Abstract
The host-parasite relationship is based on a series of interplays between host defense mechanisms and parasite survival strategies. Progress has been made in understanding the role of host immune response in amebiasis. While host cells elaborate diverse mechanisms for pathogen expulsion, amebae have also developed complex strategies to modulate host immune response and facilitate their own survival. This paper will give an overview of current research on the mutual interactions between host and Entamoeba histolytica in human and experimental amebiasis. Understanding this crosstalk is crucial for the effective design and implementation of new vaccines and drugs for this leading parasitic disease.
Introduction
Amebiasis, a disease caused by protozoan parasite Entamoeba histolytica, is estimated to result in 50 million cases of colitis and liver abscess and up to 100 thousand deaths each year (1). The majority of infections occur in areas of poor sanitation and nutrition. For example, a 40% annual incidence of E. histolytica infection was observed in a cohort of preschool children in Bangladesh (2); 80% of the children acquired infection at least once, and 53% of them got a repeat infection over the 4-year observation (3). It was reported in a serologic study that anti-E. histolytica antibodies were detected in 8.4% of the population in Mexico (4). E. histolytica infection resolves in most people with no or nonspecific symptoms; only approximately 10% to 20% of infected patients become symptomatic, manifesting in dysentery, chronic colitis, toxic megacolon, or extraintestinal disease (5).
During the establishment of infection, E. histolytica confronts a series of host innate defenses, including intestinal mucosa and epithelial barrier, phagocytes, granulocytes, and lytic serum components (6). While host cells elaborate diverse mechanisms for pathogen expulsion, amebae have also developed complex strategies to evade host defense and facilitate their own survival. Dissection of this crosstalk is essential for the development of novel immunotherapeutics for amebiasis. In this review, we summarize recent advances on the role of innate barriers and immune components involved in the host defense to E. histolytica; and the mechanisms by which E. histolytica evades immune clearance in human and experimental amebiasis.
Interactions of host innate immune system with E. histolytica
Innate cell-mediated immunity to E. histolytica
The early stage (within 1–2 days) of ALA lesion is characterized by a dominant infiltration of polymorphonuclear leukocytes (PMNs) surrounding trophozoites. In later stage (after 3 days), lymphocytes, macrophages and epithelioid cells were recruited to the sites, associated with the formation of granuloma, which contribute to the confinement of invading trophozoites (7, 8). Histopathological studies in intestinal amebiasis model also demonstrated the early recruitment and destruction of leukocytes following amebic invasion (9). The following will be focused on the crosstalk of invasive ameba with each individual cell type.
1. Neutrophil
Neutrophils are frequently observed as the earliest infiltrating cells during the acute phase of host response to amebic invasion (8, 10). It is possible that they are recruited by the chemotactic activity of an amebic membrane-bound peptide (11, 12) and chemokines secreted by epithelial cells exposed to E. histolytica (13). C5a and C3a fragments produced by activated complement on the parasite surface might also contribute to neutrophil chemotaxis (14). As the consequence of interaction with trophozoites, neutrophils are activated with the release of reactive oxygen species and anti-microbial peptides. Many in vitro studies have reported neutrophil amebicidal activity after stimulation by IFN-γ, TNF-α, LPS or amebic antigens (15, 16). Depletion of neutrophils with anti-Gr-1 neutralizing antibodies resulted in exacerbated amebic hepatic (17, 18) and intestinal (19, 20) lesions in SCID (17), CBA (19) and BALB/c (18, 20) mice, suggesting that these cells might play a protective role in early resistance to amebiasis. It is worth noting that these antibodies might recognize and deplete other granulocytes like eosinophils, which were found infiltrating and degranulating in some colon biopsies from amebic colitis patients (Haque R and Petri WA, unpublished work).
However, the above rationale was challenged by observations that neutrophils not only failed to destroy E. histolytica, but in fact were lysed or inactivated by virulent ameba (21). There are several mechanisms whereby ameba can interfere with the proper functioning of neutrophils (Table. 1). By disruption of NADPH oxidase activities, ameba could inhibit the respiratory burst of neutrophils and resist the oxidative stress (22–24). Peroxiredoxin, a 29 kDa surface protein conferring resistance to host reactive oxygen defences, was shown to be associated with the virulence of ameba (25–27). It was reported in a recent study that E. histolytica could induce neutrophil apoptosis, associated with ERK1/2 activation and mediated by NADPH oxidase-generated ROS (24). The destruction of neutrophils by E. histolytica results in the release of cytotoxic oxidase and lytic peptidases, leading to the host tissue damage.
