Abstract
We have previously shown that mice lacking the IL-12-specific receptor subunit β2 (IL-12Rβ2) develop more severe experimental autoimmune encephalomyelitis than wild-type (WT) mice. The mechanism underlying this phenomenon is not known; nor is it known whether deficiency of IL-12Rβ2 impacts other autoimmune disorders similarly. In the present study we demonstrate that IL-12Rβ2−/− mice develop earlier onset and more severe disease in the streptozotocin-induced model of diabetes, indicating predisposition of IL-12Rβ2-deficient mice to autoimmune diseases. T cells from IL-12Rβ2−/− mice exhibited significantly higher proliferative responses upon TCR stimulation. The numbers of naturally occurring CD25+CD4+ regulatory T cells (Tregs) in the thymus and spleen of IL-12Rβ2−/− mice were comparable to those of WT mice. However, IL-12Rβ2−/− mice exhibited a significantly reduced capacity to develop Tregs upon stimulation with TGF-β, as shown by significantly lower numbers of CD25+CD4+ T cells that expressed Foxp3. Functionally, CD25+CD4+ Tregs derived from IL-12Rβ2−/− mice were less efficient than those from WT mice in suppressing effector T cells. The role of IL-12Rβ2 in the induction of Tregs was confirmed using small interfering RNA. These findings suggest that signaling via IL-12Rβ2 regulates both the number and functional maturity of Treg cells, which indicates a novel mechanism underlying the regulation of autoimmune diseases by the IL-12 pathway.
Interleukin-12, a heterodimeric cytokine produced by activated APCs (1), has been shown to play an important role in the differentiation and expansion of Th1 CD4+ cells, which primarily elicit inflammatory responses (1, 2). IL-12 exerts its effects through a receptor (IL-12R) composed of two chains, IL-12Rβ1 and IL-12Rβ2 (3). IL-12Rβ1 subunit also participates in another heterodimeric receptor, the IL-23 receptor, whereas the only known function of IL-12Rβ2 is to act as transducer of IL-12 signals. This makes IL-12Rβ2-deficient mice a valuable tool to study the specific role of IL-12 in the immune system. We and others have found that mice lacking IL-12 or IL-12Rβ2 develop significantly more severe clinical and pathological signs of experimental autoimmune encephalomyelitis (EAE),3 a CD4+ T cell-mediated autoimmune disease of the CNS (4–7). Lymphocytes of IL-12Rβ2−/− mice produced higher levels of proinflammatory cytokines TNF-α and IL-17 (4). Furthermore, early administration of IL-12 suppresses EAE, associated with an increase in IL-10 production (8). These results suggest that IL-12 plays an immunoregulatory role in autoimmune disorders. Indeed, it has been suggested that IL-12 is a two-faced cytokine: a proinflammatory and a key immunoregulatory molecule (1, 9). Thus far, this important dichotomy has not been addressed in depth. It is also unknown whether enhanced disease in IL-12Rβ2−/− mice is specific to the EAE model, or whether IL-12Rβ2-deficiency has more universal consequences, impacting other autoimmune diseases similarly.
CD4+CD25+ regulatory T cells (Tregs) are a unique population of professional suppressor cells that constitute 5–10% of peripheral CD4+ T cells. These cells play an important role in tolerance induction and in the inhibition of autoimmune diseases (10–12). The forkhead family transcription factor Foxp3 has been shown to govern lineage-specific differentiation of Tregs in a manner independent of CD25 expression (13–17). Injection of TGF-β for 5 days during the late phase of immunization for collagen-induced arthritis or for EAE protects against the development of these autoimmune diseases (18). TGF-β induced Foxp3 gene expression in TCR-challenged CD4+CD25− naive T cells, which mediated their transition toward a Treg phenotype with potent immunosuppressive potential (19 –21). Thus, TGF-β has been shown to be critical to the generation of CD4+CD25+Foxp3+ Tregs (22, 23).
In the present study, we first addressed whether enhanced autoimmunity in IL-12Rβ2−/− mice is a general phenomenon by studying multiple low doses of streptozotocin (STZ)-induced diabetes, an autoimmune disorder in which autoreactive T cells attack pancreatic islets (24). Compared with wild-type (WT) mice, IL-12Rβ2−/− mice exhibited earlier onset and more severe STZ-induced diabetes, with pronounced insulitis. To address the mechanism underlying enhanced autoimmunity in mice lacking IL-12 responsiveness, we tested our hypothesis that CD4+ T cells from IL-12Rβ2−/− mice have a reduced capacity to differentiate into Tregs. Our results indicate that IL-12Rβ2 plays an important role in the development of CD4+CD25+ Tregs.
Materials and Methods
Animals
Female 8-wk-old mice homozygous for IL-12Rβ2 mutation and their WT control (both on B6 × 129 background) were purchased from The Jackson Laboratory. The IL-12Rβ2 gene mutation was created and screened by RT-PCR and Southern blot analysis as described (25). All work was performed in accordance with the guidelines for animal use and care at Thomas Jefferson University.
STZ-diabetic model induction
Autoimmune diabetes can be induced experimentally by treating susceptible strains of mice with multiple low doses of STZ (24, 26). To induce this disease, mice were injected i.p. with freshly made STZ (Sigma-Aldrich) at 50 mg/kg/day in citrate-saline buffer, pH 4.5 for 5 consecutive days, following the protocol described previously (24, 26). STZ-injected mice were bled via tail veins every 5 days and blood glucose levels were determined using Accu-Check Advantage Blood glucose meter (Roche). Significantly hyperglycemic animals (plasma glucose >250 mg/dl) were considered diabetic.
