Abstract
Neurogenin 3 is essential for enteroendocrine cell development; however, it is unknown whether this transcription factor is sufficient to induce an endocrine program in the intestine or how it affects the development of other epithelial cells originating from common progenitors. In this study, the mouse villin promoter was used to drive Neurogenin 3 expression throughout the developing epithelium to measure the affect on cell fate. Although the general morphology of the intestine was unchanged, transgenic founder embryos displayed increased numbers of cells expressing the pan-endocrine marker chromogranin A. Accordingly, expression of several hormones and pro-endocrine transcription factors were increased in the transgenics suggesting that Neurogenin 3 stimulated a program of terminal enteroendocrine cell development. To test whether increased endocrine cell differentiation affected the development of other secretory cell lineages, we quantified goblet cells, the only other secretory cell formed in embryonic intestine. The Neurogenin 3-expressing transgenics had decreased numbers of goblet cells in correspondence to the increase in endocrine cells, with no change in the total secretory cell numbers. Thus, our data suggest that Neurogenin 3 can redirect the differentiation of bipotential secretory progenitors to endocrine rather than goblet cell fate.
Keywords: transgenic mice, intestine development, notch signaling, stem cell, cell fate, enteroendocrine cell
Introduction
Multipotential stem cells in the intestine give rise to two general cell lineages to form the epithelium. The absorptive or columnar lineage forms enterocytes, the predominant cell type, which is responsible for absorption of nutrients. The secretory or granulocytic lineage forms three distinct cell types, including goblet cells, enteroendocrine cells and Paneth cells, which are responsible for secretion of mucus, hormones and antimicrobial peptides, respectively. With the exception of Paneth cells, which do not emerge until crypt formation after birth, these various cell types are first established in fetal development during intestinal organogenesis. Cell specification first occurs around the time of morphological transformation from a pseudostratified to a columnar epithelium with the emergence of villi, and then continues throughout the lifespan of the organism with constant replenishment of the epithelium from stem cells in the crypts. Notch signaling appears to play a critical role in the fate decision between the absorptive and secretory lineages. Disruption of Notch signaling in the intestine results in loss of enterocytes and increased numbers of secretory cells (Jensen et al., 2000; van Es et al., 2005). The Notch-regulated basic-helix-loop-helix (bHLH) transcription factor Math1 (mouse atonal homologue 1) is thought to be the key determinant of secretory cell development, as demonstrated by the loss of all intestinal secretory cell types in mice carrying a Math1 null mutation (Yang et al., 2001).
The mechanisms regulating the development of the various secretory cell types from multipotential precursors in the intestine are not fully understood. Mature endocrine cells arise from Neurogenin 3 (Neurog3)-expressing progenitor cells in the proliferative zone near the resident stem cell population (Bjerknes and Cheng, 2006; Jenny et al., 2002; Schonhoff et al., 2004). The importance of this bHLH transcription factor for specification of the enteroendocrine cell lineages is demonstrated by loss of all intestinal endocrine cells in mice carrying a Neurog3 null mutation (Jenny et al., 2002). A similar finding, near complete loss of enteroendocrine cells, was also recently reported for human patients with NEUROG3 gene mutations (Wang et al., 2006). Endocrine progenitors normally form numerous distinct mature cell types, each defined by its specific hormone product, including cholecystokinin (CCK), secretin, somatostatin, serotonin and many others (Hocker and Wiedenmann, 1998). Although Neurog3 is not expressed in mature endocrine cells, it is known to stimulate pro-endocrine transcription factors, such as Neurod1, Pax4, Pax6, Nkx2.2, and IA-1 (Heremans et al., 2002; Huang et al., 2000; Mellitzer et al., 2006; Smith et al., 2003; Treff et al., 2006). These and other pro-endocrine transcription factors are thought to be responsible for differentiation and maintenance of the mature endocrine populations. For example, Neurod1 is known to stimulate cell cycle withdrawal and transcription of the hormone secretin (Mutoh et al., 1998; Naya et al., 1997). Mice with a Neurod1 null mutation are missing secretin-and CCK-cells, although they maintain many other enteroendocrine cells, including serotonin- and glucagon-expressing cells (Mutoh et al., 1998; Naya et al., 1997). Neurog3 is also the key determining factor for endocrine cell development in the pancreas as demonstrated by the loss of pancreatic endocrine cell precursors and mature pancreatic endocrine cells in Neurog3-deficient mice (Jenny et al., 2002). Analysis of these null mice has also shown that Neurog3 is required for the development of a subset of endocrine cells in the glandular stomach (Jenny et al., 2002; Lee et al., 2002).
