal models clearly indicate that impairment of the IGF-IR signaling system compromises the development and maintenance of different neural components in the brain, and may contribute to the pathology of the CNS.
4. IGF-IR IN NEURODEGENERATIVE DISORDERS
4.1. IGF-I - TNFα signaling interplay and HIV associated dementia (HAD)
Despite of the use of highly active antiretroviral therapy (HAART) HIV-1 infection of the brain is associated with various manifestations of neurological disorders ranging from mild cognitive and motor impairments to clear dementia (65–68). The major productive reservoir of HIV-1 infection in the brain are perivascular macrophages and, to a lesser extent, microglia (69, 70). HIV-1 can also infect astrocytes, and there are sporadic reports of HIV-1 detection in oligodendrocytes and neurons, however in these three cell types the infection is not productive (71, 72). Pathological changes associated with the presence of HIV-1 in the brain involve mainly the white matter and are characterized by perivascular cuffs of lymphocytes, parenchymal microglial nodules, multinucleated giant cells, and reactive astrocytes, which define HIV encephalitis (HIVE). Interestingly, pathological changes in the gray matter are less pronounced, and neuronal apoptosis is considered as a rare event. HIV-1 -activated macrophages, microglia and astrocytes have been shown to release proinflammatory cytokines such as tumor necrosis factor-α (TNFα), transforming growth factor–β (TGF-β), monocyte chemo-attractant protein–1 (MCP-1), and interleukin-1β (IL-1β), all of which are suspected of altering neuronal function and survival (66, 73–75). In addition to cytokine -mediated neurotoxicity, HIVE may also involve the failure of neuroprotective mechanisms. In particular, the IGF-IR signaling system, which is known to play a significant role in neuroprotection, may be insufficiently deployed. There are several findings that point to a role for IGF-I in neuro-AIDS. Decreased levels of IGF-I together with growth hormone resistance have been found to be contributing factors in AIDS wasting syndrome (AWS); and the neuronal expression of IGF-I receptor (IGF-IR) appears to be insufficient as a compensatory mechanism to counteract neuronal degeneration (76, 77). In addition, reduced levels of serum IGF-I have been observed in HIV-infected children, especially those with Failure to Thrive (FTT), (78, 79). As IGF-I is a principal mediator of the action of human growth hormone, its role in anabolic effects has prompted studies on IGF-I levels in HIV-infected patients, and the use of both IGF-I and growth hormone in the treatment of cachectic patients (80–83). Although some improvements in body mass have been noted, results of some of these studies suggest partial resistance to growth hormone and IGF-I therapies in the setting of HIV wasting syndrome (78). Decreased levels of IGF-I in the CNS, or alternatively development of IGF-I/insulin resistance may compromise neuronal survival during HIV infection. In this respect, TNFα inhibits some of the known aspects of the IGF-IR and insulin receptor (IR) in diabetes (84, 85), and TNFα has been shown to inhibit IGF-I-stimulated protein synthesis in myoblasts (78), and compromise neuronal survival (48, 86). Therefore, one could speculate that the interaction between TNFα and IGF-I signal transduction pathways may represent an important component, which could determine neuronal fate in the paradigm of HIVE.
4.1.1. IGF-I and TNFα in non-apoptotic neuronal injury
Although the molecular mechanisms involved in IGF-IR–mediated neuro-protection are associated with a strong anti-apoptotic potential of this tyrosine kinase receptor, several reports, including our recent studies suggested that neuronal damage may happen in the absence of apoptosis (87), and that IGF-I could be still protective (48, 86). This includes neuronal damage often observed in the presence of TNFα (75, 88), and possibly, TNFα–mediated serine phosphorylation of IRS-1 (89, 90). The process of IRS-1 phosphorylation on serine residues has been associated with the inactivation of IRS-1 function as a signaling molecule, which when phosphorylated on multiple tyrosine residues amplifies and diversifies the signal from insulin and IGF-I receptors (89, 91). Several serine kinases have been implicated in the process of IRS-1 inactivation. For instance, c-Jun N-terminal kinase (JNK) has been identified to mediate IRS-1 phosphorylation at Ser302 and Ser307 (89, 92). Other studies demonstrated that PKCζ (93), MAP kinases p42/p44 and p38 (94), and PI3-kinase (95, 96) could be also involved, further stressing the importance of IRS-1 phosphorylation during neuronal outgrowth and neuronal injury.
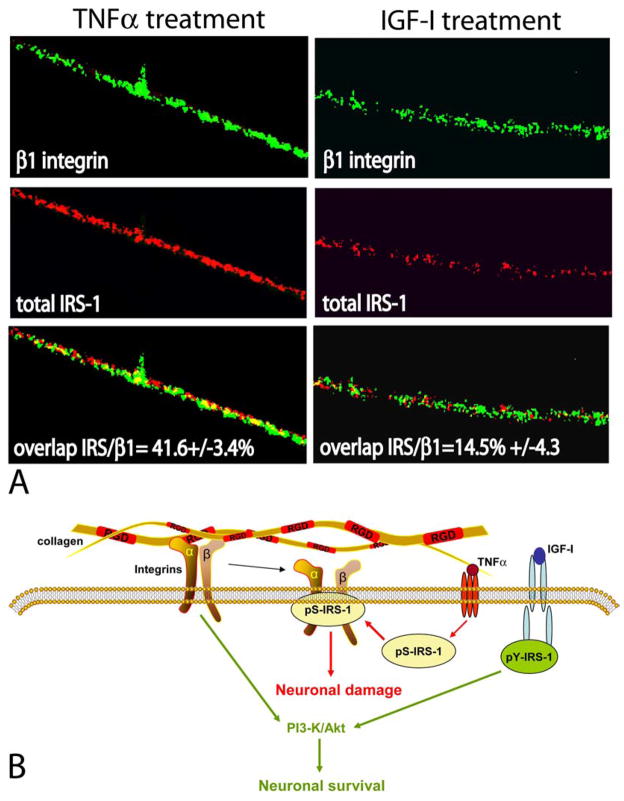
Only a few reports have implicated serine phosphorylation of IRS-1 in the process of TNFα–mediated neuronal damage (90, 97). One such study demonstrated that cerebellar granule neurons lost the ability of responding to IGF-I survival signal when treated with low doses of TNFα. IRS-2 serine phosphorylation, and a loss of signaling connection between IGF-IR and PI3-kinase were suspected for this TNFα–mediated IGF-I resistance. We have demonstrated that differentiated PC12 neurons, which overexpress ectopic IGF-IR, were able to maintain neuronal processes in the presence of TNFα much better than parental PC12 neurons (48). Although initially, we did not observe any significant changes in the phosphorylation status of cytosolic IRS-1 and IRS-2, our results suggested that TNFα–mediated degeneration of neuronal processes was linked with the interaction between IRS-1 and α1β1 integrin (86). Such an interaction has been already proposed by Vuori and Ruoslahti, who first demonstrated the binding between IRS-1 and αvβ3 integrin in cells cultured on vitronectin (98). More recently, reciprocal interactions between the insulin receptor - IRS-1 signaling axis and α5β1-integrin showed both the improvement of insulin-mediated attachment to fibronectin, and increased IRS-1 phosphorylation (99). In addition, extensive studies on cell adhesion and motility of LNCaP prostate cancer cells demonstrated the formation of IRS-1–α5β1 integrin complex under conditions in which IRS-1 is predominantly phosphorylated on serine residues (33). The association between β1-integrin and IGF-IR has also been demonstrated in myeloma cells (100), and several other reports confirmed the presence of a functional cross-talk between integrins and the IGF-IR signaling system (101–103). Finally, our recent study demonstrates an inverse relationship between neuronal stability and TNFα–mediated serine phosphorylation of IRS-1, and subsequent binding of pS-IRS-1 to α1β1-integrin in the membrane rafts of differentiated PC12 neurons (104) (Figure 4).
Figure 4.
Functional implications of the pS-IRS-1 -β 1-integrin membrane complex. Panel A: Double immunolabeling of IRS-1 and β1-integrin in differentiated PC12 neurons. Fluorescent images were collected from inverted fluorescent microscope equipped with motorized Z-axis and deconvolution software (SlideBook4). Anti-β1-integrin mouse monoclonal antibody and anti-mouse FITC-conjugated secondary antibody (green fluorescence), as well as anti-IRS-1, rabbit polyclonal antibody, and anti-rabbit rhodamine-conjugated secondary antibody (red fluorescence), were utilized. Note the presence of a strong co-localization between IRS-1 and β1-integrin detected in neuronal processes after TNFα treatment and much less of the co-localization after IGF-I treatment (yellow fluorescence). The percentage of co-localization between IRS-1 and β1-integrin is indicated with standard deviation (n=3). Original magnification x1000. Panel B: The pS-IRS-1 – β1-integrin complex formation in membrane rafts of differentiated neuronal cells impairs the binding between integrins and collagen and is thought to compromise stability of neuronal processes. This protein – protein interaction is facilitated by TNFα which triggers accumulation of pS-IRS-1 in membrane rafts and is attenuated by IGF-I, which facilitates tyrosine phosphorylation of IRS-1, impairs formation of the complex and supports neuronal survival.
4.1.2. Functional interplay between TNFα, IGF-I and the family of a disintegtin and metalloproteases (ADAMs) in neuronal injury and regeneration
Activation of proinflammatory cytokines including TNFα and their effects on nervous system have been repeatedly demonstrated in vitro (48, 86, 105, 106), and in neurodegenerative disorders such as HAD (75, 107, 108), Multiple Sclerosis, Alzheimer’s disease, and neuronal injuries that develop after ischemia and type 2 diabetes (105, 109–111). TNFα is synthesized as a trimeric type II membrane-associated precursor (112). Upon cleavage, the mature form of TNFα is released from the cell surface and can either enter the blood stream or can stay in the extracellular environment (113). Recently, a considerable effort has been made to identify the TNFα convertase/sheddase. ADAM17 (a disintegrin and metalloproteinase 17, also referred as TNFα converting enzyme or TACE) is presently considered as the major sheddase for TNFα (114, 115). A targeted deletion of ADAM17 in transgenic mice confirmed its critical role in the shedding of TNFα (116). In addition, other members of the family, ADAM9 and ADAM10, which are abundantly expressed in the nervous system, have been also confirmed as efficient TNFα convertases (117). These neuronal ADAMs, are also responsible for cleavage –mediated activation of Notch and its ligand Delta (117–120); cleavage of collagen IV, and amyloid precursor protein (APP) - decreasing formation of the toxic β-amyloid (117); and, what is closely related with the topic of this review, tissue release of IGF-I by cleaving IGF-I binding proteins (121, 122). Importantly, ADAM10 is also known to participate in axonal outgrowth, and when eliminated by a single knockout, leads to severe developmental changes including underdevelopment of the CNS (117).
4.1.3. Contribution of HIV and diabetes to the development of HAD
Deregulation of glucose homeostasis has been recently considered as a serious risk factor for cognitive impairment among HIV –infected individuals (123, 124). This impairment of the mental status became even more important in view of recent findings that protease inhibitors, which are the major components of highly active antiretroviral therapy (HAART), may cause glucose intolerance and often lead to the development of type 2 diabetes mellitus (124, 125). Two potential mechanisms are associated with this unexpected metabolic impairment: (i) direct interaction between protease inhibitors and glucose transporter GLUT4 (126); and (ii) interference with cellular retinoic acid binding protein type I (CRABP-I), which in normal circumstances is expected to support PPARγ–mediated downregulation of free fatty acids and TNFα (127, 128). As a result of this HIV -associated metabolic abnormality, insulin resistance and type-2 diabetes may develop, leading to weight loss, atypical fat distribution (125), and later to serious organ damage, which includes eyes, kidneys, peripheral nervous system and the brain (129). The slowly progressing alterations in cerebral function and structure that occur in diabetes are often referred as diabetic encephalopathy. The clinical manifestation of diabetic encephalopathy includes changes in cognitive function including moderate impairment of verbal memory and mental speed (130). These pathologic changes are very similar to those observed in HIV associated dementia; and in fact, the incidence of dementia doubles in elderly individuals who are both diabetic and HIV positive (124, 131).