Histopathology
Mice were sacrificed and pancreata were harvested at day 40 after diabetes induction. Five-μm pancreatic sections were stained with H&E and slides were assessed in a blinded fashion for inflammation (27). The incidence and severity of insulitis were analyzed in four paraffin sections per pancreas, separated by 150 μm, and stained with H&E. Mononuclear cell (MNC) infiltration and insulitis were scored as follows: noninfiltrated, peri-insulitis (MNCs surrounding islets and ducts but no infiltration of the islet architecture), moderate insulitis (MNCs infiltrating <50% of the islet architecture), and severe insulitis (>50% of the islet tissue infiltrated by lymphocytes and/or loss of islet architecture) (27).
Cell preparation from lymphoid organs
To study Treg differentiation of CD4+ T cells in IL-12Rβ2−/− and WT mice, naive mice were sacrificed and MNCs were harvested from spleen and thymus. Erythrocytes in the cell pellet of the spleen were hemolyzed by NH4Cl-Tris buffer for 5 min at room temperature, followed by washing. CD4+ T cells from spleen were isolated using magnetic microbeads (Miltenyi Biotec). CD4+CD25+ and CD4+CD25− T cells were purified by CD4+CD25+ Treg isolation kit (Miltenyi Biotec). The purity of cell sub-populations was confirmed by FACSAria (BD Biosciences) analysis and was consistently >98%.
CFSE assays
CFSE was obtained from Invitrogen. Purified T cell populations of interest were washed and resuspended in PBS containing 5 μM CFSE. After incubation for 3–5 min at room temperature, a 1/5th volume of FBS was added for 30 s, and labeled cells were washed and subjected to proliferation assays. CD4+, CD4+CD25+, and CD4+CD25− T cells (1 × 106 cells/well) were cultured in the presence or absence of 1.0 μg/ml anti-CD3 and 0.2 μg/ml anti-CD28 combined with 50 U/ml mouse rIL-2. After 72 h of culture, stained cells were analyzed with a FACSAria (BD Biosciences).
TGF-β treatment and proliferation assay by [3H]thymidine incorporation
CD4+CD25+ cells were generated in vitro, following a previously described protocol (21). In brief, freshly isolated spleen cells from IL-12Rβ2−/− and WT mice were initially stimulated by 1.0 μg/ml anti-CD3 and 0.2 μg/ml anti-CD28 for 48 h in the presence or absence of 5 ng/ml rTGF-β1 (PeproTech), followed by a resting phase for another 96 h with 50 U/ml mouse rIL-2. Cell pellets were harvested for flow cytometric analysis, and supernatants were collected for cytokine detection. Purified CD4+CD25+ and CD4+CD25− T cells were cocultured with spleen cells from WT mice at 1:1 ratio under the stimulation of 1.0 μg/ml anti-CD3 and 0.2 μg/ml anti-CD28. After 60 h of incubation, cells were pulsed for 12 h with 1 μCi of [3H]thymidine (sp. act. 42 Ci/mmol). Thymidine incorporation was measured using a scintillation counter.
Flow cytometry
FITC-labeled anti-CD25, FITC-labeled anti-IFN-γ, PE-labeled anti-IL-10, PE-labeled anti-CD4, PerCP-cy5.5-labeled anti-CD4, allophycocyanin-labeled anti-CD4, and allophycocyanin-Cy7-labeled anti-CD8 mAbs were purchased from BD Biosciences. PE-labeled anti-Foxp3 mAb was purchased from eBioscience. Donkey-anti-mouse IL-12Rβ2 mAb, Donkey IgG control, and FITC-labeled anti-donkey IgG mAb were purchased from Santa Cruz Biotechnology. For immunostaining, single cell suspensions were prepared from spleen, thymus, and cultured cells. One million cells were resuspended in the staining buffer (PBS, 1% FCS, 0.02% NaN3) and incubated with Ab for 30 min at 4°C after Fc Block. For intracellular staining, cells were treated with 1 μl/ml GolgiPlug (BD Biosciences) for 4 h before staining. After surface staining, cells were fixed and permeabilized using the Cytofix/Cytoperm system (BD Biosciences). After permeabilization, cells were resuspended in permeabilization buffer and stained with intracellular mAb for 30 min in 4°C. All flow cytometric analyses were performed using appropriate isotype controls. Data were acquired on a FACSAria (BD Biosciences) and analyzed using FlowJo Software.
Cytokine production
For cytokine detection, supernatants were collected 48 h of cultures, and levels of IL-2, IL-4, IL-10, IFN-γ, MCP-1 and TNF-α were measured using a cytometric bead array (CBA) kit (BD Biosciences). Levels of IL-17 and GM-CSF were measured by ELISA (BD Biosciences for IL-17 and eBioscience for GM-CSF) as per manufacturer’s instructions.
Small interfering RNA (siRNA) administration
siRNA targeting IL-12Rβ2 (ID: 158269) and negative control siRNA were designed and synthesized by Ambion (silencer). IL-12Rβ2 and negative control siRNA were administered to naive B6 mice in PBS i.v. at 10 mg/kg following the manufacturer’s protocol (28, 29). Splenic MNCs were collected 24 h after injection and cultured with Con A (2 μg/ml), IL-2 (10 ng/ml), and IL-12 (20 ng/ml) for 4 days (30). IL-12Rβ2 expression was analyzed by flow cytometry.
Statistics
Student’s t test was used for comparing parameters among different groups. All tests were two-sided. p values < 0.05 were considered significant.
Results
IL-12Rβ2−/− mice are more susceptible to STZ-induced diabetes
To investigate the role of IL-12Rβ2 in the pathogenesis of STZ-induced diabetes, we injected IL-12Rβ2−/− mice and their WT controls with a low dose of STZ. Two of ten WT mice (20%) developed hyperglycemia. In contrast, 6 of 10 (60%) IL-12Rβ2−/− mice developed hyperglycemia (Fig. 1A). IL-12Rβ2−/− mice developed diabetes 10 days earlier (day 15) than WT mice (day 25; Fig. 1A). Furthermore, more severe hyperglycemia (average blood glucose) was observed in IL-12Rβ2−/− mice than in WT mice (Fig. 1B).