Ectopic expression studies in transgenic mouse models have shown that Neurog3 is sufficient to induce some aspects of pancreatic endocrine cell development. A predominant formation of glucagon-expressing cells was observed in mice carrying transgenes that activate Neurog3 expression in the early pancreatic endodermal bud (Apelqvist et al., 1999; Schwitzgebel et al., 2000). Similar results were seen in early chick embryos electroporated with a mouse Neurog3 construct, where endodermal expression stimulated cell migration to form islet-like cell clusters that express glucagon and somatostatin (Grapin-Botton et al., 2001). Thus, ectopic expression of Neurog3 appears to stimulate early pancreatic progenitors to differentiate along certain pancreatic islet cell lineages. However, whether Neurog3 is sufficient to promote endocrine cell differentiation in the intestine has not been investigated. In this study we generated transgenic mice that express Neurog3 throughout the developing intestinal epithelium. We observed a widespread induction of enteroendocrine cells. Surprisingly, the increase in endocrine cell number was countered by a decrease in the number of goblet cells, suggesting that Neurog3 affects the choice of a bipotential secretory cell to adopt endocrine rather than goblet cell fate.
Materials and methods
Generation of Vil-Neurog3 transgenic embryos
The Vil-Neurog3 transgene construct contained the mouse Neurog3 cDNA under the control of the mouse villin enhancer/promoter followed by SV40 sequences that provided a polyA+ site (Figure 1A). To engineer the transgene, a DNA fragment containing the 646 bp Neurog3 coding sequence was isolated from pCS2+ mNeurog3 (Vojtek et al., 2003) after BamHI/XbaI digestion and shuttled into pBluescript SK before cloning into the villin expression plasmid pBSII-12.4KbVill/ΔATG (Madison et al., 2002) with ClaI/SacII digestion. Following verification of the construct by sequencing, the 14.0 kb transgene was excised from the vector with PmeI and microinjected into F2 zygotes from C57BL/6 X SJL parents by the University of Michigan Transgenic Animal Model Core. Potential transgenic founders were harvested at E18.5 and screened for the transgene by PCR amplification of genomic DNA (298 bp product) (Lopez-Diaz et al., 2006) using the following primers: V1S 5′ GTAACAGGCACTAAGGGAGCCAATGTAGAC and Neurog3PCR 5′ ACACTTGGATGGTGAGCGCATCCAAGGGAT.
Figure 1.
Generation of Vil-Neurog3 transgenics. A) The transgene construct included the mouse Neurog3 coding region (grey box) regulated by the 12.4 kb mouse villin enhancer/promoter with SV40 sequences (white box) providing polyA+ recognition sequences. B) Total Neurog3 expression (transgene and endogenous) measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR) showed transgenic embryos 71, 76 and 179 to be the highest expressing. The data are represented as fold-change in mRNA concentration in comparison to nontransgenic (Ntg) littermate controls (mean ± SEM). C–E) Neurog3 immunostaining demonstrated a marked increase in Neurog3-expressing cells in Vil-Neurog3 transgenics. In Ntg controls, Neurog3 expression is limited to the nuclei of rare epithelial cells in the proliferating intervillus zone (C, arrowheads). In contrast, transgenic founder embryos (71 (D) and 179 (E)) showed a large number of Neurog3 positive cells in the villus epithelium as well as the intervillus zone (arrowheads). Nuclear DAPI staining was pseudocolored red to show colocalization (yellow) with the Neurog3 antibody (green). Bar = 50 μm.