4.1.4. IGF-IR and reactive oxygen species (ROS)
Hyperglycemia and diabetes mellitus are associated with the increase in reactive oxygen species (ROS) production at the cellular level (132, 133), which plays an important role in the development of diabetic complications (134, 135). ROS dependent signals have been also linked to defects in genomic maintenance and accelerated aging (136). We have shown recently a strong IGF-IR anti-oxidant phenotype in mesangial cells maintained at high glucose concentration (132, 137). This novel IGF-IR function was closely coupled with the improvement of cell survival. These observations are in agreement with the studies in which longevity in mice was directly linked to increased resistance to oxidative stress, in which the adaptor protein p66Shc, one of the IGF-IR signaling molecules, have emerged as major genetic determinants of longevity and oxidative stress, in mammals (138, 139). In this respect, our preliminary experiments indicate that both high glucose (25mM for 16 hours) and prolonged exposure to TNFα (50nM for 3–5 days) elevated the accumulation of ROS in differentiated neuronal cultures of PC12 cells. The accumulated ROS were found in neuronal processes and in perinuclear cytoplasm, and paralleled with gradual loss of neuronal processes. In the presence of IGF-I, however, differentiated PC12 neurons did not accumulate ROS above the control levels, and were partially protected from TNFα–mediated retraction of neuronal processes. On the other hand, multiple reports in the literature strongly indicate that IGF-IR is associated with accelerated aging, and that reduced IGF-I expression promotes longevity (139–142). In contrast to the enhanced longevity, age related decrease in IGF-I has been associated with increased risk of cardiovascular disease (143, 144), premature arteriosclerosis and diabetes (145), as well as neurodegenerative disorders (146–148).
Our search for a potential mechanism involved in this IGF-IR –mediated antioxidant function pointed to the p66Shc – FOXO3a signaling axis. This is in a strong agreement with multiple reports, which support a dominant role for wild type p66Shc protein in the intracellular pathways that are involved in generation of mitochondrial ROS, and the conversion of oxidative stress to apoptosis (149–151). Recently, better understanding of p66Shc has emerged, based on evidence that the p66Shc protein functionally interacts with the mammalian Forkhead homolog, FOXO3a (150). In the proposed mechanism, ROS induced phosphorylation of p66Shc at a critical SH2 Ser-36 residue, leads to the phosphorylation dependent inactivation of FOXO3a, and associated transcriptional repression of one of the most prominent anti-ROS enzymes, manganese superoxide dismutase (MnSOD) (152, 153). Therefore, answer to the question, how activated IGF-IR affects functional interplay between FOXO3a and p66shc could be very helpful in clarifying our understanding of the complicated relationship between IGF-IR signaling system, ROS metabolism, and aging.
4.2. IGF-I and Alzheimer’s disease (AD)
A strong line of experimental evidence supports the role of IGF-I in neuronal outgrowth, survival and differentiation (154–156), and in modulation of neuronal excitability and synaptic plasticity (157, 158). Therefore, it is conceivable that IGF-IR signaling pathways may be dysregulated, or inefficiently deployed in neurodegenerative disorders. AD is a progressive neurodegenerative disease associated with cognitive and behavioral dysfunction, occurring predominantly later in life, and characterized by cell loss with increased activation of the pro-apoptotic genes and signaling pathways, impaired energy metabolism, mitochondrial dysfunction, and chronic oxidative stress (159, 160). Neurofibrillary tangles and senile plaque accumulation in the hippocampus and neocortex are the main pathological features of AD. Senile plaques are manly build by amyloid β-peptide (Aβ), which is formed by γ-secretase –mediated cleavage of the amyloid precursor protein (APP) (161, 162). Although most of the secreted fragments of APP have positive effects on cell survival, neuronal outgrowth, and synaptic plasticity (163); the accumulation of Aβ in the brain is associated with neuronal damage, and represents a high risk factor in the development of AD (164).
A considerable line of evidence supports the involvement of abnormal IGF-I and insulin function in Aβ deposition (155). It has been demonstrated for instance that IGF-I and insulin increase the level of APP through the activation of PI3-K signaling pathway (165), and that IGF-I promotes the cleavage of APP through the stimulation of α-secretases, and in this way could contribute to the reduction of the harmful Aβ (166). In addition, IGF-I appears to stimulate elimination of brain amyloids through a complex process that includes stimulation of Aβ release and its subsequent clearance from the brain parenchyma (166).
In AD transgenic mouse model, IGF-I was demonstrated to induce clearance of Aβ from the brain by upregulating levels of transport proteins such as albumin and transthyretin (166); and it has been suggested that disrupted IGF-I signaling in cells involved in blood brain barrier (BBB) may contribute to amyloidosis (167). In this process, choroid plexum epithelium has been shown to represent the primary target of IGF-I-mediated clearance of brain Aβ, and disruption of the IGF-IR signaling in the choroid plexus was sufficient to trigger pathological changes similar to those founded in AD brains including brain amyloidosis and Tau-hyperphosphorilation (168). These observations have been indirectly supported by the fact that the choroid plexus in AD patients shows amyloid deposits and tangles (169).
As a consequence of the reduced IGF-I signaling at the BBB, brain Aβ clearance is diminished. Because Aβ has been also shown to antagonize insulin and IGF-I receptor binding in neurons (170), even more severe deficiencies in the IGF-I and insulin signaling may develop, further compromising neuronal survival and function. Therefore, the loss of sensitivity to IGF-I, and partial BBB dysfunction, which occur during normal aging, can contribute to the development of AD amyloidosis (171). This hypothesis was supported by the observation that AD patients have lower levels of transthyretin, an amyloid carrier in cerebro-spinal fluid (CSF) compared to normal aged individuals (172).
Although all these findings strongly suggest the involvement of IGF-I-resistance at the BBB as a pathogenic event in AD, a direct cause of this resistance remains to be clarified. In this respect, alterations in IGF-BPs, and activated cytokine linked to inflammatory responses at the BBB, could attenuate IGF-I signaling in this critically important compartment of the brain (173–175).
The impairment of IGF-I signaling system observed in the brain of AD patients has been also associated with the pathological development of neurofibrillary tangles (NFT), which contain microtubule-associated protein, Tau, in the form of filamentous aggregates. The Tau was originally discovered as a heat stable protein that facilitates assembly of the microtubules in vitro (176). Further studies demonstrated that Tau phosphorylation is a physiological process required for cytoskeleton assembly and stabilization. The physiological roles of Tau involve regulation of neurite extension (177), axonal transport, and microtubules stability and dynamics (178, 179). In neurodegenerative diseases in which Tau pathology has been observed, Tau is hyper-phosphorylated (180), and this aberrant phosphorylation characterized Tau proteins isolated from AD brains (181).
The kinases, which are responsible for physiological phosphorylation of Tau include Erks, and cyclin dependent kinase 5 (Cdk-5), both of which are known to be activated by IGF-I and insulin (182–184). On the other hand, impaired insulin or IGF-I signaling may result in hyper-phosphorylation of Tau due to the reduced activation of PI3–kinase/Akt signaling axis, which results in the release of the inhibition of multifunctional serine/threonine kinase GSK3β. Unlike other protein kinases, GSK3β is normally constitutively active, and is primarily regulated through the phosphorylation dependent inactivation (185). IGF-I and insulin have been shown to mediate this phosphorylation dependent GSK3β inactivation (186, 187), and possibly contribute to the attenuation of Tau hyper-phosphorylation.
Although the mechanisms of increased GSK3β activation in AD can be readily explained on the basis of impaired IGF-I/insulin signaling, coexisting increased levels of Erks (188), Akt (189, 190) and Cdk-5 (191) found in AD brains should compensate for this abnormal Tau phosphorylation. However, GSK3β can also be activated in response to the accumulation of oxidative stress (192, 193), which is commonly seen in the brains of AD patients (194, 195). Therefore, impaired IGF-I/insulin signaling systems can synergize with oxidative stress in the process of GSK3β activation, leading to the aberrant Tau phosphorylation, and the formation of neurofibrillary tangles. Importantly, the potential role of attenuated IGF-I responses in AD is supported by the evidence that IGF-I treatment resulted in enhanced cognitive performance, increased levels of synaptic proteins, and reduced astrogliosis associated with Aβ plaques (196). In addition, Mini-Mental State Examination scores and serum levels of IGF-I in AD have been evaluated, and show that lower levels of serum IGF-I correlate well with cognitive impairments (146).
5. IGF-IR AND BRAIN TUMORS
5.1. IGF-I in cellular transformation
The relevance of IGF-IR in normal growth and development has been best demonstrated by the studies in mice with targeted disruption of the IGF-IR gene (54). These mice are 45% of the size of wild type littermates at birth, and die shortly due to severe organ hypoplasia (54). In vitro, mouse embryo fibroblasts (MEF) derived from the IGF-IR (−/−) embryos (R- cells) have been shown to grow more slowly than wild-type fibroblasts in monolayer cultures, and were unable to proliferate under anchorage-independent conditions (197). Cell growth in anchorage-independence indicates transformation in vitro and tumorigenicity in vivo; and interestingly, R- cells demonstrated unusual resistance to cellular transformation by a number of oncogenic proteins, including SV40 large T-antigen, activated RAS, overexpression of epidermal growth factor receptor (EGFR) (198), and by human polyomavirus JC large T-antigen (199).
In close relation to these multiple observations, downregulation of the IGF-IR expression (200), or inhibition of its function (201, 202), results in the reversion of the transformed phenotype and massive apoptosis, especially when the cells were cultured in anchorage-independence (202). Accordingly, the effects of IGF-IR inhibition were much more pronounced against metastatic cancer in comparison to the inhibition of the primary tumor growth (203, 204). Similarly, downregulation of the IGF-IR had only modest effects on cell growth in monolayer. The obtained growth inhibition ranged between 10 and 15%, suggesting that the IGF-IR is not an absolute requirement for cell growth in monolayer; however its function in supporting anchorage-independent growth and survival are much more apparent (202, 205, 206).
IGF-IR activation or overexpression is associated with an increased propensity for invasion and metastasis by inducing tyrosine phosphorylation of IRS-1 that can influence the interaction between E-cadherin and β-catenin, enhancing β-catenin transcriptional activity and disconnecting E-cadherin from the actin cytoskeleton (207). The loss of E-cadherin expression or function is well recognized as causing disruption of cell–cell contacts and release of invasive tumor cells from primary epithelial tumors (208). Similarly, tumor cell motility and invasive potential are influenced by the crosstalk between the IGF-IR signaling axis and integrins (209), and by IGF-induced secretion of matrix metalloproteinases (210). The importance of these findings was supported by the experiments in which overexpressed IGF-IR conferred metastatic phenotype in a murine model of pancreatic cancer (211), and by the studies in murine hemopoietic and human prostate cancer cells that express low levels of the IGF-IR and lack IRS-1 (212, 213).