FIGURE 1.
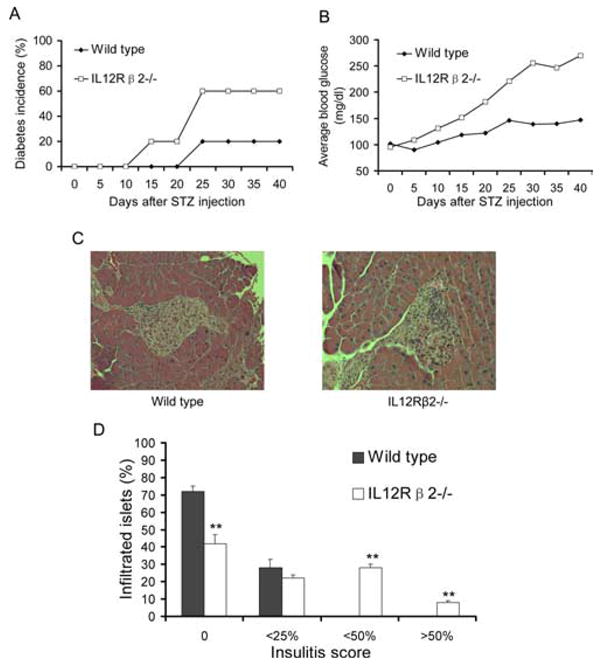
IL-12Rβ2−/− mice and susceptibility to STZ diabetes. IL-12Rβ2−/− mice and WT mice were injected i.p. with freshly made STZ at 50 mg/kg/day for 5 continuous days. Blood samples were harvested via the tail vein every 5 days after STZ injection, and plasma glucose levels were determined. Significantly hyperglycemic animals (plasma glucose >250 mg/dl) were considered diabetic. A and B, Diabetes incidence (A) and average level of blood glucose (B), n = 10 in each group. Mice were sacrificed 40 days after diabetes induction. Pancreatic sections were stained with H&E. C, WT mice with hyperglycemia (left) vs IL-12Rβ2−/− mice with hyperglycemia after STZ injection (right). D, Quantification of infiltrated islets and ranking of insulitis were performed. The degree of mononuclear cell infiltration (insulitis score) was measured using the following rankings: no infiltration (0), peri-insulitis (<25%), moderate insulitis (<50%), and severe insulitis (>50%). **, p < 0.01. One representative experiment of two is shown.
Severe pancreatic inflammation in STZ-induced diabetic IL-12Rβ2−/− mice
Mice injected with STZ were sacrificed on day 40, and 5 μm thick sections of the pancreas were stained with H&E. Most pancreatic islets of WT mice were either normal or mildly infiltrated by leukocytes. In contrast, severe insulitis and massive islet destruction were observed in IL-12Rβ2−/− mice (Fig. 1, C and D). Thus, a direct correlation was found between clinical and pathological features of STZ-induced diabetes in WT and IL-12Rβ2−/− mice.
Increased proportions of mature T cell populations in thymus and normal CD25+ and Foxp3 expression in IL-12Rβ2−/− mice
We analyzed T cell subpopulation phenotypes in thymus and spleen of WT and IL-12Rβ2−/− mice. In the thymus of IL-12Rβ2−/− mice we found more CD4+ and CD8+ single positive T cells (mature; p < 0.01; Fig. 2) but fewer CD4+CD8+ double positive T cells (immature) than in WT mice (data not shown). However, there was no significant difference in Foxp3 gene expression between WT and IL-12Rβ2−/− mice (data not shown). There were also no differences in the total number and percentage of CD4+, CD8+, or CD4+CD25+ populations in spleens of naive WT and IL-12Rβ2−/− mice (data not shown).
FIGURE 2.
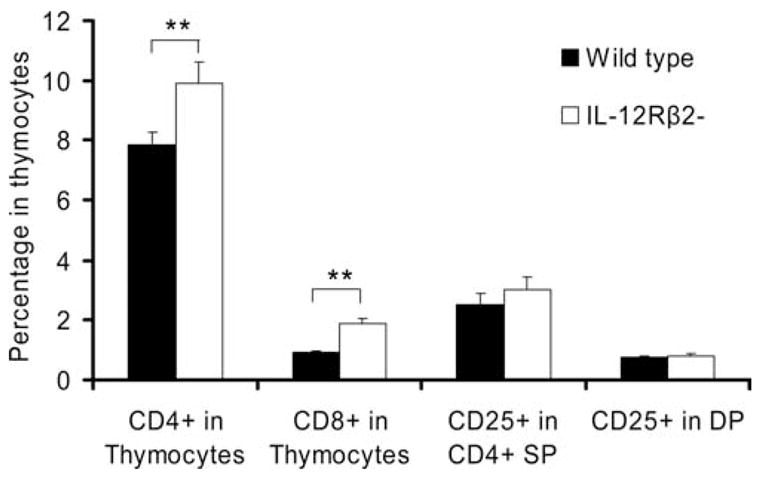
Phenotypic characterization of thymocytes from IL-12Rβ2−/− and WT mice. MNCs (1 × 106) from thymus were harvested from naive WT and IL-12Rβ2−/− mice and stained with anti-CD4 and anti-CD8 mAb. Cells were then intracellularly stained with anti-Foxp3 mAb and analyzed by flow cytometry. SP = CD4+ single positive, DP = CD4+CD8+ double positive. Columns refer to mean values and bars to SD (n = 5 each group). **, p < 0.01. One representative experiment of three is shown.