Table 1.
| E. histolytica components | Possible impact on neutrophils | reference |
|---|---|---|
| Live trophozoites | Induce apoptosis of neutraphils | 24 |
| E. histolytica iron-containing superoxide dismutase | Detoxify reactive oxygen species (ROS) by forming H2O2 | 23 |
| NADPH:flavin oxidoreductase (Eh34) | Detoxify ROS by forming H2O2 | 23 |
| Peroxiredoxin (Eh29) | Remove H2O2 | 25–27 |
The conflicting results on the role of neutrophils in amebiasis may be attributed to the context in host genetic background, parasite pathogeneicity, and activation state of neutrophils. Experimental manipulations including challenge dose may also make difference for the results of the investigations.
2. Macrophage
The role of macrophages as effector cells during amebic infection has been documented in both animal models and patient studies. Like neutrophils, macrophages acquire amebicidal activity after in vitro stimulation with IFN-γ, TNF-α, or colony stimulating factor-1 (28–30). Different surface components of trophozoites were shown to be recognized by macrophages via TLR-2 and TLR-4 signaling (31). Upregulated TLR-2 expression was observed in macrophages exposed to Gal/GalNac lectin of E. histolytica, resulting in NF-κB activation and proinflammatory cytokine production (32). Macrophages lacking TLR-2 and TLR-4 showed impaired response to E. histolytica lipopeptidophosphoglycan (LPPG), suggesting an essential role of pattern recognition for the macrophage response (31). As an important cytotoxicity mediator, nitric oxide (NO) was found to be capable of inhibiting cysteine proteinases and alcohol dehydrogenase 2, virulence factors of the parasite (33). Inducible nitric oxide synthase (iNOS)-deficient mice were more susceptible to ALA and E. histolytica-induced hepatocytic apoptosis (34), suggesting that NO plays a critical role in host defense against amebiasis.
Despite the sensitivity of E. histolytica to NO-mediated cytotoxicity, a suppression of cell-mediated immunity with impaired macrophage function has frequently been observed in human and experimental amebiasis, suggesting that amebae have developed strategies to modulate macrophage responses. The amebic modulation is multifactorial. For instance, exposure to E. histolytica trophozoites or amebic components has resulted in suppressed respiratory burst ((ROI: H2O2, O2−, OH·) (35) and reduced NO production (36) by macrophages. Inhibition of NO production was mediated via the competitive consumption of NOS substrate L-arginine by a putative arginase expressed in E. histolytica, which converts L-arginine into L-ornithine (37). A decrease in TNF-α secretion was also observed due to rapid degradation of c-fos and TNF-α transcripts by ameba (38); Secreted or whole soluble amebic proteins inhibited IFN-γ-induced MHC-II expression on macrophages, suggesting an impaired amebic antigen presentation capacity in these cells (39).
The amebic molecules and mechanisms involved in the subversion of macrophage function are still poorly understood. It is suggested that the mechanisms involved depend at least partially on prostaglandin E2 (PGE2), an immunoregulator produced by E. histolytica or macrophages exposed to amebic proteins (40, 41). In vertebrates, PGE2 is synthesized by cyclooxygenase (COX). Expression of COX isoforms has been detected in E. histolytica trophozoites (40) as well as ameba-exposed macrophages (41). PGE2 elevates cAMP levels in macrophages, triggering the PKA pathway, which in turn inhibits the expression of Ia molecules, the release of Th1 cytokines, NADPH-mediated oxidative burst, as well as NO synthesis via PKC pathway (39, 41). The COX inhibitor indomethacin partially restores Ia expression on the surface of macrophages (39). An immunosuppressor synthesized by ameba, Monocyte Locomotion Inhibitory Factor (MLIF), also contributes to the modulation of host immune responses. MLIF is a soluble pentapeptide with anti-inflammatory properties, inhibiting the production of NO (42) and pro-inflammatory chemokines (43) by human leukocytes. More recently, MLIF was found to disturb the balance of pro- and anti-inflammatory cytokines, specifically, inhibit IL-1 while favor IL-10 production by CD4+ T cells (44).
3. NK cell and NKT cell
NK cells and NKT cells are known to play an important role in host defense by production of IFN-γ and cytolytic peptides after activation. NK cells are detectable early during infection in ALA mouse models (8, 45). Elevated cytotoxic activity of NK cells was found in mice infected with pathogenic ameba compared to those infected with non-pathogenic strains (46), suggesting the involvement of NK cell-mediated defense in this model. One recent study reported the gender-dependent differences regarding to the control of ALA in C57BL/6 mice. Compared to susceptible male mice, increased IFN-γ and functional NKT cells were found in females and thought to be crucial for the enhanced resistance seen in females (45).