5.2. IGF-IR in medulloblastoma
Although brain tumors secrete IGF-I and IGF-2, and the activity of IGF-IR signaling system has been detected in glioblastomas (107–109), neuroblastoms (58), astrocytomas and meningiomas (1), the most compelling evidence of a direct involvement of the IGF-IR comes from medulloblastomas (214–218), which are malignant cerebellar tumors of the childhood. Medulloblastomas represent nearly 25% of all pediatric intracranial neoplasms. These highly malignant tumors arise from the cerebellum and affect mainly children between ages five and fifteen. Although the etiology of medulloblastomas has not been fully elucidated, several reports show that the IGF-IR signaling system is highly active in medulloblastoma cell lines (214, 215), in medulloblastoma animal models (215, 217–220) and in medulloblastoma clinical samples (199, 216). First, Kurihara et al. detected IGF-I binding sites in one medulloblastoma specimen (221), and later, the presence of IGF-IR protein and its transcript have been confirmed by Western blot and RT-PCR, respectively (214). The results from our laboratory illustrated that medulloblastoma cell lines and over twenty medulloblastoma biopsies were characterized by an abundant presence of the IGF-IR, and its major signaling molecule, IRS-1 (215, 216 and Figure 5). Medulloblastoma cell lines and medulloblastoma biopsies were strongly positive when immunolabeled with anti-pY1316-IGF-IR antibody, which recognizes phosphorylated (active) form of the IGF-IR (222). In addition, growth and survival of medulloblastoma cell lines cultured in anchorage-independence were strongly dependent on the presence of exogenous IGF-I (215, 216). In this condition, different strategies aiming against the IGF-IR function were very efficient in eliminating medulloblastoma cells in vitro and in experimental animals (215, 217, 223). Other examples of the IGF-IR involvement include strong synergy between Sonic hedgehog/Patched and the IGF-IR signaling systems, which resulted in a significant increase in medulloblastoma tumor formation in RCAS/tv-a transgenic mice (218); and the detection of nuclear IRS-1 in medulloblastoma cell lines and medulloblastoma biopsies positive for the human polyomavirus JC (224).
Figure 5.
Expression of IGF-1 and IRS-1 in Medulloblastomas. Immunohistochemical experiments demonstrate the presence of IGF-IR in the cytoplasm with a membrane-associated pattern in both T-Antigen positive and T-Antigen negative human medulloblastoma samples. The IGF-IR docking molecule, IRS-1 is expressed in the cytoplasm of neoplastic cells in T-Antigen negative cases; however it is prominently nuclear in T-Antigen expressing tumor cells. Original magnification x1000.
Growing evidence is accumulating in support of a viral component as a potential etiologic factor in medulloblastomas (216, 225). In this respect, human neurotropic polyomavirus JC encodes within its early genome a regulatory protein, JC T-antigen. JC T-antigen beside its critical role in the viral cycle has transforming properties in vitro (226, 227) and is tumorigenic in experimental animals (228–230). Recent studies revealed association of JCV genome with spontaneous medulloblastomas in humans, and the detection of JCV T-antigen in human tumor cells (216, 231). This may raise serious epidemiological concerns, since more than 80% of human population becomes asymptomatically infected by this ubiquitous polyomavirus (232). Since the presence of IGF-IR has been repeatedly shown as a strong requirement for T-antigen –mediated cellular transformation (233), it encouraged investigations of a potential functional interaction between the IGF-IR system and JCV T-antigen in the paradigm of medulloblastoma. Several important findings have been established in the course of these studies: (i) insulin receptor substrate 1 (IRS-1), which is the major signaling molecule for the IGF-IR, translocates to the nucleus in the presence of the JC T-antigen (224); (ii) nuclear IRS-1 was detected in T-antigen positive medulloblastoma cell lines, and in T-antigen positive biopsies from patients diagnosed with medulloblastoma (224 and Figure 5); (iii) IRS-1 domain responsible for a direct JC T-antigen binding was localized within the N-terminal portion of IRS-1 molecule, and the binding was independent from IRS-1 tyrosine phosphorylation and was strongly inhibited by IRS-1 serine phosphorylation (224); (iv) competition for the IRS-1 – T-antigen binding by a dominant negative mutant of IRS-1 (PH/PTB domain), inhibited growth and survival of JC T-antigen –transformed cells in anchorage-independent culture conditions (224). These multiple finding suggest that not only IGF-IR, but also its major signaling partner, IRS-1, can be considered as a potential therapeutic target especially in those medulloblastoma cases which express viral oncoprotein, JCV T-antigen.
5.3. IGF-IR in DNA repair and genomic integrity
During transformation, an increase in the cell’s propensity to accumulate mutations may develop as a result of dysregulation of normal mechanisms controlling faithful and unfaithful DNA repair. Scattered reports indicate that the IGF-IR may have functions affecting DNA repair. These include enhanced radioresistance proportional to the IGF-IR protein level in both mouse embryo fibroblasts and breast tumor cells (234), enhanced DNA repair via the IGF-I activated p38 signaling pathway in response to UV-mediated DNA damage (235, 236), and delayed UVB-induced apoptosis via IGF-I-mediated activation of Akt, resulting in enhanced repair of DNA cyclobutane thymidine dimers in keratinocytes (237). In contrast, one report notes a delay in DNA repair of potentially lethal radiation damage observed following IGF-I and insulin treatments of A549 cells (238).
Another demonstration of a potential involvement of the IGF-IR in DNA repair has been provided by experimental studies focused on the effects of IGF-I stimulation on DNA repair of double-strand breaks (DSBs). DSBs are usually formed after cell exposure to ionizing radiation, endogenous free radicals, anticancer drugs including cisplatin and mitomycin C, and can be inflicted spontaneously during DNA replication, when replication forks encounter other DNA lesions, including single strand breaks and intra-strand cross-links (239–242). Importantly, even a small number of DSBs can initiate a strong pro-apoptotic signal when damaged DNA is left unrepaired. Therefore, in addition to anti-apoptotic pathways, cell survival relies also on the efficiency of DNA repair (243). Early events in the detection of DSBs include activation of protein kinases ATM (ataxia telangiectasia mutated), ATR (ATM -related), and DNA-PK, which all have been shown to phosphorylate histone H2AX (γ-H2AX) within mega-base pair regions surrounding DSBs, “attracting” different components of the DNA repair machinery (244, 245). To prevent DNA damage -induced apoptosis, DNA breaks must be repaired. In cells replicating DNA, homologous recombination DNA repair (HRR) seems to predominate, while quiescent cells utilize non-homologous end joining NHEJ (240). The choice between DNA repair mechanisms can be controlled, at least partially, by the availability of DNA template. Cells that proliferate have an advantage of using newly synthesized template supplied in the process of DNA synthesis. Cells arrested in G1 could potentially utilize HRR; however the homology search is much more complicated and requires the access to the template on the homologous chromosome. Alternatively, quiescent cells may simply link DNA ends without any template using DNA binding Ku70/Ku80 complex and DNA-PK, followed by DNA ligation with XRCC4-ligase 4 (246, 247). This quick fix, however, may cost the cell a gain or loss of several base pairs. Therefore DNA repair by NHEJ can be considered as an error prone mechanism, which may lead to the accumulation of spontaneous mutations after DNA damage (240–242).
The major enzymatic component of HRR in eukaryotic cells is Rad51. Rad51 is a structural and functional homologue of bacterial RecA recombinase (248–250). Following detection of DSBs, the breast cancer susceptibility gene product (BRCA2) is suspected to mediate translocation of Rad51 to the sites of damaged DNA (251, 252). In parallel, the ATM -activated 5′-3′ endonuclease complex (Rad50 - MRE11 - NBS1) exposes both 3′ ends of DNA at the break (253, 254). The ends are initially protected by the ssDNA binding protein RPA, which is subsequently replaced by Rad51 in a process that involves the initial binding of Rad52 into the ssDNA – RPA complex (255, 256). As a result, newly formed Rad51 nucleoprotein filaments are directly involved in homology search and strand invasion, which in eukaryotic cells require ATPase activity from Rad54 (248, 257, 258).
Our recent studies indicate that activated IGF-IR enhances HRR, by a mechanism that controls translocation of Rad51 to the sites of damaged DNA (nuclear foci) (1). This effect involves a direct cytosolic interaction between Rad51 and IRS-1. The binding is direct, occurs within the perinuclear region of the cell, involves the N-terminal portion of IRS-1, and was attenuated by IGF-I –mediated IRS-1 tyrosine phosphorylation. Importantly, cells with low levels of the IGF-IR, or cells expressing IGF-IR mutants that fail to phosphorylate IRS-1, retain Rad51 within the perinuclear compartment, and show significantly less DNA repair by HRR (1). These results indicate a novel function for the IGF-IR/IRS-1 signaling axis, which involves intracellular trafficking of Rad51 from cytosol to the sites of damaged DNA - a crucial step in the process of DNA repair by homologous recombination.
5.4. Nuclear translocation of IRS-1 and cancer
IRS-1 has been considered for a long time as a typical cytosolic protein implicated in insulin receptor and IGF-I receptor signaling (259, 260). Beside its metabolic and growth promoting function, IRS-1 has been also suspected to play a role in malignant transformation, presumably by amplifying the IGF-IR signal. The first convincing evidence indicating transforming potential of IRS-1 was demonstrated in R− cells, which are mouse embryo fibroblasts from mice with targeted disruption of the IGF-IR gene. The R- cells, which are resistant to SV40 T-antigen –mediated transformation (261), acquired transformed phenotype following co-transfection with IRS-1 and T-antigen containing expression vectors (261, 262). In a similar manner, T-antigen from human polyomavirus JC (JCV T-antigen) also required the presence of IGF-IR/IRS-1 signaling axis for transformation (199). Interestingly, we have found nuclear IRS-1 in cells expressing JCV T-antigen (224), and in JCV T-antigen positive medulloblastoma clinical samples (199, 216, 224 and Figure 5). This first demonstration of nuclear IRS-1 was further confirmed by others. For instance, nuclear IRS-1 have been found in cells transfected with v-src, SV40 T-antigen, in fibroblasts stimulated with IGF-I (263, 264), in hepatocytes (265), in 32D cells (266), and in osteoblasts (267). In other reports, nuclear IRS-1 was detected in breast cancer cells in association with estrogen receptor alpha (ERα) (268, 269). Although the mechanism by which IRS-1 is translocated to the nucleus is not well established, it could utilize its own putative nuclear localization signal (263). On the other hand, abundant presence of nuclear IRS-1 in cells expressing proteins with high affinity to the nucleus, such as JCV T-antigen (224), SV40 T-antigen (264), and ERα (268) may indicate that IRS-1 requires association with other proteins for its efficient nuclear translocation. Biological relevance of nuclear IRS-1 has been studied quite extensively during last several years. In fibroblasts stimulated with IGF-I, nuclear IRS-1 has been found in association with upstream binding factor (UBF1 - regulator of RNA polymerase I), which coincided with increased rRNA synthesis (270). In addition, a direct connection between IRS-1 and homologous recombination dependent DNA repair (HRR) have been recently proposed (1). This new signaling interplay involves peri-nuclear binding between hypo-phosphorylated IRS-1 and the major enzymatic component of HRR, Rad51. IGF-I–mediated IRS-1 tyrosine phosphorylation attenuated IRS-1 binding to Rad51 allowing efficient translocation of Rad51 to the sites of damaged DNA, which lead to the increased contribution of HRR in DNA repair of double strand breaks. In JCV T-antigen positive cell however, IRS-1 translocates to the nucleus, where it has been found in the complex with Rad51 at the sites of damaged DNA (271, 272). Importantly, the presence of nuclear IRS-1-Rad51 complexes was strongly associated with the inhibition of HRR and with the increased incidence of spontaneous mutations in cells expressing JCV T-antigen (271).
Acknowledgments
We would like to acknowledge Jessica Otte for her technical assistance. This work was supported by grants from NIH: RO1CA095518 to KR, RO1 MH71162 to FP, and PO1 NS36466 to KK, LDV, and KR.