Hyperproliferation of effector CD4+ T cells and hypoproliferation of CD4+ Tregs of IL-12Rβ2−/− mice in vitro
To investigate T cell proliferative responses, CD4+CD25+ and CD4+CD25− T cells were isolated from spleens of WT and IL-12Rβ2−/− mice and labeled with CFSE. After stimulation with anti-CD3 and anti-CD28 for 3 days, flow cytometric analyses showed that significantly more CD4+CD25+ Tregs from IL-12Rβ2−/− mice remained undivided (17.1%) than those from WT mice (2.1%). In contrast, the CD4+CD25− population (effector) of IL-12Rβ2−/− mice demonstrated significantly higher proliferative responses (3.4% of CD25− T cells remained undivided) than WT mice (9.9%; Fig. 3).
FIGURE 3.
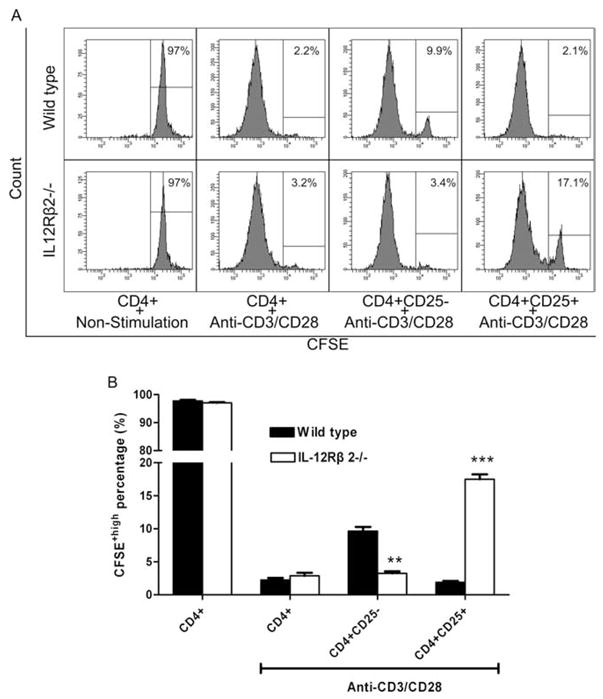
Different proliferation of Treg and effector T cells in IL-12Rβ2−/− mice and WT mice. CD4+, CD4+CD25+, and CD4+CD25− T cells were purified from spleen of naive IL-12Rβ2−/− and WT mice and were labeled with CFSE. Cells were cultured 72 h in the presence or absence of 1.0 μg/ml anti-CD3 and 0.2 μg/ml anti-CD28. A, The fluorescent intensity of live cells after 72 h of culture is displayed as a histogram. Numbers are percentages of cells that remained undivided. B, The average percentages of these cells are shown. Columns refer to mean values and bars to SD (n = 4 each group). **, p < 0.01; ***, p < 0.001. One representative experiment of three is shown.
Decreased capacity of T cells of IL-12Rβ2−/− mice to be driven into CD4+CD25+ Tregs by TGF-β
CD4+CD25+ Treg cells have been shown to specifically express Foxp3, a transcription factor whose activity is sufficient to convert naive CD4+CD25− cells to CD4+CD25+ Tregs (21). We found that ~8% of CD4+ T cells in the spleen of both WT and IL-12Rβ2−/− mice express CD25 (data not shown). To determine whether the presence of TGF-β during priming promotes the development of CD4+CD25+ Tregs and Foxp3 gene expression in vitro, splenocytes from WT and IL-12Rβ2−/− mice were stimulated with anti-CD3 and anti-CD28 for 48 h in the presence or absence of TGF-β, followed by a resting period of 96 h in fresh medium containing exogenous IL-2. As shown in Fig. 4A, TGF-β induced a significant increase in CD4+CD25+ cells and Foxp3 gene expression in both WT and IL-12Rβ2−/− mice. However, much higher increases were observed in WT mice than in IL-12Rβ2−/− mice for CD4+CD25+ cells (20- vs 5-fold), Foxp3 gene expression in CD4+CD25+ cells (40- vs 10-fold), and Foxp3 gene expression in CD4+CD25− cells (10- vs 2-fold), respectively (Fig. 4B). These results indicate that IL-12Rβ2−/− mice have diminished response to TGF-β-induced Treg generation compared with WT mice.
FIGURE 4.

T cells phenotype after TGF-β treatment of cells from IL-12Rβ2−/− mice and WT mice. Spleen cells from IL-12Rβ2−/− and WT mice were stimulated with anti-CD3 and anti-CD28 in the presence or absence of 5 ng/ml TGF-β for 48 h and rested in fresh medium supplemented with 50 U/ml IL-2 for 96 h. Cells were harvested and stained, and the percentages of T cell subpopulations were determined by flow cytometry. A, Number of cells per 10,000 CD4+ cells after staining. B, The fold increase was assessed by using the numbers in TGF-β-treated groups divided by the numbers in untreated groups. Columns refer to mean values and bars to SD (n = 5 each group). **, p < 0.01; *, p < 0.05. One representative experiment of three is shown.