Tissue morphology and immunohistochemistry
Intestine was dissected from potential E18.5 Vil-Neurog3 transgenic founders, and the proximal region (one cm distal to the pylorus) was paraffin embedded after fixing overnight in 4% paraformaldehyde. Sections (4 μm) were stained with H&E, alcian blue and PAS (Newcomer Supply Company), alkaline phosphatase (Red Alkaline Phosphatase Kit, Vector Laboratories), or immunostained using the following primary antibodies: rabbit anti-CgA (1:500; 94188/5 gift from J. F. Rehfeld), goat anti-5HT (serotonin) (1:500, Immunostar #20079) and rabbit anti-CCK (1:1000, Chemicon #AB1972) followed by appropriate secondary antibodies conjugated to Cy2 or Cy3 (1:500, Jackson ImmunoResearch Laboratories). After deparaffinization and rehydration, sections were subjected to antigen retrieval with Antigen Unmasking Solution (Vector Labs) at 100°C for 10 min, cooled, rinsed in PBS and incubated in TPBS (0.01% Triton X-100 in phosphate buffered saline (PBS)) for 5 min. Blocking was performed for 30 min at room temperature with TPBS containing 10% donkey serum and 1% bovine serum albumin (BSA). Primary antibodies were incubated overnight at 4°C in PBS containing 1% BSA and 0.1% Triton X-100. Slides were rinsed in TPBS, blocked for a second time and incubated with secondary antibodies in 1% BSA and 0.1% Triton X-100 for 30 min, rinsed in PBS and mounted with coverslips in ProLong® Gold (Molecular Probes) containing 1 μg/ml 4′, 6-diamidino-2-phenylindole (DAPI). Microscopy was performed with a Nikon E800 equipped with a SPOT digital camera or a Zeiss LSM 510 confocal microscope.
Neurog3 immunostaining used a mouse monoclonal antibody (Zahn et al., 2004) (1:4000, F25A1B3 concentrated) obtained from the NICHD Developmental Studies Hybridoma Bank, (University of Iowa) with tyramide signal amplification (TSA Kit #2, Molecular Probes-Invitrogen). Briefly, deparaffinized and rehydrated paraffin sections were subjected to antigen retrieval, endogenous peroxidase activity was quenched with 3% H2O2 for 1 hr., blocked as recommended by the TSA kit, and incubated overnight with Neurog3 monoclonal antibody in blocking solution at 4 °C. After rinsing with PBS, slides where incubated with HRP-conjugated goat-anti-mouse IgG (1:100, provided in TSA kit) and Alexa Fluor® 488 tyramide amplification was performed according to manufacturer’s recommendations.
Morphometric analysis
For each founder embryo the complete region of intact tissue on a stained paraffin section was captured in contiguous digital images (5–15 fields). A single individual blinded to genotype performed the morphometric analysis using Image J (1.34s by Wayne Rasband, NIH, USA; http://rsb.info.nih.gov/ij/) to measure the total area of epithelial tissue contained in each composite image, and count positively stained cells within the region. Data were expressed as number of positive cells/area of epithelium (μm2).
Analysis of gene expression
RNA from a small intestine segment (~ 2 cm region distal to the paraffin embedded segment) was isolated and DNase-treated using the RNeasy Mini kit (Qiagen). Gene expression was determined by qRT-PCR analysis of transgenic founders and Ntg littermate controls. Reverse transcription used the Iscript cDNA synthesis kit (Bio-Rad) with 20–100 ng/μl RNA, and qRT-PCR was performed using an Icycler (Bio-Rad) with SYBR Green dye (Molecular Probes) as previously described (Jain et al., 2006). The qRT-PCR primers are listed in Supplemental Table 1. Expression levels were determined for individual embryos with N=3 assays per sample. Expression levels were normalized to the expression of Gapdh, which remained the same in transgenic and Ntg controls.
Statistical analysis
Quantitative data were presented as mean ± SEM and analyzed using Student’s t-test with P < 0.05 considered significant.