References
- 1.Trojanek J, Ho T, Del Valle L, Nowicki M, Wang JY, Lassak A, Peruzzi F, Khalili K, Skorski T, Reiss K. Role of the insulin-like growth factor I/insulin receptor substrate 1 axis in Rad51 trafficking and DNA repair by homologous recombination. Mol Cell Biol. 2003;23:7510–24. doi: 10.1128/MCB.23.21.7510-7524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiss K, Valentinis B, Tu X, Xu SQ, Baserga R. Molecular markers of IGF-I-mediated mitogenesis. Exp Cell Res. 1998;242:361–72. doi: 10.1006/excr.1998.4113. [DOI] [PubMed] [Google Scholar]
- 3.Baserga R, Sell C, Porcu P, Rubini M. The role of the IGF-I receptor in the growth and transformation of mammalian cells. Cell Prolif. 1994;27:63–71. doi: 10.1111/j.1365-2184.1994.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 4.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55:249–52. [PubMed] [Google Scholar]
- 5.LeRoith D, Baserga R, Helman L, Roberts CT., Jr Insulin-like growth factors and cancer. Ann Intern Med. 1995;122:54–9. doi: 10.7326/0003-4819-122-1-199501010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. Embo J. 1986;5:2503–12. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward CW, Garrett TP. Structural relationships between the insulin receptor and epidermal growth factor receptor families and other proteins. Curr Opin Drug Discov Devel. 2004;7:630–8. [PubMed] [Google Scholar]
- 8.Schumacher R, Mosthaf L, Schlessinger J, Brandenburg D, Ullrich A. Insulin and insulin-like growth factor-1 binding specificity is determined by distinct regions of their cognate receptors. J Biol Chem. 1991;266:19288–95. [PubMed] [Google Scholar]
- 9.Germain-Lee EL, Janicot M, Lammers R, Ullrich A, Casella SJ. Expression of a type I insulin-like growth factor receptor with low affinity for insulin-like growth factor II. Biochem J. 1992;281(Pt 2):413–7. doi: 10.1042/bj2810413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Roith D, Scavo L, Butler A. What is the role of circulating IGF-I? Trends Endocrinol Metab. 2001;12:48–52. doi: 10.1016/s1043-2760(00)00349-0. [DOI] [PubMed] [Google Scholar]
- 11.Duan C. Specifying the cellular responses to IGF signals: roles of IGF-binding proteins. J Endocrinol. 2002;175:41–54. doi: 10.1677/joe.0.1750041. [DOI] [PubMed] [Google Scholar]
- 12.Favelyukis S, Till JH, Hubbard SR, Miller WT. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat Struct Biol. 2001;8:1058–63. doi: 10.1038/nsb721. [DOI] [PubMed] [Google Scholar]
- 13.White MF. The IRS-1 signaling system. Curr Opin Genet Dev. 1994;4:47–54. doi: 10.1016/0959-437x(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 14.Furlanetto RW, Dey BR, Lopaczynski W, Nissley SP. 14-3-3 proteins interact with the insulin-like growth factor receptor but not the insulin receptor. Biochem J. 1997;327:765–71. doi: 10.1042/bj3270765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baserga R, Porcu P, Rubini M, Sell C. Cell cycle control by the IGF-1 receptor and its ligands. Adv Exp Med Biol. 1993;343:105–12. doi: 10.1007/978-1-4615-2988-0_11. [DOI] [PubMed] [Google Scholar]
- 16.Baserga R, Resnicoff M, D’Ambrosio C, Valentinis B. The role of the IGF-I receptor in apoptosis. Vitam Horm. 1997;53:65–98. doi: 10.1016/s0083-6729(08)60704-9. [DOI] [PubMed] [Google Scholar]
- 17.Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, Calabretta B, Baserga R. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol. 1999;19:7203–15. doi: 10.1128/mcb.19.10.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers MG, Jr, White MF. The new elements of insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes. 1993;42:643–50. doi: 10.2337/diab.42.5.643. [DOI] [PubMed] [Google Scholar]
- 19.Myers MG, Jr, Sun XJ, White MF. The IRS-1 signaling system. Trends Biochem Sci. 1994;19:289–93. doi: 10.1016/0968-0004(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 20.White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl 2):S2–17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 21.Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, 3rd, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–90. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 22.Lavan BE, Lane WS, Lienhard GE. The 60-kDa phosphotyrosine protein in insulin-treated adipocytes is a new member of the insulin receptor substrate family. J Biol Chem. 1997;272:11439–43. doi: 10.1074/jbc.272.17.11439. [DOI] [PubMed] [Google Scholar]
- 23.Fantin VR, Sparling JD, Slot JW, Keller SR, Lienhard GE, Lavan BE. Characterization of insulin receptor substrate 4 in human embryonic kidney 293 cells. J Biol Chem. 1998;273:10726–32. doi: 10.1074/jbc.273.17.10726. [DOI] [PubMed] [Google Scholar]
- 24.Morrione A, Valentinis B, Li S, Ooi JY, Margolis B, Baserga R. Grb10: A new substrate of the insulin-like growth factor I receptor. Cancer Res. 1996;56:3165–7. [PubMed] [Google Scholar]
- 25.Beitner-Johnson D, V, Blakesley A, Shen-Orr Z, Jimenez M, Stannard B, Wang LM, Pierce J, LeRoith D. The proto-oncogene product c-Crk associates with insulin receptor substrate-1 and 4PS. Modulation by insulin growth factor-I (IGF) and enhanced IGF-I signaling. J Biol Chem. 1996;271:9287–90. doi: 10.1074/jbc.271.16.9287. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto K, Altschuler D, Wood E, Horlick K, Jacobs S, Lapetina EG. Association of phosphorylated insulin-like growth factor-I receptor with the SH2 domains of phosphatidylinositol 3-kinase p85. J Biol Chem. 1992;267:11337–43. [PubMed] [Google Scholar]
- 27.Seely BL, Reichart DR, Staubs PA, Jhun BH, Hsu D, Maegawa H, Milarski KL, Saltiel AR, Olefsky JM. Localization of the insulin-like growth factor I receptor binding sites for the SH2 domain proteins p85, Syp, and GTPase activating protein. J Biol Chem. 1995;270:19151–7. doi: 10.1074/jbc.270.32.19151. [DOI] [PubMed] [Google Scholar]
- 28.Arbet-Engels C, Tartare-Deckert S, Eckhart W. C-terminal Src kinase associates with ligand-stimulated insulin-like growth factor-I receptor. J Biol Chem. 1999;274:5422–8. doi: 10.1074/jbc.274.9.5422. [DOI] [PubMed] [Google Scholar]
- 29.Carson JP, Kulik G, Weber MJ. Antiapoptotic signaling in LNCaP prostate cancer cells: a survival signaling pathway independent of phosphatidylinositol 3′-kinase and Akt/protein kinase B. Cancer Res. 1999;59:1449–53. [PubMed] [Google Scholar]
- 30.Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majewski M, Nieborowska-Skorska M, Salomoni P, Slupianek A, Reiss K, Trotta R, Calabretta B, Skorski T. Activation of mitochondrial Raf-1 is involved in the antiapoptotic effects of Akt. Cancer Res. 1999;59:2815–9. [PubMed] [Google Scholar]
- 32.Salomoni P, Wasik MA, Riedel RF, Reiss K, Choi JK, Skorski T, Calabretta B. Expression of constitutively active Raf-1 in the mitochondria restores antiapoptotic and leukemogenic potential of a transformation-deficient BCR/ABL mutant. J Exp Med. 1998;187:1995–2007. doi: 10.1084/jem.187.12.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiss K, Wang JY, Romano G, Tu X, Peruzzi F, Baserga R. Mechanisms of regulation of cell adhesion and motility by insulin receptor substrate-1 in prostate cancer cells. Oncogene. 2001;20:490–500. doi: 10.1038/sj.onc.1204112. [DOI] [PubMed] [Google Scholar]
- 34.Peruzzi F, Prisco M, Morrione A, Valentinis B, Baserga R. Anti-apoptotic signaling of the IGF-I receptor through mitochondrial translocation of c-Raf and Nedd4. J Biol Chem. 2001;14:14. doi: 10.1074/jbc.M103188200. [DOI] [PubMed] [Google Scholar]
- 35.Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–38. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 36.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X (L) Cell. 1996;87:619–28. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 37.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 38.Digicaylioglu M, Garden G, Timberlake S, Fletcher L, Lipton SA. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc Natl Acad Sci U S A. 2004;101:9855–60. doi: 10.1073/pnas.0403172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bondy CA, Lee WH. Patterns of insulin-like growth factor and IGF receptor gene expression in the brain. Functional implications. Ann N Y Acad Sci. 1993;692:33–43. doi: 10.1111/j.1749-6632.1993.tb26203.x. [DOI] [PubMed] [Google Scholar]
- 40.Aguado F, Sanchez-Franco F, Rodrigo J, Cacicedo L, Martinez-Murillo R. Insulin-like growth factor I-immunoreactive peptide in adult human cerebellar Purkinje cells: co-localization with low-affinity nerve growth factor receptor. Neuroscience. 1994;59:641–50. doi: 10.1016/0306-4522(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 41.De Keyser J, Wilczak N, De Backer JP, Herroelen L, Vauquelin G. Insulin-like growth factor-I receptors in human brain and pituitary gland: an autoradiographic study. Synapse. 1994;17:196–202. doi: 10.1002/syn.890170309. [DOI] [PubMed] [Google Scholar]
- 42.Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K. A role for IGF-1 in the rescue of CNS neurons following hypoxic- ischemic injury. Biochem Biophys Res Commun. 1992;182:593–9. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- 43.D’Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci U S A. 1993;90:10989–93. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–5. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 45.Heck S, Lezoualc’h F, Engert S, Behl C. Insulin-like growth factor-1-mediated neuroprotection against oxidative stress is associated with activation of nuclear factor kappaB. J Biol Chem. 1999;274:9828–35. doi: 10.1074/jbc.274.14.9828. [DOI] [PubMed] [Google Scholar]
- 46.Russell JW, Feldman EL. Insulin-like growth factor-I prevents apoptosis in sympathetic neurons exposed to high glucose. Horm Metab Res. 1999;31:90–6. doi: 10.1055/s-2007-978704. [DOI] [PubMed] [Google Scholar]
- 47.Vincent AM, Mobley BC, Hiller A, Feldman EL. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol Dis. 2004;16:407–16. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Ying Wang J, Peruzzi F, Lassak A, Del Valle L, Radhakrishnan S, Rappaport J, Khalili K, Amini S, Reiss K. Neuroprotective effects of IGF-I against TNFalpha-induced neuronal damage in HIV-associated dementia. Virology. 2003;305:66–76. doi: 10.1006/viro.2002.1690. [DOI] [PubMed] [Google Scholar]
- 49.Zheng WH, Kar S, Quirion R. Insulin-like growth factor-1-induced phosphorylation of transcription factor FKHRL1 is mediated by phosphatidylinositol 3-kinase/Akt kinase and role of this pathway in insulin-like growth factor-1-induced survival of cultured hippocampal neurons. Mol Pharmacol. 2002;62:225–33. doi: 10.1124/mol.62.2.225. [DOI] [PubMed] [Google Scholar]
- 50.Sara VR, Carlsson-Skwirut C, Bergman T, Jornvall H, Roberts PJ, Crawford M, Hakansson LN, Civalero I, Nordberg A. Identification of Gly-Pro-Glu (GPE), the aminoterminal tripeptide of insulin-like growth factor 1 which is truncated in brain, as a novel neuroactive peptide. Biochem Biophys Res Commun. 1989;165:766–71. doi: 10.1016/s0006-291x(89)80032-4. [DOI] [PubMed] [Google Scholar]
- 51.Palmiter RD, Norstedt G, Gelinas RE, Hammer RE, Brinster RL. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983;222:809–14. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- 52.Mathews LS, Hammer RE, Behringer RR, D’Ercole AJ, Bell GI, Brinster RL, Palmiter RD. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology. 1988;123:2827–33. doi: 10.1210/endo-123-6-2827. [DOI] [PubMed] [Google Scholar]