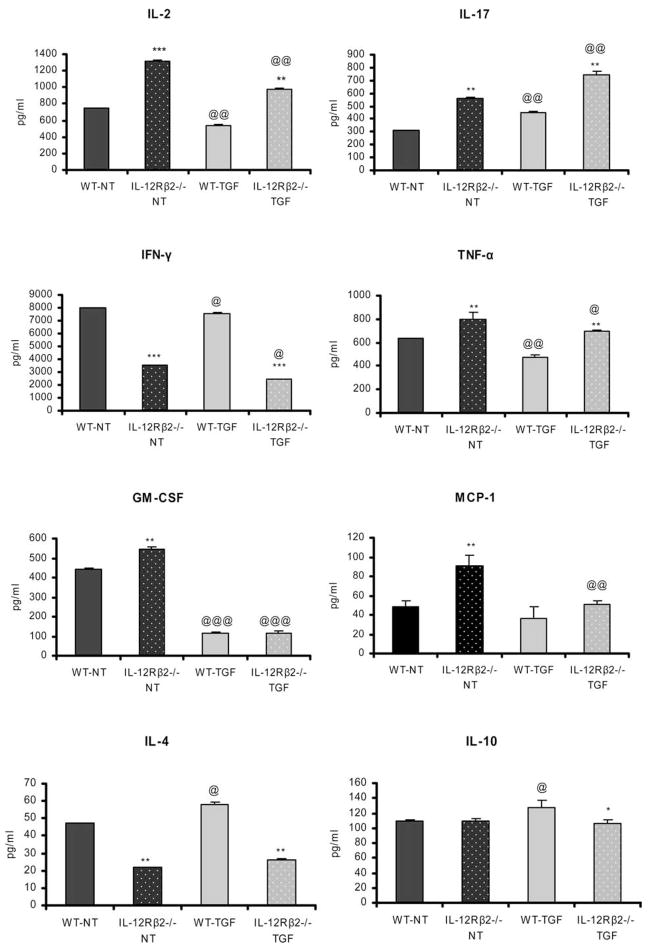
We then determined the secretion of proinflammatory cytokines IL-2, TNF-α, IFN-γ, MCP-1, GM-CSF, IL-17, and anti-inflammatory cytokines IL-4, IL-5, and IL-10 into supernatants of the cell cultures described above. CD4+ T cells of IL-12Rβ2−/− mice produced higher levels of IL-2, TNF-α, MCP-1, GM-CSF, and IL-17, but lower levels of IFN-γ and IL-4. No difference was found in IL-5 levels between the two groups (data not shown). Significantly increased IL-10 production was observed upon TGF-β stimulation of cells from WT mice but not from IL-12Rβ2−/− cells (Fig. 5), consistent with our observations that IL-12Rβ2-deficient T cells are more resistant to Treg induction.
FIGURE 5.
Cytokine levels in supernatants of splenocyte cultures with or without TGF-β treatment. Spleen cells from IL-12Rβ2−/− and WT mice were stimulated with anti-CD3 and anti-CD28 in the presence or absence of 5 ng/ml TGF-β for 48 h. Supernatants were collected and cytokine levels were assayed using CBAs (for IL-2, IL-4, IL-10, IFN-γ, MCP-1, and TNF-α) and ELISA (for IL-17 and GM-CSF). Columns refer to mean values and bars to SD (n = 4 each group). WT: wild type mice; NT: non-TGF-β treated; TGF: TGF-β treated. * Represents the comparison of IL-12Rβ2−/− with WT groups; @ represents the comparison of TGF-β treated with non-treated in the same strain. *, @ p < 0.05; **, @@ p < 0.01; ***, @@@ p < 0.001. One representative experiment of three is shown.
Reduced suppressive function of Tregs from IL-12Rβ2−/− mice
To determine the functional activity of naturally occurring and TGF-β-induced CD4+CD25+ Tregs from WT and IL-12Rβ2−/− mice, we examined the ability of these cells to suppress proliferation of anti-CD3/CD28-stimulated WT spleen cells as effector cells. These CD4+CD25+ T cells were cocultured with effector cells at a ratio of 1:1. Coculture with CD4+CD25− T cells was set up in parallel as controls. As shown in Fig. 6, coculture with CD25+ T cells of either WT or IL-12Rβ2−/− mice induced significant inhibition of effector T cells. Naturally occurring CD4+CD25+ cells from WT mice had a greater suppressive effect than in those from IL-12Rβ2−/− mice (comparison between columns 4 and 8, p < 0.01). Greater suppressive effects were also seen in all groups of TGF-β-treated CD4+CD25+ T cells than those without TGF-β treatment. However, after TGF-β treatment, there was no significant difference between the suppressive effects of IL-12Rβ2 from WT and IL-12Rβ2−/− mice (Fig. 6), suggesting that TGF-β has the capacity to overcome the negative influence of IL-12Rβ2 deficiency on the function of IL-12Rβ2. Together, these data indicate a significant reduction of Treg function in IL-12Rβ2 mice compared with their WT counterpart.
FIGURE 6.

Coculture of CD4+CD25+/− T cells from IL-12Rβ2−/− mice vs WT mice. CD4+CD25+/− T cells of IL-12Rβ2−/− and WT mice with or without TGF-β treatment were cocultured with normal WT splenocytes at ratio = 1:1 in presence of 1.0 μg/ml anti-CD3 and 0.2 μg/ml anti-CD28. The ratio of CD25+/− cells: effector cells was 1:1. After 96 h culture, proliferative responses of effector cells were determined by incorporation of [H3]thymidine. Columns refer to mean values and bars to SD (n = 4 each group). WT: Wild type mice. ##, p < 0.01 of comparison between columns 4 and 6. **, ##, p < 0.01. One representative experiment of three is shown.
IL-12Rβ2 siRNA knockdown
To confirm that Treg function is impaired in the absence of IL-12Rβ2, we used siRNA to knockdown IL-12Rβ2 expression in vivo. Flow cytometric analysis (Fig. 7A) showed that the expression of IL-12Rβ2 on CD4+ T cells was clearly knocked down when compared with those in mice that received control siRNA (9 vs 50%). When the capacity of these cells to differentiate into Treg by TGF-β treatment was determined, similar results were obtained as in IL-12Rβ2−/− mice (Figs. 4 and 5) in terms of CD25+ and Foxp3+ expression (Fig. 7B) and cytokine production (Fig. 7C), thus confirming the results derived from IL-12Rβ2−/− mice.
FIGURE 7.