Results and Discussion
Generation of Vil-Neurog3 transgenics
To determine whether overexpression of Neurog3 in the developing intestinal epithelium is sufficient to trigger a program of endocrine cell differentiation, we engineered mouse embryos that expressed Neurog3 under the control of the villin promoter (Fig. 1A). Previous studies demonstrated that the villin transgene promoter fragment is expressed throughout the epithelium, including stem and progenitor cells, with expression first detected at embryonic day 12.5 (E12.5) (Madison et al., 2002). We studied Vil-Neurog3 transgenic founders at late embryonic development (E18.5), focusing on the proximal small intestine, the site of highest villin transgene expression. Seven expressing transgenics were identified by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR); the highest expressing transgenic animals (71, 76 and 179) contained a 100–200 fold increase in total Neurog3 mRNA compared to nontransgenic (Ntg) littermate controls (Fig. 1B). Immunostaining for Neurog3 showed increased numbers of Neurog3-positive cells in the epithelium of Vil-Neurog3 transgenics, including positive cells on the villi in addition to the normal pattern of expression in rare cells in the proliferative intervillus zone (Fig. 1C–E).
Increased endocrine cell development in Vil-Neurog3 transgenics
The morphology of the Vil-Neurog3 transgenic intestine was grossly normal with typical villus structure (Fig. 2A, E). However, immunostaining for the pan-endocrine marker chromogranin A (CgA) showed a marked increase in endocrine cells (Fig. 2B, F). Morphometric analysis revealed that the high expressing transgenics, 71 and 179, had 8.7-fold increases in CgA positive cells, while the other transgenics (76, 120, 124 and 151) had smaller but still significant differences, ranging from 1.8- to 3.4-fold increased endocrine cell number when compared to Ntg (Fig. 2I). Increased CgA expression was also shown by qRT-PCR, with 16- and 9- fold increased mRNA abundance in the intestine of transgenics 71 and 179, respectively (Fig. 3A). In addition to increased numbers, the distribution of CgA positive cells was altered. Normally both endocrine cells and goblet cells appear singly in the intestinal epithelium and are not in close proximity. This pattern likely reflects Notch-mediated lateral inhibition (Apelqvist et al., 1999; Bjerknes and Cheng, 2005). However, in Vil-Neurog3 transgenics endocrine cells were frequently clustered (Fig. 2F insert), suggesting that transgenic expression of Neurog3 altered the lateral inhibition process that orchestrates the normal pattern of secretory cell distribution.
Figure 2.
Increased endocrine cells in Vil-Neurog3 transgenics. Paraffin sections from transgenic founder embryos and Ntg controls were H&E stained for analysis of cellular morphology (A, E) and for endocrine cells by immunostaining, including antibodies to chromogranin A (CgA) (B, F), serotonin (C, G), and cholecystokinin (CCK) (D, H). Shown are Ntg 50 (A–D) and transgenic 179 (E–H). Confirmed endocrine cells, which are epithelial cells staining for hormone in the basolateral region, are marked with arrowheads. Confocal microscope analysis of CgA staining showed that endocrine cells in the transgenic mice frequently lie next to each other (3 adjacent cells are shown in F insert) instead of the normal pattern of scattered single cells. Costaining for serotonin (green) and CCK (red) demonstrated that endocrine cells induced by Neurog3 expression contained a single product and thus are similar to normal, terminally differentiated endocrine cells (H insert). Immunostained cells were counted and displayed as fold-change in cell number in comparison to Ntg50 (mean ± SEM), including CgA positive cells (I), serotonin-positive cells (J) and CCK-positive cells (K). Bar = 50 μm.
Figure 3.
Endocrine gene expression is increased in Vil-Neurog3 transgenics. qRT-PCR analysis of intestine RNA from transgenic founder embryos (71, 76 and 179) and Ntg littermate controls, including the pan-endocrine marker CgA (A), the serotonin converting enzyme tryptophan hydroxylase 1 (Tph-1) (B), secretin (C), CCK (D), somatostatin (SST) (E), and glucagon (F). Data were normalized to Gapdh expression in the same samples and reported as fold change (mean ± SEM) from levels in Ntg littermates (*P < 0.05).
Increased expression of hormone products was also observed by immunostaining and measurement of endocrine-specific transcripts by qRT-PCR. The number of serotonin expressing cells was increased 13-, 3-, and 17-fold in transgenics 71, 76 and 179, respectively (Fig. 2C, G, J). Accordingly, mRNA concentration of the serotonin converting enzyme tryptophan hydroxylase 1 (Tph1) was increased as much as 30-fold (Fig. 3B). Secretin mRNA abundance was also increased 2 to 4-fold (Fig. 3C). To test whether individual endocrine cells in Vil-Neurog3 transgenics had the normal characteristic of expression of a single hormone product, Tg 179 was immunostained for both serotonin and CCK in the same section. The results showed staining of each antibody in distinct cell populations, which is the normal pattern for mature endocrine cells (Fig 2H, insert). This suggests that terminal endocrine cell differentiation took place in the presence of Neurog3 overexpression.