- 53.Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy CJ, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:8630–5. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 55.Camarero G, Avendano C, Fernandez-Moreno C, Villar A, Contreras J, de Pablo F, Pichel JG, Varela-Nieto I. Delayed inner ear maturation and neuronal loss in postnatal Igf-1-deficient mice. J Neurosci. 2001;21:7630–41. doi: 10.1523/JNEUROSCI.21-19-07630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng CM, Reinhardt RR, Lee WH, Joncas G, Patel SC, Bondy CA. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc Natl Acad Sci U S A. 2000;97:10236–41. doi: 10.1073/pnas.170008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arsenijevic Y, Weiss S, Schneider B, Aebischer P. Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2. J Neurosci. 2001;21:7194–202. doi: 10.1523/JNEUROSCI.21-18-07194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23:7710–8. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–40. doi: 10.1016/s1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 60.Aberg ND, Blomstrand F, Aberg MA, Bjorklund U, Carlsson B, Carlsson-Skwirut C, Bang P, Ronnback L, Eriksson PS. Insulin-like growth factor-I increases astrocyte intercellular gap junctional communication and connexin43 expression in vitro. J Neurosci Res. 2003;74:12–22. doi: 10.1002/jnr.10734. [DOI] [PubMed] [Google Scholar]
- 61.Jiang F, Frederick TJ, Wood TL. IGF-I synergizes with FGF-2 to stimulate oligodendrocyte progenitor entry into the cell cycle. Dev Biol. 2001;232:414–23. doi: 10.1006/dbio.2001.0208. [DOI] [PubMed] [Google Scholar]
- 62.Arsenijevic Y, Weiss S. Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: distinct actions from those of brain-derived neurotrophic factor. J Neurosci. 1998;18:2118–28. doi: 10.1523/JNEUROSCI.18-06-02118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164:111–22. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–74. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luciano CA, Pardo CA, McArthur JC. Recent developments in the HIV neuropathies. Curr Opin Neurol. 2003;16:403–9. doi: 10.1097/01.wco.0000073943.19076.98. [DOI] [PubMed] [Google Scholar]
- 66.Peruzzi F, Bergonzini V, Aprea S, Reiss K, Sawaya BE, Rappaport J, Amini S, Khalili K. Cross talk between growth factors and viral and cellular factors alters neuronal signaling pathways: implication for HIV-associated dementia. Brain Res Brain Res Rev. 2005;50:114–25. doi: 10.1016/j.brainresrev.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–21. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 68.McArthur JC, Sacktor N, Selnes O. Human immunodeficiency virus-associated dementia. Semin Neurol. 1999;19:129–50. doi: 10.1055/s-2008-1040831. [DOI] [PubMed] [Google Scholar]
- 69.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–15. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–41. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 71.Torres-Munoz J, Stockton P, Tacoronte N, Roberts B, Maronpot RR, Petito CK. Detection of HIV-1 gene sequences in hippocampal neurons isolated from postmortem AIDS brains by laser capture microdissection. J Neuropathol Exp Neurol. 2001;60:885–92. doi: 10.1093/jnen/60.9.885. [DOI] [PubMed] [Google Scholar]
- 72.Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:144–54. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–18. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- 74.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–6. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saha RN, Pahan K. Tumor necrosis factor-alpha at the crossroads of neuronal life and death during HIV-associated dementia. J Neurochem. 2003;86:1057–71. doi: 10.1046/j.1471-4159.2003.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grinspoon S, Corcoran C, Lee K, Burrows B, Hubbard J, Katznelson L, Walsh M, Guccione A, Cannan J, Heller H, Basgoz N, Klibanski A. Loss of lean body and muscle mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. J Clin Endocrinol Metab. 1996;81:4051–8. doi: 10.1210/jcem.81.11.8923860. [DOI] [PubMed] [Google Scholar]
- 77.Grinspoon S, Corcoran C, Stanley T, Katznelson L, Klibanski A. Effects of androgen administration on the growth hormone-insulin-like growth factor I axis in men with acquired immunodeficiency syndrome wasting. J Clin Endocrinol Metab. 1998;83:4251–6. doi: 10.1210/jcem.83.12.5305. [DOI] [PubMed] [Google Scholar]
- 78.Jain S, Golde DW, Bailey R, Geffner ME. Insulin-like growth factor-I resistance. Endocr Rev. 1998;19:625–46. doi: 10.1210/edrv.19.5.0348. [DOI] [PubMed] [Google Scholar]
- 79.Laue L, Pizzo PA, Butler K, Cutler GB., Jr Growth and neuroendocrine dysfunction in children with acquired immunodeficiency syndrome. J Pediatr. 1990;117:541–5. doi: 10.1016/s0022-3476(05)80685-7. [DOI] [PubMed] [Google Scholar]
- 80.Lo JC, Mulligan K, Noor MA, Schwarz JM, Halvorsen RA, Grunfeld C, Schambelan M. The effects of recombinant human growth hormone on body composition and glucose metabolism in hiv-infected patients with fat accumulation. J Clin Endocrinol Metab. 2001;86:3480–7. doi: 10.1210/jcem.86.8.7785. [DOI] [PubMed] [Google Scholar]
- 81.Mynarcik DC, McNurlan MA, Steigbigel RT, Fuhrer J, Gelato MC. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr. 2000;25:312–21. doi: 10.1097/00042560-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 82.Mynarcik DC, Frost RA, Lang CH, DeCristofaro K, McNurlan MA, Garlick PJ, Steigbigel RT, Fuhrer J, Ahnn S, Gelato MC. Insulin-like growth factor system in patients with HIV infection: effect of exogenous growth hormone administration. J Acquir Immune Defic Syndr. 1999;22:49–55. doi: 10.1097/00042560-199909010-00006. [DOI] [PubMed] [Google Scholar]
- 83.Frost RA, Fuhrer J, Steigbigel R, Mariuz P, Lang CH, Gelato MC. Wasting in the acquired immune deficiency syndrome is associated with multiple defects in the serum insulin-like growth factor system. Clin Endocrinol (Oxf) 1996;44:501–14. doi: 10.1046/j.1365-2265.1996.705526.x. [DOI] [PubMed] [Google Scholar]
- 84.Brindley DN, Wang CN, Mei J, Xu J, Hanna AN. Tumor necrosis factor-alpha and ceramides in insulin resistance. Lipids. 1999;34(Suppl):S85–8. doi: 10.1007/BF02562240. [DOI] [PubMed] [Google Scholar]
- 85.Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–5. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 86.Wang JY, Grabacka M, Marcinkiewicz C, Staniszewska I, Peruzzi F, Khalili K, Amini S, Reiss K. Involvement of alpha1beta1 integrin in insulin-like growth factor-1-mediated protection of PC12 neuronal processes from tumor necrosis factor-alpha-induced injury. J Neurosci Res. 2006;83:7–18. doi: 10.1002/jnr.20712. [DOI] [PubMed] [Google Scholar]
- 87.Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–8. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 88.Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006 doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 89.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–9. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venters HD, Tang Q, Liu Q, VanHoy RW, Dantzer R, Kelley KW. A new mechanism of neurodegeneration: a proinflammatory cytokine inhibits receptor signaling by a survival peptide. Proc Natl Acad Sci U S A. 1999;96:9879–84. doi: 10.1073/pnas.96.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.White MF. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Recent Prog Horm Res. 1998;53:119–38. [PubMed] [Google Scholar]
- 92.Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem. 2004;279:35298–305. doi: 10.1074/jbc.M405203200. [DOI] [PubMed] [Google Scholar]
- 93.Ravichandran LV, Esposito DL, Chen J, Quon MJ. Protein kinase C-zeta phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. J Biol Chem. 2001;276:3543–9. doi: 10.1074/jbc.M007231200. [DOI] [PubMed] [Google Scholar]
- 94.Fujishiro M, Gotoh Y, Katagiri H, Sakoda H, Ogihara T, Anai M, Onishi Y, Ono H, Abe M, Shojima N, Fukushima Y, Kikuchi M, Oka Y, Asano T. Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3-L1 adipocytes. Mol Endocrinol. 2003;17:487–97. doi: 10.1210/me.2002-0131. [DOI] [PubMed] [Google Scholar]
- 95.Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci U S A. 1997;94:9660–4. doi: 10.1073/pnas.94.18.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paz K, Liu YF, Shorer H, Hemi R, LeRoith D, Quan M, Kanety H, Seger R, Zick Y. Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. J Biol Chem. 1999;274:28816–22. doi: 10.1074/jbc.274.40.28816. [DOI] [PubMed] [Google Scholar]
- 97.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 98.Vuori K, Ruoslahti E. Association of insulin receptor substrate-1 with integrins. Science. 1994;266:1576–8. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- 99.Guilherme A, Torres K, Czech MP. Cross-talk between insulin receptor and integrin alpha5 beta1 signaling pathways. J Biol Chem. 1998;273:22899–903. doi: 10.1074/jbc.273.36.22899. [DOI] [PubMed] [Google Scholar]
- 100.Tai YT, Podar K, Catley L, Tseng YH, Akiyama M, Shringarpure R, Burger R, Hideshima T, Chauhan D, Mitsiades N, Richardson P, Munshi NC, Kahn CR, Mitsiades C, Anderson KC. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of beta1-integrin and phosphatidylinositol 3′-kinase/AKT signaling. Cancer Res. 2003;63:5850–8. [PubMed] [Google Scholar]
- 101.Lynch L, Vodyanik PI, Boettiger D, Guvakova MA. Insulin-like growth factor I controls adhesion strength mediated by alpha5beta1 integrins in motile carcinoma cells. Mol Biol Cell. 2005;16:51–63. doi: 10.1091/mbc.E04-05-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hermanto U, Zong CS, Li W, Wang LH. RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Mol Cell Biol. 2002;22:2345–65. doi: 10.1128/MCB.22.7.2345-2365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maile LA, Clemmons DR. The alphaVbeta3 integrin regulates insulin-like growth factor I (IGF-I) receptor phosphorylation by altering the rate of recruitment of the Src-homology 2-containing phosphotyrosine phosphatase-2 to the activated IGF-I receptor. Endocrinology. 2002;143:4259–64. doi: 10.1210/en.2002-220395. [DOI] [PubMed] [Google Scholar]
- 104.Wang JY, Gualco E, Peruzzi F, Sawaya BE, Passiatore G, Marcinkiewicz C, Staniszewska I, Ferrante P, Amini S, Khalili K, Reiss K. Interaction between serine phosphorylated IRS-1 and beta1-integrin affects the stability of neuronal processes. J Neurosci Res. 2007;85:2360–73. doi: 10.1002/jnr.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dorr J, Roth K, Zurbuchen U, Deisz R, Bechmann I, Lehmann TN, Meier S, Nitsch R, Zipp F. Tumor-necrosis-factor-related apoptosis-inducing-ligand (TRAIL)-mediated death of neurons in living human brain tissue is inhibited by flupirtine-maleate. J Neuroimmunol. 2005;167:204–9. doi: 10.1016/j.jneuroim.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 106.Huang Y, Erdmann N, Peng H, Zhao Y, Zheng J. The role of TNF related apoptosis-inducing ligand in neurodegenerative diseases. Cell Mol Immunol. 2005;2:113–22. [PubMed] [Google Scholar]
- 107.Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest. 2006;36:447–58. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 108.Konsman JP, Drukarch B, Van Dam AM. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci (Lond) 2007;112:1–25. doi: 10.1042/CS20060043. [DOI] [PubMed] [Google Scholar]
- 109.Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis. 2001;8:590–9. doi: 10.1006/nbdi.2001.0414. [DOI] [PubMed] [Google Scholar]
- 110.Munoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog Neurobiol. 1998;56:307–40. doi: 10.1016/s0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 111.Tarkowski E, Liljeroth AM, Minthon L, Tarkowski A, Wallin A, Blennow K. Cerebral pattern of pro- and anti-inflammatory cytokines in dementias. Brain Res Bull. 2003;61:255–60. doi: 10.1016/s0361-9230(03)00088-1. [DOI] [PubMed] [Google Scholar]