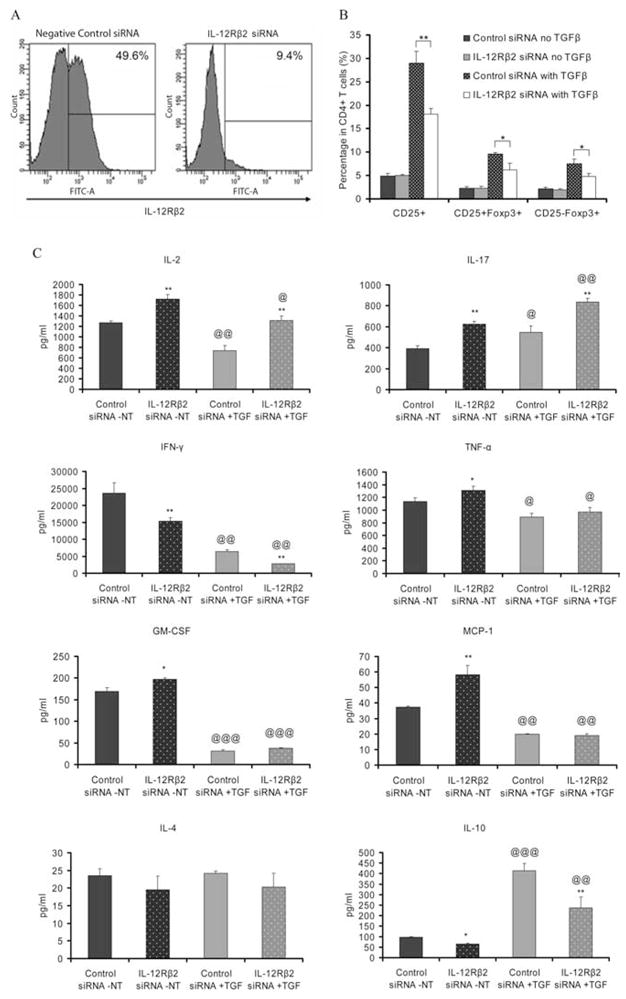
siRNA knockdown of IL-12Rβ2. IL-12Rβ2-specific siRNA and negative control siRNA were injected i.v. to naive C57BL/6 mice at 10 mg/kg (n = 5 each group). Mice were sacrificed 24h after injection and splenocytes were collected. A, Cells were cultured with Con A (2 μg/ml), IL-2 (10 ng/ml), and IL-12 (20 ng/ml) for 4 days then stained with donkey-anti-mouse IL-12Rβ2 mAb (1st; donkey IgG as isotype control) and FITC-labeled anti-donkey IgG (2nd) mAb. IL-12Rβ2 expression in viable lymphocytes was determined by flow cytometry. B, Spleen cells from two groups of mice (n = 5 each group) were stimulated with anti-CD3 and anti-CD28 in the presence or absence of 5 ng/ml TGF-β, as described in Figs. 4 and 5. Cells were harvested, stained, and the percentages of T cell subpopulations were determined by flow cytometry. C, Supernatants were collected and cytokine levels were assayed using CBAs (for IL-2, IL-4, IL-10, IFN-γ, MCP-1, and TNF-α) and ELISA (for IL-17 and GM-CSF). Columns refer to mean values and bars to SD (n = 5 mice in each group). NT: non-TGF-β treated; TGF: TGF-β treated. * Represents the comparison of IL-12Rβ2 siRNA treated with negative control siRNA treated groups; @ represents the comparison of TGF-β treated with non-treated in the same strain. *, @ p < 0.05; **, @@ p < 0.01; ***, @@@ p < 0.001. Data are one representative of two independent experiments.
Discussion
Previous studies have shown that IL-12Rβ2−/− mice develop more severe EAE than WT mice, which is characterized by earlier disease onset, more severe paralysis, increased rates of mortality, and more extensive demyelination and inflammatory infiltration in the CNS (4). These findings led us to ask whether this increased susceptibility to EAE is model specific, or whether perhaps a lack of IL-12Rβ2 predisposes to higher susceptibility to autoimmune diseases. Our observation in the STZ-induced diabetic mouse model suggests the latter to be the case: that IL-12Rβ2 deficiency results in a higher universal susceptibility to autoimmune diseases.
To investigate the mechanism of increased susceptibility to autoimmune diseases in IL-12Rβ2−/− mice, we have profiled the CD4+ T cell populations in IL-12Rβ2−/− mice and WT control mice. Characterization of cytokine production demonstrated that IL-12Rβ2−/− mice secrete higher levels of proinflammatory cytokines IL-2, TNF-α, GM-CSF, and IL-17, a major proinflammatory cytokine implicated in autoimmune disorders (4, 31–35). Furthermore, the addition of IL-12 to CD4+ T cells cultured with IL-23 inhibited IL-17 production in a dose-dependent manner (34). These data suggest that a lack of IL-12 responsiveness in IL-12Rβ2−/− mice may eliminate physiological down-regulation of IL-17 production by IL-12 and promote unopposed up-regulation of IL-17 by IL-23 (4, 36). However, it is now clear how IL-12 responsiveness exerts this immunoregulatory effect on proinflammatory responses, and, especially, whether this immunoregulatory effect is mediated via an induction of Tregs.
Mammalian immune responses are balanced by the interplay between effector T cells and suppressive T cells (37–39). Effector T cells serve to enhance immunoreactivity and, in contrast, suppressor T cells inhibit effector immune responses (38, 40). CD25 has been identified as a reliable marker for suppressor T cells, and naturally occurring CD4+CD25+ T cells are considered to be Tregs which have primarily immunosuppressive functions (10 –12). Our results showed hyperproliferation of CD4+CD25− T cells and hypoproliferation of CD4+CD25+ T cells in IL-12Rβ2-deficient mice in response to anti-CD3/anti-CD28 stimulation, indicating that IL-12Rβ2−/− mice have more CD4+CD25− effector T cells but fewer CD4+CD25+ Tregs than WT mice upon activation. Thus, the immune response of IL-12Rβ2−/− mice is likely to be biased toward the effector phenotype, which may explain increased immunoreactivity leading to earlier and more severe disease compared with WT mice.