Increased expression of pro-endocrine transcription factors in Vil-Neurog3 transgenics
Consistent with enhanced secretin gene transcription, expression of the bHLH transcription factor Neurod1 was increased in Vil-Neurog3 transgenics (Fig. 4A). Neurod1, a downstream target of Neurog3, is thought to participate in the terminal differentiation of endocrine cells in the intestine and pancreas. It has been shown to stimulate secretin gene transcription (Mutoh et al., 1997) and to induce cell cycle arrest by induction of the gene encoding the cyclin-dependent kinase inhibitor p21 (Mutoh et al., 1998). Moreover, Neurod1-deficient mice have abnormal enteroendocrine cell differentiation, with loss of secretin- and CCK-cells, although serotonin and other endocrine cell populations are retained (Naya et al., 1997). Our data are consistent with these earlier studies, which suggest that Neurod1 is a key transcription factor specifying subpopulations of enteroendocrine cells by increasing hormone gene expression and regulating cell cycle arrest. However, unlike Neurog3, Neurod1 may not be sufficient for induction of endocrine cell development since our parallel studies with Villin-Neurod1 transgenic mouse embryos did not reveal increased endocrine cell development or hormone marker expression, including CCK and secretin (data not shown).
Figure 4.
Increased gene expression of pro-endocrine transcription factors in Vil-Neurog3 transgenics. qRT-PCR analysis of Neurod1 (A), Pax 4 (B) and the general epithelial transcription factor Pdx-1 (C) in intestine RNA from transgenic founder embryos (71, 76 and 179) and Ntg littermate controls. Data were normalized to Gapdh expression in the same samples and reported as fold change (mean ± SEM) from levels in Ntg littermates (*P < 0.05).
We also observed increased expression of the paired-box homeoprotein Pax4 in Vil-Neurog3 transgenics (Fig. 4B). This pro-endocrine transcription factor has been shown to be upregulated by Neurog3 in cell culture studies (Smith et al., 2003). Pax4-deficient mice have disrupted development of specific endocrine cell populations in pancreas and intestine, with loss of insulin- and somatostatin-cells in the islet and more widespread loss of enteroendocrine cells, including CCK, secretin, serotonin, GIP and peptide YY cells (Larsson et al., 1998). Our data supports the conclusion that Pax4 is a Neurog3 target that contributes to the maturation of enteroendocrine cells from Neurog3 expressing progenitors. In contrast, Pdx-1 expression, which is a general epithelial cell marker in the proximal intestine, was not changed in Vil-Neurog3 transgenics (Fig. 4C).
Interestingly, there was significant variation in the expression of specific hormones among the transgenic founders. Transgenic 179 had increased numbers of immunopositive CCK cells and increased CCK mRNA, while transgenics 71 and 76 did not differ from Ntg controls (Fig. 2D, H, K; Fig. 3D). Somatostatin mRNA was increased in the two highest expressing transgenics 71 and 179, but was normal in 76, while glucagon and gastrin mRNA were normal in both 71 and 179 (Fig. 3E, F and data not shown). The basis for this variation is not clear, but it may be a consequence of differences in expression of pro-endocrine transcription factors among the various transgenic founders. Indeed, there were substantial differences in the levels of Neurod1 and Pax4 (Fig. 4). Perhaps the variable level of transgene expression contributes to the differences in phenotype among the transgenic founders. In addition, although not examined in this study, it is also expected that founder embryos will vary in the timing of activation of the Vil-Neurog3 transgene, since this has been observed in other studies using the villin promoter (Gumucio, unpublished). Future studies of transgenic lines that differ in expression might help to assess what affect timing and level of expression might have on developmental decisions.