4. Mast cell
The essential role of mast cells in host control of infection has been shown in animal models infected with various bacterial and parasitic pathogens (47–49). In these models, mast cell deficient W/Wv mice exhibit delayed or failed clearance of pathogens and resistance is restored by mast cell reconstitution. Activated mast cells can kill pathogens by release of toxic proteases and mediators; cytokines produced by mast cells (ie. IL-6, TNF-α) can recruit phagocytes and influence lymphocytic development and functions; mast cells also mediate IgE-associated acquired immunity that contributes to type I immune responses (47). However, mast cell activation is not always associated with pathogen resistance and can correlate with the severity of inflammation and failed pathogen clearance (49–50).
Compared to bacterial and other parasitic pathogens, limited information is available regarding the mast cell-mediated immune response in amebiasis. Our previous study has reported an increased mast cell infiltration associated with substantially up-regulated mast cell protease genes in infected mouse ceca at 10 weeks post challenge (51). However, the fundamental question that whether mast cells contribute to parasite clearance or play a pathologic role in tissue damage remains unanswered.
Complement-mediated lysis of E. histolytica
After trophozoites penetrate the epithelial layer they presumably come into contact with host plasma components, such that soluble factors that can potentially destroy invading parasites or target them for killing by effector cells. The alternative and classic pathways of complement provide defense against the invading parasites in blood stream. The activation of the alternative pathway is initiated at least in part via cleavage of C3 and C5 by the parasite’s 56-kDa neutral cysteine proteinase (52). C3a and C5a are strong chemoattractants involved in the recruitment of inflammatory cells and activation of phagocytic cells, all of which may contribute to parasite expulsion or tissue damage (53). The cysteine proteinase of E. histolytica, which is capable of cleaving C3 and C5, may degrade the anaphylatoxins C3a and C5a to evade host immunity (53). Moreover, the Gal/GalNac lectin heavy subunit of E. histolytica inhibits the assembly of C8 and C9 into the C5b-9 membrane attack complex, thereby preventing complement-mediated lysis of the parasite (54).
Interactions of intestinal epithelial cells with E. histolytica
Defining the role of intestinal epithelial cells (IECs) in parasitic infection is an exciting and rapidly expanding area. Increasing evidence indicates that IECs serve as the effectors of mucosal immune system, responding to pathogens by secretion of proinflammatory mediators and acting as antigen presenting cells (55–57). Coculture of epithelial cell lines with E. histolytica trophozoites resulted in increased production of TNF-α, IL-1α, IL-6, IL-8, GROa, and GM-CSF by the epithelial cells (57). Similar results were observed in the SCID mouse-human intestinal xenograft (SCID-Hu-INT) model, where E. histolytica infection resulted in elevated production of IL-1β and IL-8 by the human intestinal xenograft (56). This ameba-induced epithelial inflammatory cascade was at least in part dependent on the signaling through NF-κB (56). However the role of NF-κB in amebiasis is not entirely clear. Chadee’s group (58) has reported that amebic proteins could actually inhibit NF-κB activation in IEC primed by macrophage secretions. This protective effect on IEC was mediated by the stress protein Hsp, known to be upregulated by many stimuli and to promote cell survival (59). Finally, it remains unclear whether NF-κB activation and other defensive functions are mediated by pattern recognition receptors like TLRs. It is generally thought that normal epithelium has very low expression of PRRs which results in a tolerant mucosal response to commensal antigens; Whereas under pathological conditions, the expression of PRRs is upregulated, leading to amplified immune response with consequent elimination of pathogens and probable mucosal destruction.
Natural resistance to experimental amebiasis in murine models
Hamano et al. (60) recently demonstrated that the resistance to intestinal amebiasis of the C57BL/6 strain was conferred by non-hemopoietic cells, suggesting that the epithelial response is critical. Notably, there was a requirement for hemopoietic IL-10 in this natural resistance, as IL-10 deficient mice or mice whose hemopoietic cells were deficient in IL-10 became susceptible to infection. Since IL-10 deficient mice manifest abundant defects in their epithelium a two-step protection model was proposed from these results, whereby the epithelium mediates the initial response (and protection requires IL-10), followed by acute inflammation, whereby either response can exhibit protective or deleterious immunity.