- 112.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 113.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–7. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 114.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Becherer JD, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–6. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 115.Zheng Y, Saftig P, Hartmann D, Blobel C. Evaluation of the contribution of different ADAMs to tumor necrosis factor alpha (TNFalpha) shedding and of the function of the TNFalpha ectodomain in ensuring selective stimulated shedding by the TNFalpha convertase (TACE/ADAM17) J Biol Chem. 2004;279:42898–906. doi: 10.1074/jbc.M403193200. [DOI] [PubMed] [Google Scholar]
- 116.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 117.Yang P, Baker KA, Hagg T. The ADAMs family: coordinators of nervous system development, plasticity and repair. Prog Neurobiol. 2006;79:73–94. doi: 10.1016/j.pneurobio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Dallas DJ, Genever PG, Patton AJ, Millichip MI, McKie N, Skerry TM. Localization of ADAM10 and Notch receptors in bone. Bone. 1999;25:9–15. doi: 10.1016/s8756-3282(99)00099-x. [DOI] [PubMed] [Google Scholar]
- 119.Muraguchi T, Takegami Y, Ohtsuka T, Kitajima S, Chandana EP, Omura A, Miki T, Takahashi R, Matsumoto N, Ludwig A, Noda M, Takahashi C. RECK modulates Notch signaling during cortical neurogenesis by regulating ADAM10 activity. Nat Neurosci. 2007;10:838–45. doi: 10.1038/nn1922. [DOI] [PubMed] [Google Scholar]
- 120.Six E, Ndiaye D, Laabi Y, Brou C, Gupta-Rossi N, Israel A, Logeat F. The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase. Proc Natl Acad Sci U S A. 2003;100:7638–43. doi: 10.1073/pnas.1230693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Govoni KE, Amaar YG, Kramer A, Winter E, Baylink DJ, Mohan S. Regulation of insulin-like growth factor binding protein-5, four and a half lim-2, and a disintegrin and metalloprotease-9 expression in osteoblasts. Growth Horm IGF Res. 2006;16:49–56. doi: 10.1016/j.ghir.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mohan S, Thompson GR, Amaar YG, Hathaway G, Tschesche H, Baylink DJ. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry. 2002;41:15394–403. doi: 10.1021/bi026458q. [DOI] [PubMed] [Google Scholar]
- 123.Valcour VG, Sacktor NC, Paul RH, Watters MR, Selnes OA, Shiramizu BT, Williams AE, Shikuma CM. Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging With HIV cohort. J Acquir Immune Defic Syndr. 2006;43:405–10. doi: 10.1097/01.qai.0000243119.67529.f5. [DOI] [PubMed] [Google Scholar]
- 124.Valcour VG, Shikuma CM, Shiramizu BT, Williams AE, Watters MR, Poff PW, Grove JS, Selnes OA, Sacktor NC. Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;38:31–6. doi: 10.1097/00126334-200501010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Larson R, Capili B, Eckert-Norton M, Colagreco JP, Anastasi JK. Disorders of glucose metabolism in the context of human immunodeficiency virus infection. J Am Acad Nurse Pract. 2006;18:92–103. doi: 10.1111/j.1745-7599.2006.00109.x. [DOI] [PubMed] [Google Scholar]
- 126.Hertel J, Struthers H, Horj CB, Hruz PW. A structural basis for the acute effects of HIV protease inhibitors on GLUT4 intrinsic activity. J Biol Chem. 2004;279:55147–52. doi: 10.1074/jbc.M410826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barbaro G. HIV infection, highly active antiretroviral therapy and the cardiovascular system. Cardiovasc Res. 2003;60:87–95. doi: 10.1016/s0008-6363(02)00828-3. [DOI] [PubMed] [Google Scholar]
- 128.Barbaro G. Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Curr HIV Res. 2006;4:79–85. doi: 10.2174/157016206775197664. [DOI] [PubMed] [Google Scholar]
- 129.Biessels GJ, Gispen WH. The impact of diabetes on cognition: what can be learned from rodent models? Neurobiol Aging. 2005;26(Suppl 1):36–41. doi: 10.1016/j.neurobiolaging.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 130.Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–80. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- 131.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes OA, Grove J, Liu Y, Abdul-Majid KB, Gartner S, Sacktor N. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol. 2004;157:197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 132.Kang BP, Urbonas A, Baddoo A, Baskin S, Malhotra A, Meggs LG. IGF-1 inhibits the mitochondrial apoptosis program in mesangial cells exposed to high glucose. Am J Physiol Renal Physiol. 2003;285:F1013–24. doi: 10.1152/ajprenal.00209.2003. [DOI] [PubMed] [Google Scholar]
- 133.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 134.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 135.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:22–8. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–9. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- 137.Chintapalli J, Yang S, Opawumi D, Goyal SR, Shamsuddin N, Malhotra A, Reiss K, Meggs LG. Inhibition of wild-type p66ShcA in mesangial cells prevents glycooxidant-dependent FOXO3a regulation and promotes the survival phenotype. Am J Physiol Renal Physiol. 2007;292:F523–30. doi: 10.1152/ajprenal.00215.2006. [DOI] [PubMed] [Google Scholar]
- 138.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci U S A. 2003;100:2112–6. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–7. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 140.Holzenberger M. The GH/IGF-I axis and longevity. Eur J Endocrinol. 2004;151(Suppl 1):S23–7. doi: 10.1530/eje.0.151s023. [DOI] [PubMed] [Google Scholar]
- 141.Shimokawa I, Higami Y, Tsuchiya T, Otani H, Komatsu T, Chiba T, Yamaza H. Life span extension by reduction of the growth hormone-insulin-like growth factor-1 axis: relation to caloric restriction. Faseb J. 2003;17:1108–9. doi: 10.1096/fj.02-0819fje. [DOI] [PubMed] [Google Scholar]
- 142.Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA, Borlak J, Ertl G, Bauersachs J. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–43. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 143.Anversa P. Aging and longevity: the IGF-1 enigma. Circ Res. 2005;97:411–4. doi: 10.1161/01.RES.0000182212.09147.56. [DOI] [PubMed] [Google Scholar]
- 144.Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol. 2007;292:H1398–403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 145.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–20. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 146.Watanabe T, Miyazaki A, Katagiri T, Yamamoto H, Idei T, Iguchi T. Relationship between serum insulin-like growth factor-1 levels and Alzheimer’s disease and vascular dementia. J Am Geriatr Soc. 2005;53:1748–53. doi: 10.1111/j.1532-5415.2005.53524.x. [DOI] [PubMed] [Google Scholar]
- 147.Garcia-Segura LM, Diz-Chaves Y, Perez-Martin M, Darnaudery M. Estradiol, insulin-like growth factor-I and brain aging. Psychoneuroendocrinology. 2007;32(Suppl 1):S57–61. doi: 10.1016/j.psyneuen.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 148.Trejo JL, Carro E, Garcia-Galloway E, Torres-Aleman I. Role of insulin-like growth factor I signaling in neurodegenerative diseases. J Mol Med. 2004;82:156–62. doi: 10.1007/s00109-003-0499-7. [DOI] [PubMed] [Google Scholar]
- 149.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–33. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 150.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 151.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, Puri C, Tacchetti C, Ferrini M, Mannucci R, Nicoletti I, Lanfrancone L, Giorgio M, Pelicci PG. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004;279:25689–95. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 152.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO’s road. Sci STKE 2003. 2003;RE5 doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 153.Gotoh N, Toyoda M, Shibuya M. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol Cell Biol. 1997;17:1824–31. doi: 10.1128/mcb.17.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–33. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer’s disease. Trends Neurosci. 2003;26:404–6. doi: 10.1016/S0166-2236(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 156.Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 157.Blair LA, Marshall J. IGF-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–9. doi: 10.1016/s0896-6273(00)80950-2. [DOI] [PubMed] [Google Scholar]
- 158.Gutierrez-Ospina G, Saum L, Calikoglu AS, Diaz-Cintra S, Barrios FA, D’Ercole AJ. Increased neural activity in transgenic mice with brain IGF-I overexpression: a (3H)2DG study. Neuroreport. 1997;8:2907–11. doi: 10.1097/00001756-199709080-00021. [DOI] [PubMed] [Google Scholar]
- 159.Torres-Aleman I. Targeting insulin-like growth factor-1 to treat Alzheimer’s disease. Expert Opin Ther Targets. 2007;11:1535–42. doi: 10.1517/14728222.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 160.Reid PC, Urano Y, Kodama T, Hamakubo T. Alzheimer’s disease: cholesterol, membrane rafts, isoprenoids and statins. J Cell Mol Med. 2007;11:383–92. doi: 10.1111/j.1582-4934.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Allsop D, Landon M, Kidd M. The isolation and amino acid composition of senile plaque core protein. Brain Res. 1983;259:348–52. doi: 10.1016/0006-8993(83)91273-8. [DOI] [PubMed] [Google Scholar]
- 162.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–90. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 163.Mattson MP. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev. 1997;77:1081–132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- 164.Clark CM, Karlawish JH. Alzheimer disease: current concepts and emerging diagnostic and therapeutic strategies. Ann Intern Med. 2003;138:400–10. doi: 10.7326/0003-4819-138-5-200303040-00010. [DOI] [PubMed] [Google Scholar]
- 165.Adlerz L, Holback S, Multhaup G, Iverfeldt K. IGF-1-induced processing of the amyloid precursor protein family is mediated by different signaling pathways. J Biol Chem. 2007;282:10203–9. doi: 10.1074/jbc.M611183200. [DOI] [PubMed] [Google Scholar]
- 166.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390–7. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 167.Carro E, I-Aleman Torres. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur J Pharmacol. 2004;490:127–33. doi: 10.1016/j.ejphar.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 168.Carro E, Trejo JL, Spuch C, Bohl D, Heard JM, Torres-Aleman I. Blockade of the insulin-like growth factor I receptor in the choroid plexus originates Alzheimer’s-like neuropathology in rodents: new cues into the human disease? Neurobiol Aging. 2006;27:1618–31. doi: 10.1016/j.neurobiolaging.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 169.Miklossy J, Kraftsik R, Pillevuit O, Lepori D, Genton C, Bosman FT. Curly fiber and tangle-like inclusions in the ependyma and choroid plexus--a pathogenetic relationship with the cortical Alzheimer-type changes? J Neuropathol Exp Neurol. 1998;57:1202–12. doi: 10.1097/00005072-199812000-00012. [DOI] [PubMed] [Google Scholar]
- 170.Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22:RC221. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Willis PE, Chadan S, Baracos V, Parkhouse WS. Acute exercise attenuates age-associated resistance to insulin-like growth factor I. Am J Physiol. 1997;272:E397–404. doi: 10.1152/ajpendo.1997.272.3.E397. [DOI] [PubMed] [Google Scholar]