Our results stand in interesting contrast to studies on the ability of Neurog3 to induce endocrine cell development in the developing pancreas. Transgenic mice expressing Neurog3 from the Pdx-1 promoter, which is highly expressed in the pancreatic endodermal buds, results in the precocious differentiation of glucagon-expressing endocrine cells (Apelqvist et al., 1999; Schwitzgebel et al., 2000). In addition, Pdx-Neurog3 transgenics exhibited a profound loss of exocrine cells, the predominant tissue type of the mature pancreas (Apelqvist et al., 1999; Schwitzgebel et al., 2000). In contrast, our studies of Neurog3 expression from the villin promoter showed increased endocrine cell development without disturbing the gross organogenesis of the intestine; the predominant enterocyte lineage remained largely undisturbed (Fig. 5A–C). In addition, our study shows that Neurog3 transgene expression in the developing intestine induces the development of different endocrine cell populations than expression in the early pancreatic endoderm where Neurog3 induced primarily glucagon-expressing cells (Apelqvist et al., 1999; Grapin-Botton et al., 2001; Schwitzgebel et al., 2000). We did not observe increased glucagon expression in Vil-Neurog3 transgenics (Fig. 3F). Thus, Neurog3 overexpression can have different consequences, promoting the differentiation of distinct endocrine cell lineages depending on the specific progenitor cell in which it is expressed or differences in the cellular environment for organ development.
Figure 5.
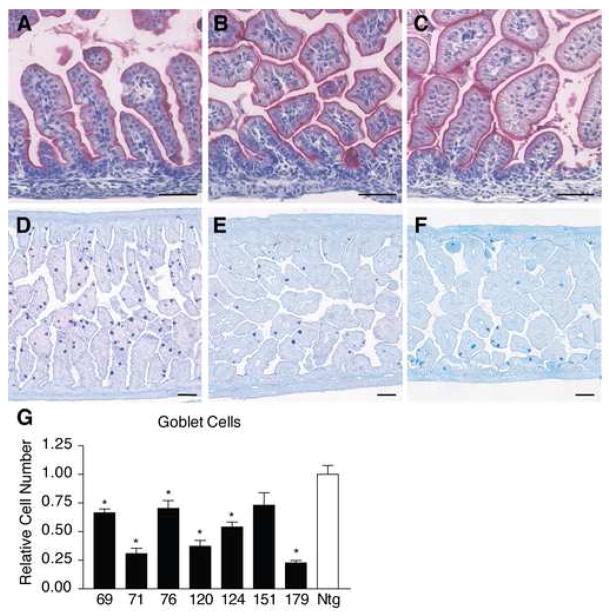
Normal enterocytes and decreased numbers of goblet cells in Vil-Neurog3 transgenics. A–C) Enterocytes were visualized by alkaline phosphatase staining of paraffin sections from Ntg 50 (A), Tg 71 (B) and Tg 179 (C) showing similar staining. D–E) Goblet cells were visualized by PAS/alcian blue staining of paraffin sections from Ntg 50 (A), Tg 71 (B) and Tg 179 (C) showing dramatic reductions in stained cells in the transgenics. G) Goblet cell numbers were counted in each of the Vil-Neurog3 founders and displayed as relative cell numbers (mean ± SEM) compared to Ntg 50 (*P < 0.05).
Fewer goblet cells in Vil-Neurog3 transgenics
To test whether increased endocrine cell numbers affected the differentiation of other secretory cells we examined goblet cells in the Vil-Neurog3 transgenic embryos. Since Paneth cells are not formed until after birth, secretory cells in the E18.5 transgenics are composed of endocrine and goblet cells. Remarkably, there was a visible reduction in goblet cells in the Vil-Neurog3 transgenics (Fig. 5D–F). Morphometric analysis showed a 1.5- to 4.7- fold decrease in the various transgenics when compared to Ntg (Fig. 5G). Furthermore, the decrease corresponded roughly to the increase in endocrine cells, with transgenics 71 and 179 exhibiting the largest increase in endocrine cells and the greatest reduction in goblet cells. The total number of secretory cells was estimated by adding the number of endocrine cells to the number of goblet cells in individual embryos (Fig. 6A). This analysis revealed that transgenics 71 and 179 had similar total numbers of secretory cells as their Ntg littermates. Consistent with this was the finding that expression of the secretory cell specific transcription factor Math-1 was unchanged in these transgenics, as was expression of the Notch ligand Delta-Like 1 and the Notch-stimulated transcriptional repressor Hes-1 (data not shown). However, the distribution of secretory cell types was dramatically changed. Normally goblet cells make up 90% of the total secretory cell population in E18.5 proximal intestine, but in the highly expressing Vil-Neurog3 transgenics this number was reduced to approximately 20%, with endocrine cells increasing from 10% in Ntg intestine to 80% of the secretory cell population in the Vil-Neurog3 transgenics (Fig. 6A). Although Paneth cells are not formed at E18.5, early cryptdin expression is detectable. Quantitation of cryptdin gene expression by qRT-PCR showed substantial variability from embryo to embryo, even among nontransgenics, but no consistent changes in the Vil-Neurog3 transgenic embryos (data not shown).