Interactions of host adaptive immune system with E. histolytica
Humoral immune response to E. histolytica
Humoral immune responses to E. histolytica have been characterized for years. Seroepidemiologic studies indicate that 81–100% of patients with invasive amebiasis develop specific circulatory antibodies within 7 days of infection (61). Our earlier prospective study on a cohort of pre-school children in Dhaka, Bangladesh has demonstrated that mucosal IgA response directed at the carbohydrate recognition domain (CRD) of the parasite Gal/GalNAc lectin is linked to protection from both infection and disease (2, 3) (Figure 2). Epitope-specific mouse monoclonal antibodies against the Gal/GalNac lectin have been shown to inhibit adherence of ameba to target cells in vitro and prevent colonization (62, 63). In contrast to mucosal IgA, serum IgA has not been correlated to protection. A higher lectin-specific IgG level was found in ALA and intestinal amebiasis patients as compared with asymptomatic controls (61). In our study, we observed an increased frequency of new E. histolytica infections in children with serum IgG antibodies to the lectin (64). These findings suggested that systemic anti-lectin antibody response might not offer direct protection from amebiasis. Furthermore, passive transfer of serum from vaccinated mice has not transferred protection from intestinal infection (unpublished work, A. Asgharpour and E. Houpt).
Fig 2. IgA anti-CRD is Associated with Immunity to E. histolytica Infection.
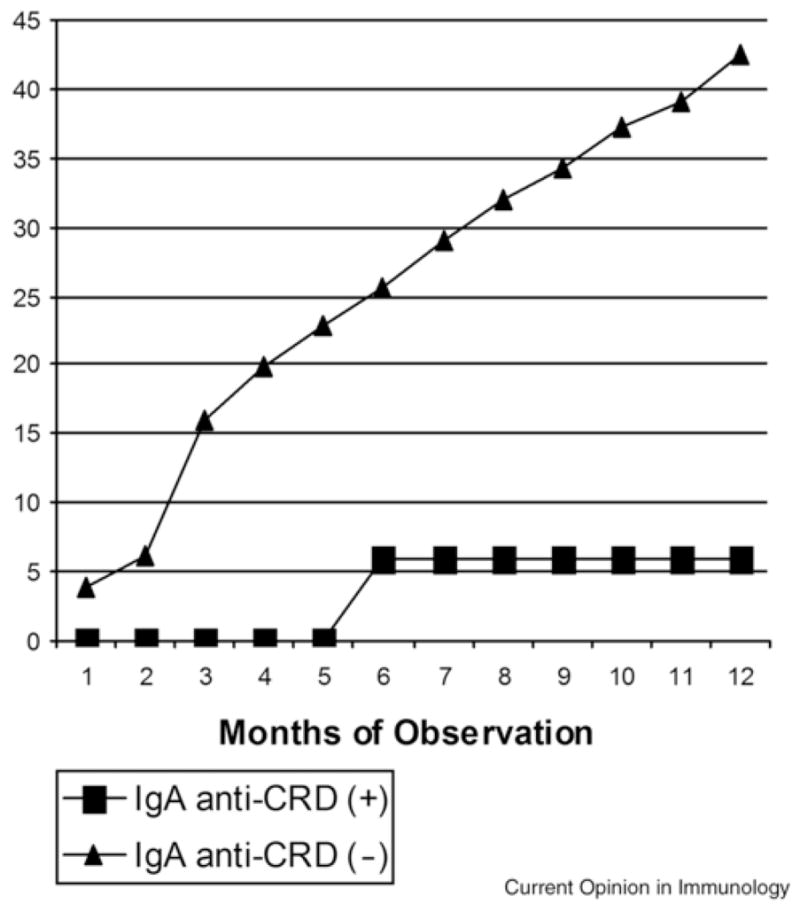
Children with fecal IgA antibodies against the Gal/GalNAc lectin carbohydrate recognition domain (IgA anti-CRD (+); n = 81) had a lower incidence of new intestinal E. histolytica infection compared to children lacking this response (IgA anti-CRD (−); (n=149). The two groups were statistically significantly different (p ≤ 0.04) at every time point. The average duration of protection was 437 days (95% CI 346–528 days). (Haque et al. 2002, 2006).
In recent years progress has been made in identification of possible vaccine candidates, the necessary routes of vaccination, and the immune correlates required for protection against amebiasis. Different proteins associated with amebic virulence have been proposed as candidate vaccine targets (reviewed in 65). The purified native Gal/GalNAc lectin has been demonstrated to be efficacious to protect gerbils and mice from amebic liver abscess (66) and intestinal infection (67), respectively. Protection from amebic colitis was associated with a positive fecal anti-lectin IgA response (67). Vaccination with the recombinant lectin fragment (LecA) elicited a substantial IgA response to the antigen, but the degree of protection was weaker compared to those vaccinated with native lectin (67).