- 172.Serot JM, Christmann D, Dubost T, Couturier M. Cerebrospinal fluid transthyretin: aging and late onset Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1997;63:506–8. doi: 10.1136/jnnp.63.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Engelhardt B, Wolburg-Buchholz K, Wolburg H. Involvement of the choroid plexus in central nervous system inflammation. Microsc Res Tech. 2001;52:112–29. doi: 10.1002/1097-0029(20010101)52:1<112::AID-JEMT13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 174.Petty MA, Lo EH. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68:311–23. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 175.Remarque EJ, Bollen EL, Weverling-Rijnsburger AW, Laterveer JC, Blauw GJ, Westendorp RG. Patients with Alzheimer’s disease display a pro-inflammatory phenotype. Exp Gerontol. 2001;36:171–6. doi: 10.1016/s0531-5565(00)00176-5. [DOI] [PubMed] [Google Scholar]
- 176.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–62. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Caceres A, Kosik KS. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990;343:461–3. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- 178.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–62. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 179.Probst A, Gotz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VM, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Burki K, Goedert M. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. 2000;99:469–81. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- 180.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–59. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 181.Schmidt ML, Zhukareva V, Newell KL, Lee VM, Trojanowski JQ. Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer’s disease. Acta Neuropathol. 2001;101:518–24. doi: 10.1007/s004010000330. [DOI] [PubMed] [Google Scholar]
- 182.Lovestone S, Reynolds CH, Latimer D, Davis DR, Anderton BH, Gallo JM, Hanger D, Mulot S, Marquardt B, Stabel S, et al. Alzheimer’s disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Curr Biol. 1994;4:1077–86. doi: 10.1016/s0960-9822(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 183.Pei JJ, Grundke-Iqbal I, Iqbal K, Bogdanovic N, Winblad B, Cowburn RF. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer’s disease neurofibrillary degeneration. Brain Res. 1998;797:267–77. doi: 10.1016/s0006-8993(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 184.Garver TD, Oyler GA, Harris KA, Polavarapu R, Damuni Z, Lehman RA, Billingsley ML. Tau phosphorylation in brain slices: pharmacological evidence for convergent effects of protein phosphatases on tau and mitogen-activated protein kinase. Mol Pharmacol. 1995;47:745–56. [PubMed] [Google Scholar]
- 185.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wilker E, Lu J, Rho O, Carbajal S, Beltran L, DiGiovanni J. Role of PI3K/Akt signaling in insulin-like growth factor-1 (IGF-1) skin tumor promotion. Mol Carcinog. 2005;44:137–45. doi: 10.1002/mc.20132. [DOI] [PubMed] [Google Scholar]
- 187.Brywe KG, Mallard C, Gustavsson M, Hedtjarn M, Leverin AL, Wang X, Blomgren K, Isgaard J, Hagberg H. IGF-I neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3beta? Eur J Neurosci. 2005;21:1489–502. doi: 10.1111/j.1460-9568.2005.03982.x. [DOI] [PubMed] [Google Scholar]
- 188.Zhu X, Lee HG, Raina AK, Perry G, Smith MA. The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals. 2002;11:270–81. doi: 10.1159/000067426. [DOI] [PubMed] [Google Scholar]
- 189.Pei JJ, Khatoon S, An WL, Nordlinder M, Tanaka T, Braak H, Tsujio I, Takeda M, Alafuzoff I, Winblad B, Cowburn RF, Grundke-Iqbal I, Iqbal K. Role of protein kinase B in Alzheimer’s neurofibrillary pathology. Acta Neuropathol. 2003;105:381–92. doi: 10.1007/s00401-002-0657-y. [DOI] [PubMed] [Google Scholar]
- 190.Rickle A, Bogdanovic N, Volkman I, Winblad B, Ravid R, Cowburn RF. Akt activity in Alzheimer’s disease and other neurodegenerative disorders. Neuroreport. 2004;15:955–9. doi: 10.1097/00001756-200404290-00005. [DOI] [PubMed] [Google Scholar]
- 191.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–22. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 192.Dokken BB, Saengsirisuwan V, Kim JS, Teachey MK, Henriksen EJ. Oxidative stress-induced insulin resistance in rat skeletal muscle: role of glycogen synthase kinase-3. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00578.2007. [DOI] [PubMed] [Google Scholar]
- 193.Endo H, Nito C, Kamada H, Yu F, Chan PH. Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3beta survival signaling. J Cereb Blood Flow Metab. 2007;27:975–82. doi: 10.1038/sj.jcbfm.9600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Polidori MC, Griffiths HR, Mariani E, Mecocci P. Hallmarks of protein oxidative damage in neurodegenerative diseases: focus on Alzheimer’s disease. Amino Acids. 2007;32:553–9. doi: 10.1007/s00726-006-0431-x. [DOI] [PubMed] [Google Scholar]
- 195.Shi Q, Gibson GE. Oxidative stress and transcriptional regulation in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:276–91. doi: 10.1097/WAD.0b013e31815721c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carro E, Trejo JL, Gerber A, Loetscher H, Torrado J, Metzger F, Torres-Aleman I. Therapeutic actions of insulin-like growth factor I on APP/PS2 mice with severe brain amyloidosis. Neurobiol Aging. 2006;27:1250–7. doi: 10.1016/j.neurobiolaging.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 197.Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604–12. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332:F105–26. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 199.Del Valle L, Wang JY, Lassak A, Peruzzi F, Croul S, Khalili K, Reiss K. Insulin-like growth factor I receptor signaling system in JC virus T antigen-induced primitive neuroectodermal tumors-medulloblastomas. J Neurovirol. 2002;8(Suppl 2):138–47. doi: 10.1080/13550280290101111. [DOI] [PubMed] [Google Scholar]
- 200.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–7. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 201.Sachdev D, Li SL, Hartell JS, Fujita-Yamaguchi Y, Miller JS, Yee D. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 2003;63:627–35. [PubMed] [Google Scholar]
- 202.Urbanska K, Trojanek J, Del Valle L, Eldeen MB, Hofmann F, Garcia-Echeverria C, Khalili K, Reiss K. Inhibition of IGF-I receptor in anchorage-independence attenuates GSK-3beta constitutive phosphorylation and compromises growth and survival of medulloblastoma cell lines. Oncogene. 2007;26:2308–17. doi: 10.1038/sj.onc.1210018. [DOI] [PubMed] [Google Scholar]
- 203.Dunn SE, Ehrlich M, Sharp NJ, Reiss K, Solomon G, Hawkins R, Baserga R, Barrett JC. A dominant negative mutant of the insulin-like growth factorI receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 1998;58:3353–61. [PubMed] [Google Scholar]
- 204.Long L, Rubin R, Baserga R, Brodt P. Loss of the metastatic phenotype in murine carcinoma cells expressing an antisense RNA to the insulin-like growth factor receptor. Cancer Res. 1995;55:1006–9. [PubMed] [Google Scholar]
- 205.Resnicoff M, Coppola D, Sell C, Rubin R, Ferrone S, Baserga R. Growth inhibition of human melanoma cells in nude mice by antisense strategies to the type 1 insulin-like growth factor receptor. Cancer Res. 1994;54:4848–50. [PubMed] [Google Scholar]
- 206.Resnicoff M, Sell C, Rubini M, Coppola D, Ambrose D, Baserga R, Rubin R. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 1994;54:2218–22. [PubMed] [Google Scholar]
- 207.Playford MP, Bicknell D, Bodmer WF, Macaulay VM. Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of beta-catenin. Proc Natl Acad Sci U S A. 2000;97:12103–8. doi: 10.1073/pnas.210394297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann N Y Acad Sci. 2004;1014:155–63. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 209.Shen MR, Hsu YM, Hsu KF, Chen YF, Tang MJ, Chou CY. Insulin-like growth factor 1 is a potent stimulator of cervical cancer cell invasiveness and proliferation that is modulated by alphavbeta3 integrin signaling. Carcinogenesis. 2006;27:962–71. doi: 10.1093/carcin/bgi336. [DOI] [PubMed] [Google Scholar]
- 210.Zhang D, Samani AA, Brodt P. The role of the IGF-I receptor in the regulation of matrix metalloproteinases, tumor invasion and metastasis. Horm Metab Res. 2003;35:802–8. doi: 10.1055/s-2004-814143. [DOI] [PubMed] [Google Scholar]
- 211.Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–53. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 212.Reiss K, Wang JY, Romano G, Furnari FB, Cavenee WK, Morrione A, Tu X, Baserga R. IGF-I receptor signaling in a prostatic cancer cell line with a PTEN mutation. Oncogene. 2000;19:2687–94. doi: 10.1038/sj.onc.1203587. [DOI] [PubMed] [Google Scholar]
- 213.Valentinis B, Navarro M, Zanocco-Marani T, Edmonds P, McCormick J, Morrione A, Sacchi A, Romano G, Reiss K, Baserga R. Insulin receptor substrate-1, p70S6K, and cell size in transformation and differentiation of hemopoietic cells. J Biol Chem. 2000;275:25451–9. doi: 10.1074/jbc.M002271200. [DOI] [PubMed] [Google Scholar]
- 214.Patti R, Reddy CD, Geoerger B, Grotzer MA, Raghunath M, Sutton LN, Phillips PC. Autocrine secreted insulin-like growth factor-I stimulates MAP kinase- dependent mitogenic effects in human primitive neuroectodermal tumor/medulloblastoma. Int J Oncol. 2000;16:577–84. doi: 10.3892/ijo.16.3.577. [DOI] [PubMed] [Google Scholar]
- 215.Wang JY, Del Valle L, Gordon J, Rubini M, Romano G, Croul S, Peruzzi F, Khalili K, Reiss K. Activation of the IGF-IR system contributes to malignant growth of human and mouse medulloblastomas. Oncogene. 2001;20:3857–68. doi: 10.1038/sj.onc.1204532. [DOI] [PubMed] [Google Scholar]
- 216.Del Valle L, Enam S, Lassak A, Wang JY, Croul S, Khalili K, Reiss K. Insulin-like Growth Factor I Receptor Activity in Human Medulloblastomas. Clin Cancer Res. 2002;8:1822–30. [PubMed] [Google Scholar]
- 217.Reiss K. Insulin-like growth factor-I receptor - a potential therapeutic target in medulloblastomas. Expert Opin Ther Targets. 2002;6:539–44. doi: 10.1517/14728222.6.5.539. [DOI] [PubMed] [Google Scholar]
- 218.Rao G, Pedone CA, Valle LD, Reiss K, Holland EC, Fults DW. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23:6156–62. doi: 10.1038/sj.onc.1207818. [DOI] [PubMed] [Google Scholar]
- 219.Hartmann W, Koch A, Brune H, Waha A, Schuller U, Dani I, Denkhaus D, Langmann W, Bode U, Wiestler OD, Schilling K, Pietsch T. Insulin-like growth factor II is involved in the proliferation control of medulloblastoma and its cerebellar precursor cells. Am J Pathol. 2005;166:1153–62. doi: 10.1016/S0002-9440(10)62335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Browd SR, Kenney AM, Gottfried ON, Yoon JW, Walterhouse D, Pedone CA, Fults DW. N-myc can substitute for insulin-like growth factor signaling in a mouse model of sonic hedgehog-induced medulloblastoma. Cancer Res. 2006;66:2666–72. doi: 10.1158/0008-5472.CAN-05-2198. [DOI] [PubMed] [Google Scholar]
- 221.Kurihara M, Tokunaga Y, Ochi A, Kawaguchi T, Tsutsumi K, Shigematsu K, Niwa M, Mori K. (Expression of insulin-like growth factor I receptors in human brain tumors: comparison with epidermal growth factor receptor by using quantitative autoradiography) No To Shinkei. 1989;41:719–25. [PubMed] [Google Scholar]
- 222.Rubini M, D’Ambrosio C, Carturan S, Yumet G, Catalano E, Shan S, Huang Z, Criscuolo M, Pifferi M, Baserga R. Characterization of an antibody that can detect an activated IGF-I receptor in human cancers. Exp Cell Res. 1999;251:22–32. doi: 10.1006/excr.1999.4562. [DOI] [PubMed] [Google Scholar]
- 223.Del Valle L, Gordon J, Enam S, Delbue S, Croul S, Abraham S, Radhakrishnan S, Assimakopoulou M, Katsetos CD, Khalili K. Expression of human neurotropic polyomavirus JCV late gene product agnoprotein in human medulloblastoma. J Natl Cancer Inst. 2002;94:267–73. doi: 10.1093/jnci/94.4.267. [DOI] [PubMed] [Google Scholar]