Figure 6.
Decreased goblet cell numbers match the increase in endocrine cell numbers. A) Total secretory cells were measured in embryos by adding goblet cell numbers determined from PAS/alcian blue staining to the endocrine cell numbers obtained from CgA immunostaining. Shown are cell numbers per μm2 (×104) in Ntg (50 and 223) and Tg (71 and 179) embryos. B) Model for secretory cell development in the fetal mouse intestine. Our data is consistent with a bipotential secretory progenitor cell that has the capacity to differentiate into either endocrine precursors or goblet cells. Neurog3 expression can determine which developmental pathway is chosen. The mechanism for terminal differentiation of endocrine precursors into the various hormone-producing mature cells is not known, but pro-endocrine transcription factors such as Neurod1 (Naya et al., 1997) and Pax4 (Larsson et al., 1998) among others, are likely mediators of this process.
Our studies demonstrate that Neurog3 is a critical transcription factor that determines secretory progenitor cell fate choice in intestinal cell development. The expression of Neurog3 in the intestine stimulated increased endocrine cell development to the detriment of goblet cells. This is the reverse of the cellular remodeling observed in mice with a Neurog3 null mutation. The intestine of these mutant mice have a complete absence of endocrine cells and increased numbers of goblet cells (Jenny et al., 2002). The stomachs of Neurog3-deficient mice also showed loss of specific endocrine cell populations associated with the appearance of goblet-like cells expressing intestinal markers (Lee et al., 2002). Thus, our data, together with these loss-of-function studies, are consistent with a model in which a bipotential secretory cell progenitor in the E18.5 gut differentiates into endocrine or goblet cells depending on the expression of Neurog3 (Fig. 6B).
The existence of multipotential secretory progenitors in the intestine was suggested by the loss of all endocrine, goblet and Paneth cell markers in mice containing a Math1 null mutation (Yang et al., 2001). However, since Math1 is expressed in terminally differentiated secretory cells as well as in progenitors, it is not clear what role Math1 plays in secretory cell formation versus maintenance. Whether the normal program for differentiation includes a single Math1-expressing multipotential secretory progenitor is debated. Analysis of intestinal epithelial cell development in adult mice by following clonal expansion of progenitors marked after mutagen treatment suggested the presence of bipotential progenitors capable of differentiation into enteroendocrine and columnar lineages, but no evidence for multipotential secretory progenitors capable of differentiating into more than one secretory cell type (Bjerknes and Cheng, 2006). This result suggests that multipotential precursors are committed to specific secretory lineages very early. Our data suggests that activation of Neurog3 expression may be the key trigger that commits a multipotential progenitor cell to the enteroendocrine lineage.