To investigate this phenomenon further, we used TGF-β which has been shown recently to induce Foxp3 gene expression in TCR-challenged CD4+CD25− naive T cells, thereby driving this population toward a Treg phenotype with potent immunosuppressive functions (18 –21). In response to TGF-β, the induction of CD4+CD25+ T cells was lower in IL-12Rβ2−/− mice than in WT mice. Foxp3 is highly expressed in CD4+CD25+ Tregs and is virtually undetectable in both resting and activated effector T cells, thus being a specific marker for Tregs (41). We have compared TGF-β-induced Foxp3 expression by intracellular staining of CD25+CD4+ T cells of WT and IL-12Rβ2−/− mice. As expected, CD25+ T cells expressed significantly higher levels of Foxp3 than CD25−CD4+ cells, whereas it was noted that CD25+CD4+ T cells from IL-12Rβ2−/− mice contained lower numbers of Foxp3-expressing cells. This shows that T cells from IL-12Rβ2−/− mice are more resistant to the induction of Tregs than those from WT mice, a property that may increase the susceptibility of IL-12Rβ2 mice to autoimmunity.
Given that a smaller proportion of Tregs is present in CD4+ T cells of IL-12Rβ2−/− mice than in WT mice, the question remains whether these Tregs possess the same functional immunoregulatory capacity as those from WT mice. To address this question, we analyzed the effectiveness of suppressor T cells from these two strains of mice. Our results show that CD4+CD25+ T cells of naive IL-12Rβ2−/− mice have less potent suppressive functions than those of WT mice. The reduced immunoregulatory function of CD4+CD25+ T cells has also been found in other mouse strains, such as mice lacking STAT1, which were more susceptible to EAE induction (42). Together, our studies showed both quantitative and qualitative Treg impairment in IL-12Rβ2−/− mice upon activation, providing a mechanism underlying the increased susceptibility of these mice to autoimmune diseases.
Naturally occurring CD4+CD25+ Tregs develop in the thymus (43, 44). Thus we determined the number of these cells and Foxp3 expression in thymocytes of IL-12Rβ2−/− mice and WT mice. There was no difference in absolute numbers of CD4+CD25+ T cells in the thymus and spleen of naive mice in both strains. These data, combined with our finding that peripheral CD4+ T cells of IL-12Rβ2−/− mice are more resistant to the induction of Tregs, indicate that IL-12 responsiveness is important in the development of Tregs upon activation. Furthermore, we found a similar total cell number but a greater proportion of mature CD4+ T cells in the thymus of IL-12Rβ2−/− mice compared with WT mice, indicating that T cells in the thymus of mice lacking IL-12Rβ2 may undergo accelerated maturation, thus releasing more effector T cells into the periphery. This phenomenon could be a mechanism underlying enhanced autoimmunity and is consistent with our previous finding in the EAE model, in which an increased absolute number of CD4+ T cells is present in the periphery of IL-12Rβ2−/− mice (4).
IL-12 responsiveness primarily induces Th1 cells that produce a large amount of IFN-γ (45), and the involvement of IL-12 responsiveness in the development of Tregs is probably via an IL-12/IFN-γ axis. Indeed, Sawitzki et al. found that CD25+CD4+ T cells, but not CD25−CD4+ T cells, showed a 5-fold increase in IFN-γ mRNA expression within 24 h of re-encountering alloantigen in vivo (46). The generation and function of alloantigen- and autoimmune-reactive Treg cells were dramatically impaired in IFN-γ- and IFN-γR-deficient mice (46 – 48). We also found that a significantly lower level of IFN-γ correlates with impaired capacity of IL-12Rβ2-deficient T cells to differentiate into Tregs upon TGF-β stimulation (Fig. 5). In addition, the observation that impaired development of CD4+CD25+ Tregs and increased susceptibility in mice lacking STAT1, of which IFN-γ is one of the strongest activators, provides indirect evidence for the involvement of IFN-γ in the development of Tregs (42). Our results, combined with those from others, propose an IL-12/IFN-γ/STAT1 axis in tolerance induction and the development of Tregs.
Taken together, our studies provide evidence that signaling via IL-12Rβ2 regulates both the number and functional maturity of Tregs, indicating that a novel mechanism underlies IL-12 pathway regulation of autoimmune diseases.
Acknowledgments
We thank Katherine Regan for editorial assistance.
Footnotes
This work was supported by grants from the National Institutes of Health and the National Multiple Sclerosis Society.