- 224.Lassak A, Del Valle L, Peruzzi F, Wang JY, Enam S, Croul S, Khalili K, Reiss K. Insulin Receptor Substrate 1 Translocation to the Nucleus by the Human JC Virus T-antigen. J Biol Chem. 2002;277:17231–8. doi: 10.1074/jbc.M110885200. [DOI] [PubMed] [Google Scholar]
- 225.Krynska B, Del Valle L, Croul S, Gordon J, Katsetos CD, Carbone M, Giordano A, Khalili K. Detection of human neurotropic JC virus DNA sequence and expression of the viral oncogenic protein in pediatric medulloblastomas. Proc Natl Acad Sci U S A. 1999;96:11519–24. doi: 10.1073/pnas.96.20.11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Khalili K, Krynska B, Del Valle L, Katsetos CD, Croul S. Medulloblastomas and the human neurotropic polyomavirus JC virus. Lancet. 1999;353:1152–3. doi: 10.1016/s0140-6736(99)00357-8. [DOI] [PubMed] [Google Scholar]
- 227.Khalili K, Croul S, Del Valle L, Krynska B, Gordon J. Oncogenic potential of human neurotropic virus: laboratory and clinical observations. Isr Med Assoc J. 2001;3:210–5. [PubMed] [Google Scholar]
- 228.Houff SA, London WT, DiChiro G, Padgett BL, Walker DL, Zu Rhein GM, Sever JL. Neuroradiological studies of JCV-induced astrocytomas in nonhuman primates. Prog Clin Biol Res. 1983;105:253–9. [PubMed] [Google Scholar]
- 229.Krynska B, Otte J, Franks R, Khalili K, Croul S. Human ubiquitous JCV (CY) T-antigen gene induces brain tumors in experimental animals. Oncogene. 1999;18:39–46. doi: 10.1038/sj.onc.1202278. [DOI] [PubMed] [Google Scholar]
- 230.Shollar D, Del Valle L, Khalili K, Otte J, Gordon J. JCV T-antigen interacts with the neurofibromatosis type 2 gene product in a transgenic mouse model of malignant peripheral nerve sheath tumors. Oncogene. 2004;23:5459–67. doi: 10.1038/sj.onc.1207728. [DOI] [PubMed] [Google Scholar]
- 231.Del Valle L, Gordon J, Assimakopoulou M, Enam S, Geddes JF, Varakis JN, Katsetos CD, Croul S, Khalili K. Detection of JC virus DNA sequences and expression of the viral regulatory protein T-antigen in tumors of the central nervous system. Cancer Res. 2001;61:4287–93. [PubMed] [Google Scholar]
- 232.Berger JR, Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J Neurovirol. 1995;1:5–18. doi: 10.3109/13550289509111006. [DOI] [PubMed] [Google Scholar]
- 233.Porcu P, Ferber A, Pietrzkowski Z, Roberts CT, Adamo M, LeRoith D, Baserga R. The growth-stimulatory effect of simian virus 40 T antigen requires the interaction of insulinlike growth factor 1 with its receptor. Mol Cell Biol. 1992;12:5069–77. doi: 10.1128/mcb.12.11.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Turner BC, Haffty BG, Narayanan L, Yuan J, Havre PA, Gumbs AA, Kaplan L, Burgaud JL, Carter D, Baserga R, Glazer PM. Insulin-like growth factor-I receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res. 1997;57:3079–83. [PubMed] [Google Scholar]
- 235.Heron-Milhavet L, Karas M, Goldsmith CM, Baum BJ, LeRoith D. Insulin-like growth factor-I (IGF-I) receptor activation rescues UV-damaged cells through a p38 signaling pathway. Potential role of the IGF-I receptor in DNA repair. J Biol Chem. 2001;276:18185–92. doi: 10.1074/jbc.M011490200. [DOI] [PubMed] [Google Scholar]
- 236.Heron-Milhavet L, LeRoith D. Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. J Biol Chem. 2002;277:15600–6. doi: 10.1074/jbc.M111142200. [DOI] [PubMed] [Google Scholar]
- 237.Decraene D, Agostinis P, Bouillon R, Degreef H, Garmyn M. Insulin-like growth factor-1-mediated AKT activation postpones the onset of ultraviolet B-induced apoptosis, providing more time for cyclobutane thymine dimer removal in primary human keratinocytes. J Biol Chem. 2002;277:32587–95. doi: 10.1074/jbc.M111106200. [DOI] [PubMed] [Google Scholar]
- 238.Jayanth VR, Belfi CA, Swick AR, Varnes ME. Insulin and insulin-like growth factor-1 (IGF-1) inhibit repair of potentially lethal radiation damage and chromosome aberrations and alter DNA repair kinetics in plateau-phase A549 cells. Radiat Res. 1995;143:165–74. [PubMed] [Google Scholar]
- 239.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–54. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 240.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 241.Wozniak K, Blasiak J. Recognition and repair of DNA-cisplatin adducts. Acta Biochim Pol. 2002;49:583–96. [PubMed] [Google Scholar]
- 242.Nowicki MO, Falinski R, Koptyra M, Slupianek A, Stoklosa T, Gloc E, Nieborowska-Skorska M, Blasiak J, Skorski T. BCR/ABL oncogenic kinase promotes unfaithful repair of the reactive oxygen species - dependent DNA double-strand breaks. Blood. 2004 doi: 10.1182/blood-2004-05-1941. [DOI] [PubMed] [Google Scholar]
- 243.Khanna KK, Lavin MF, Jackson SP, Mulhern TD. ATM, a central controller of cellular responses to DNA damage. Cell Death Differ. 2001;8:1052–65. doi: 10.1038/sj.cdd.4400874. [DOI] [PubMed] [Google Scholar]
- 244.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–7. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 245.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–95. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 246.Pierce AJ, Jasin M. NHEJ deficiency and disease. Mol Cell. 2001;8:1160–1. doi: 10.1016/s1097-2765(01)00424-5. [DOI] [PubMed] [Google Scholar]
- 247.Lundin C, Erixon K, Arnaudeau C, Schultz N, Jenssen D, Meuth M, Helleday T. Different roles for nonhomologous end joining and homologous recombination following replication arrest in mammalian cells. Mol Cell Biol. 2002;22:5869–78. doi: 10.1128/MCB.22.16.5869-5878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tombline G, Fishel R. Biochemical characterization of the human RAD51 protein. I. ATP hydrolysis. J Biol Chem. 2002;277:14417–25. doi: 10.1074/jbc.M109915200. [DOI] [PubMed] [Google Scholar]
- 249.Van Komen S, Petukhova G, Sigurdsson S, Sung P. Functional cross-talk among Rad51, Rad54, and replication protein A in heteroduplex DNA joint formation. J Biol Chem. 2002;277:43578–87. doi: 10.1074/jbc.M205864200. [DOI] [PubMed] [Google Scholar]
- 250.Baumann P, West SC. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci. 1998;23:247–51. doi: 10.1016/s0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 251.Lu H, Guo X, Meng X, Liu J, Allen C, Wray J, Nickoloff JA, Shen Z. The BRCA2-interacting protein BCCIP functions in RAD51 and BRCA2 focus formation and homologous recombinational repair. Mol Cell Biol. 2005;25:1949–57. doi: 10.1128/MCB.25.5.1949-1957.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Davies AA, Masson JY, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR, West SC. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell. 2001;7:273–82. doi: 10.1016/s1097-2765(01)00175-7. [DOI] [PubMed] [Google Scholar]
- 253.Petrini JH. The Mre11 complex and ATM: collaborating to navigate S phase. Curr Opin Cell Biol. 2000;12:293–6. doi: 10.1016/s0955-0674(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 254.Zhu XD, Kuster B, Mann M, Petrini JH, Lange T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat Genet. 2000;25:347–52. doi: 10.1038/77139. [DOI] [PubMed] [Google Scholar]
- 255.Chen G, Yuan SS, Liu W, Xu Y, Trujillo K, Song B, Cong F, Goff SP, Wu Y, Arlinghaus R, Baltimore D, Gasser PJ, Park MS, Sung P, Lee EY. Radiation-induced assembly of Rad51 and Rad52 recombination complex requires ATM and c-Abl. J Biol Chem. 1999;274:12748–52. doi: 10.1074/jbc.274.18.12748. [DOI] [PubMed] [Google Scholar]
- 256.Essers J, Houtsmuller AB, van Veelen L, Paulusma C, Nigg AL, Pastink A, Vermeulen W, Hoeijmakers JH, Kanaar R. Nuclear dynamics of RAD52 group homologous recombination proteins in response to DNA damage. Embo J. 2002;21:2030–7. doi: 10.1093/emboj/21.8.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sigurdsson S, Van Komen S, Petukhova G, Sung P. Homologous DNA pairing by human recombination factors Rad51 and Rad54. J Biol Chem. 2002;277:42790–4. doi: 10.1074/jbc.M208004200. [DOI] [PubMed] [Google Scholar]
- 258.Tombline G, Shim KS, Fishel R. Biochemical characterization of the human RAD51 protein. II. Adenosine nucleotide binding and competition. J Biol Chem. 2002;277:14426–33. doi: 10.1074/jbc.M109916200. [DOI] [PubMed] [Google Scholar]
- 259.Myers MG, Jr, Backer JM, Sun XJ, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White MF. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci U S A. 1992;89:10350–4. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Baskin DG, Schwartz MW, Sipols AJ, D’Alessio DA, Goldstein BJ, White MF. Insulin receptor substrate-1 (IRS-1) expression in rat brain. Endocrinology. 1994;134:1952–5. doi: 10.1210/endo.134.4.7511094. [DOI] [PubMed] [Google Scholar]
- 261.Sell C, Rubini M, Rubin R, Liu JP, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993;90:11217–21. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.D’Ambrosio C, Keller SR, Morrione A, Lienhard GE, Baserga R, Surmacz E. Transforming potential of the insulin receptor substrate 1. Cell Growth Differ. 1995;6:557–62. [PubMed] [Google Scholar]
- 263.Tu X, Batta P, Innocent N, Prisco M, Casaburi I, Belletti B, Baserga R. Nuclear translocation of insulin receptor substrate-1 by oncogenes and Igf-I. Effect on ribosomal RNA synthesis. J Biol Chem. 2002;277:44357–65. doi: 10.1074/jbc.M208001200. [DOI] [PubMed] [Google Scholar]
- 264.Prisco M, Santini F, Baffa R, Liu M, Drakas R, Wu A, Baserga R. Nuclear translocation of insulin receptor substrate-1 by the simian virus 40 T antigen and the activated type 1 insulin-like growth factor receptor. J Biol Chem. 2002;277:32078–85. doi: 10.1074/jbc.M204658200. [DOI] [PubMed] [Google Scholar]
- 265.Boylan JM, Gruppuso PA. Insulin receptor substrate-1 is present in hepatocyte nuclei from intact rats. Endocrinology. 2002;143:4178–83. doi: 10.1210/en.2002-220321. [DOI] [PubMed] [Google Scholar]
- 266.Tu X, Baffa R, Luke S, Prisco M, Baserga R. Intracellular redistribution of nuclear and nucleolar proteins during differentiation of 32D murine hemopoietic cells. Exp Cell Res. 2003;288:119–30. doi: 10.1016/s0014-4827(03)00178-2. [DOI] [PubMed] [Google Scholar]
- 267.Seol KC, Kim SJ. Nuclear matrix association of insulin receptor and IRS-1 by insulin in osteoblast-like UMR-106 cells. Biochem Biophys Res Commun. 2003;306:898–904. doi: 10.1016/s0006-291x(03)01046-5. [DOI] [PubMed] [Google Scholar]
- 268.Morelli C, Garofalo C, Sisci D, del Rincon S, Cascio S, Tu X, Vecchione A, Sauter ER, Miller WH, Jr, Surmacz E. Nuclear insulin receptor substrate 1 interacts with estrogen receptor alpha at ERE promoters. Oncogene. 2004;23:7517–26. doi: 10.1038/sj.onc.1208014. [DOI] [PubMed] [Google Scholar]
- 269.Chen J, Wu A, Sun H, Drakas R, Garofalo C, Cascio S, Surmacz E, Baserga R. Functional significance of type 1 insulin-like growth factor-mediated nuclear translocation of the insulin receptor substrate-1 and beta-catenin. J Biol Chem. 2005;280:29912–20. doi: 10.1074/jbc.M504516200. [DOI] [PubMed] [Google Scholar]
- 270.Drakas R, Tu X, Baserga R. Control of cell size through phosphorylation of upstream binding factor 1 by nuclear phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 2004;101:9272–6. doi: 10.1073/pnas.0403328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Trojanek J, Croul S, Ho T, Wang JY, Darbinyan A, Nowicki M, Valle LD, Skorski T, Khalili K, Reiss K. T-antigen of the human polyomavirus JC attenuates faithful DNA repair by forcing nuclear interaction between IRS-1 and Rad51. J Cell Physiol. 2006;206:35–46. doi: 10.1002/jcp.20425. [DOI] [PubMed] [Google Scholar]
- 272.Trojanek J, Ho T, Croul S, Wang JY, Chintapalli J, Koptyra M, Giordano A, Khalili K, Reiss K. IRS-1-Rad51 nuclear interaction sensitizes JCV T-antigen positive medulloblastoma cells to genotoxic treatment. Int J Cancer. 2006 doi: 10.1002/ijc.21828. [DOI] [PubMed] [Google Scholar]