Supplementary Material
Acknowledgments
We thank the University of Michigan Transgenic Animal Model Core for microinjection of the Vil-Neurog3 transgene, Marta Dzaman and the University of Michigan Organogenesis Morphology Core for assistance with histology and Chris Edwards for assistance with confocal microscopy. This work was funded by NIH PO1-DK06241 (L.C. Samuelson). L. Lopez-Diaz was supported by the Cellular and Molecular Biology Training Grant (T32-GM07315) and a Porter Fellowship from the American Physiological Society; K.L. VanDussen was supported by the Systems and Integrative Physiology Training Grant (T32-GM008322).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–81. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G381–7. doi: 10.1152/ajpgi.00160.2005. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol. 2006;300:722–735. doi: 10.1016/j.ydbio.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, Majithia AR, Melton DA. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 2001;15:444–54. doi: 10.1101/gad.846001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans Y, Van De Casteele M, in’t Veld P, Gradwohl G, Serup P, Madsen O, Pipeleers D, Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol. 2002;159:303–12. doi: 10.1083/jcb.200203074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocker M, Wiedenmann B. Molecular mechanisms of enteroendocrine differentiation. Ann N Y Acad Sci. 1998;859:160–74. doi: 10.1111/j.1749-6632.1998.tb11120.x. [DOI] [PubMed] [Google Scholar]
- Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol. 2000;20:3292–307. doi: 10.1128/mcb.20.9.3292-3307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RN, Brunkan CS, Chew CS, Samuelson LC. Gene expression profiling of gastrin target genes in parietal cells. Physiol Genomics. 2006;24:124–32. doi: 10.1152/physiolgenomics.00133.2005. [DOI] [PubMed] [Google Scholar]
- Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. Embo J. 2002;21:6338–47. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Larsson LI, St-Onge L, Hougaard DM, Sosa-Pineda B, Gruss P. Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech Dev. 1998;79:153–9. doi: 10.1016/s0925-4773(98)00182-8. [DOI] [PubMed] [Google Scholar]
- Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–97. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y, Brunkan CS, Keeley T, Eaton KA, Merchant JL, Chew CS, Samuelson LC. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G970–9. doi: 10.1152/ajpgi.00461.2005. [DOI] [PubMed] [Google Scholar]
- Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- Mellitzer G, Bonne S, Luco RF, Van De Casteele M, Lenne-Samuel N, Collombat P, Mansouri A, Lee J, Lan M, Pipeleers D, Nielsen FC, Ferrer J, Gradwohl G, Heimberg H. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. Embo J. 2006;25:1344–52. doi: 10.1038/sj.emboj.7601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh H, Fung BP, Naya FJ, Tsai MJ, Nishitani J, Leiter AB. The basic helix-loop-helix transcription factor BETA2/NeuroD is expressed in mammalian enteroendocrine cells and activates secretin gene expression. Proc Natl Acad Sci U S A. 1997;94:3560–4. doi: 10.1073/pnas.94.8.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh H, Naya FJ, Tsai MJ, Leiter AB. The basic helix-loop-helix protein BETA2 interacts with p300 to coordinate differentiation of secretin-expressing enteroendocrine cells. Genes Dev. 1998;12:820–30. doi: 10.1101/gad.12.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323–34. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld JR, Vasser M, Jhurani P, Ng P, Hunter JJ, Ross J, Jr, Chien KR, Lowe DG. Distinct molecular phenotypes in murine cardiac muscle development, growth, and hypertrophy. J Mol Cell Cardiol. 1998;30:2269–80. doi: 10.1006/jmcc.1998.0787. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–54. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–42. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Smith SB, Gasa R, Watada H, Wang J, Griffen SC, German MS. Neurogenin3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J Biol Chem. 2003;278:38254–9. doi: 10.1074/jbc.M302229200. [DOI] [PubMed] [Google Scholar]
- Treff NR, Vincent RK, Budde ML, Browning VL, Magliocca JF, Kapur V, Odorico JS. Differentiation of embryonic stem cells conditionally expressing neurogenin 3. Stem Cells. 2006;24:2529–37. doi: 10.1634/stemcells.2006-0082. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–63. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Vojtek AB, Taylor J, DeRuiter SL, Yu JY, Figueroa C, Kwok RP, Turner DL. Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol Cell Biol. 2003;23:4417–27. doi: 10.1128/MCB.23.13.4417-4427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, Hill ID, Vargas JH, Gershman G, Farmer DG, Reyen L, Martin MG. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–80. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–8. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Zahn S, Hecksher-Sorensen J, Pedersen IL, Serup P, Madsen O. Generation of monoclonal antibodies against mouse neurogenin 3: a new immunocytochemical tool to study the pancreatic endocrine progenitor cell. Hybrid Hybridomics. 2004;23:385–8. doi: 10.1089/hyb.2004.23.385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.