Abbreviations used in this paper: EAE, experimental autoimmune encephalomyelitis; siRNA, small interfering RNA; Treg, regulatory T cell; STZ, streptozotocin; WT, wild type; MNC, mononuclear cell; CBA, cytometric bead array.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 2006;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 2.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 3.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 4.Zhang GX, Yu S, Gran B, Li J, Siglienti I, Chen X, Calida D, Ventura E, Kamoun M, Rostami A. Role of IL-12 receptor β 1 in regulation of T cell response by APC in experimental autoimmune encephalomyelitis. J Immunol. 2003;171:4485–4492. doi: 10.4049/jimmunol.171.9.4485. [DOI] [PubMed] [Google Scholar]
- 5.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 6.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 8.Gran B, Chu N, Zhang GX, Yu S, Li Y, Chen XH, Kamoun M, Rostami A. Early administration of IL-12 suppresses EAE through induction of interferon-γ. J Neuroimmunol. 2004;156:123–131. doi: 10.1016/j.jneuroim.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Fahey AJ, Robins RA, Constantinescu CS. Curcumin modulation of IFN-β and IL-12 signalling and cytokine induction in human T cells. J Cell Mol Med. 2007;11:1129–1137. doi: 10.1111/j.1582-4934.2007.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int Immunol. 1999;11:1625–1634. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- 11.Sundstedt A, O’Neill EJ, Nicolson KS, Wraith DC. Role for IL-10 in suppresion mediated by peptide-induced regulary T cells in vivo. J Immunol. 2003;170:1240–1248. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- 12.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the foxhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 15.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 16.Stephens GL, Shevach EM. Foxp3+ regulatory T cells: selfishness under scrutiny. Immunity. 2007;27:417–419. doi: 10.1016/j.immuni.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Long E, Wood KJ. Understanding FOXP3: progress towards achieving transplantation tolerance. Transplant Rev. 84:459–461. doi: 10.1097/01.tp.0000275424.52998.ad. [DOI] [PubMed] [Google Scholar]
- 18.Santambrogio L, Hochwald GM, Leu CH, Thorbecke GJ. Antagonistic effect of endogenous and exogenous TGF-β and TNF on autoimmune diseases in mice. Immunopharmacol Immunotoxicol. 1993;15:461–478. doi: 10.3109/08923979309035240. [DOI] [PubMed] [Google Scholar]
- 19.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-β in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 20.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-β-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 21.Hyung BP, Doo JP, Eunkyeong J, Seokmann H. Acquisition of anergic and suppressive activities in transforming growth factor-β-costimulated CD4+CD25− T cells. Int Immunol. 2004;16:1203–1213. doi: 10.1093/intimm/dxh123. [DOI] [PubMed] [Google Scholar]
- 22.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance: I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-β T cell-transgenic mice. J Immunol. 2007;178:179–185. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Carper K, Zheng XX, Kuhr CS, Reyes JD, Liang Y, Perkins DL, Thomson AW, Perkins JD. The role of foxp3+ regulatory T cells in liver transplant tolerance. Transplant Proc. 2006;38:3205–3206. doi: 10.1016/j.transproceed.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 24.Lamhamedi-Cherradi SE, Zheng SJ, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmumune diseases in TRAIL−/− mice. Nat Immunol. 2003;4:255–260. doi: 10.1038/ni894. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, Wang X, Gadina M, O’Shea JJ, Presky DH, Magram J. IL-12 receptor β 2 (IL-12R β2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J Immunol. 2000;165:6221–6228. doi: 10.4049/jimmunol.165.11.6221. [DOI] [PubMed] [Google Scholar]
- 26.Herold KC, Montag AG, Buckingham F. Induction of tolerance to autoimmune diabetes with islet antigens. J Exp Med. 1992;176:1107–1114. doi: 10.1084/jem.176.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casellas A, Salavert A, Agudo J, Ayuso E, Jimenez V, Moya M, Munoz S, Franckhauser S, Bosch F. Expression of IGF-I in pancreatic islets prevents lymphocytic infiltration and protects mice from type 1 diabetes. Diabetes. 2006;55:3246–3255. doi: 10.2337/db06-0328. [DOI] [PubMed] [Google Scholar]
- 28.Lu PY, Xie F, Woodle MC. In vivo application of RNA interference: from functional genomics to therapeutics. Adv Genet. 2005;54:117–142. doi: 10.1016/S0065-2660(05)54006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sass G, Shembade ND, Haimerl F, Lamoureux N, Hashemolhosseini S, Tannapfel A, Tiegs G. TNF pretreatment interferes with mitochondrial apoptosis in the mouse liver by A20-mediated down-regulation of Bax. J Immunol. 2007;179:7042–7049. doi: 10.4049/jimmunol.179.10.7042. [DOI] [PubMed] [Google Scholar]
- 30.Chakir H, Camilucci AA, Filion LG, Webb JR. Differentiation of murine NK cells into distinct subsets based on variable expression of the IL-12R β 2 subunit. J Immunol. 2000;165:4985–4993. doi: 10.4049/jimmunol.165.9.4985. [DOI] [PubMed] [Google Scholar]
- 31.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 32.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irmler IM, Gajda M, Bräuer R. Exacerbation of antigen-induced arthritis in IFN-γ-deficient mice as a result of unrestricted IL-17 response. J Immunol. 2007;179:6228–6236. doi: 10.4049/jimmunol.179.9.6228. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal S, Ghilardi N, Xie MH, De Sauvage FJ, Gurney AL. Interleukin 23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin 17. J Biol Chem. 2002;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 36.Ireland DD, Palian BM, Reiss CS. Interleukin (IL)-12 receptor β1 or IL-12 receptor β 2 deficiency in mice indicates that IL-12 and IL-23 are not essential for host recovery from viral encephalitis. Viral Immunol. 2005;18:397–402. doi: 10.1089/vim.2005.18.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor A, Verhagen J, Akdis CA, Akdis M. T regulatory cells in allergy and health: a question of allergen specificity and balance. Int Arch Allergy Immunol Rev. 2004;135:73–82. doi: 10.1159/000080523. [DOI] [PubMed] [Google Scholar]
- 39.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 40.Torgerson TR. Regulatory T cells in human autoimmune diseases. Springer Semin Immunopathol. 2006;28:63–76. doi: 10.1007/s00281-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 41.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 42.Nishibori T, Tanabe Y, Su L, David M. Impaired development of CD4+CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J Exp Med. 2004;199:25–34. doi: 10.1084/jem.20020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol Rev. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 44.Cassis L, Aiello S, Noris M. Natural versus adaptive regulatory T cells. Contrib Nephrol Rev. 2005;146:121–131. doi: 10.1159/000082072. [DOI] [PubMed] [Google Scholar]
- 45.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 46.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-γ production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-γ. Arthritis Res Ther. 2005;7:R402–R415. doi: 10.1186/ar1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood KJ, Sawitzki B. Interferon γ: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol. 2006;27:183–187. doi: 10.1016/j.it.2006.02.008. [DOI] [PubMed] [Google Scholar]