T cell-mediated immune response to E. histolytica
The induction of effective immunity against Entamoeba must consider the activation of antigen-specific T cells which lead to secretion of appropriate cytokines and direct cytotoxicity to ameba. In our recent cohort study in Dhaka, amebic antigen stimulated IFN-γ production by PBMCs was associated with protection from future E. histolytica caused diarrhea (68), suggesting a potentially important role for Th1 cell-mediated protection (Figure 3). Furthermore, case reports have suggested that invasive amebiasis is exacerbated following interventions that suppress cell-mediated immunity, such as steroid therapy and splenectomy (69, 70). However, in an experimental amebic colitis model, CD4+ T cells have been suggested to mediate pathogenesis (51) instead of protection, therefore the pattern of CD4+ T cell response may be critical. Namely, an increase in mast cell activation and infiltration was associated with elevated Th2 cytokines in the colitis model at 10 weeks post challenge, suggesting the involvement of mast cells and a Th2 response in the progression of amebic colitis during the chronic phase (51).
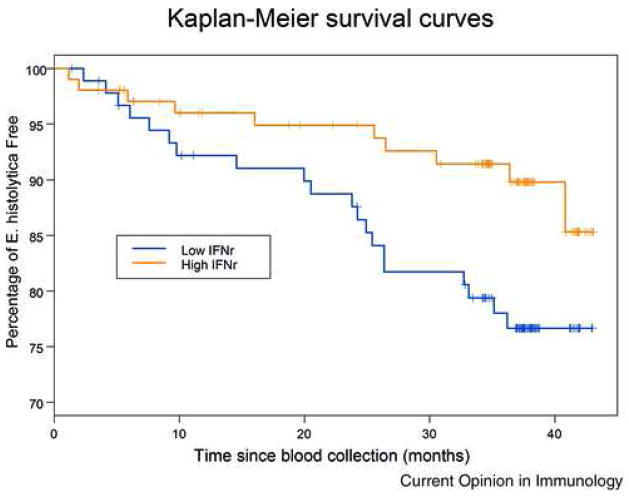
Fig. 3. High Levels of IFN-γ Predict Increased Survival Free of E. histolytica Diarrhea.
PBMCs were stimulated with soluble amebic extract and children grouped by IFN-γ production in response to SAE stimulation. Children were then followed for 44 months and incidence of E. histolytica diarrhea measured. Upper line and lower line indicate children with and without interferon gamma response above the median for all children (580 pg/ml) respectively. The two lines are significantly different: Logrank test p-value=0.03. n = 92 for the low IFN-γ, and n =103 for high IFN-γ groups (Haque et al 2006).
Growing evidence has suggested that during amebic infection T cells appear to be hyporesponsive to mitogen or antigen-stimulated proliferation (71). In vitro amebic components have favored Th2 cytokine production, with suppression of the Th1 cytokines (72). The amebic molecules conferring this suppression remain to be determined, but perhaps include MLIF activity (44). The Th1 to Th2 shift during amebic infection might result in a retarded T cell response directed to amebic antigen. Considering that ameba have mitogenic effects on lymphocytes, presumably nonspecific polyclonal activation may also disrupt antigen-specific anti-amebic immunity mediated by Th1 cells.
Non-immune host defenses and E. histolytica
1. Mucin
As the major constituent of the intestinal mucus layer, mucin forms the first line of host defense against invasion of E. histolytica (73). Mucin is a glycoprotein synthesized by goblet cells and submucosal glands, containing 80% carbohydrate by mass as O-linked polysaccharide chains. This makes it a high affinity ligand for the Gal/GalNac lectin of E. histolytica, potentially competing for trophozoite attachment to the underlying epithelium (74). Previous work has shown that invading trophozoites secrete cysteine proteases and glycosidases which help disrupt of the mucus barrier and epithelial tight junctions (75). E. histolytica trophozoites also secrete a heat stable factor that causes mucous hypersecretion from goblet cells, resulting in mucin depletion (76). The disrupted mucus layer does not inhibit amebic adherence to target epithelial cells as effectively as does the native polymer, indicative of a loss of protective function.
The potential capacity for synthetic alterations of mucins might add resistance to degradation. Although the chemical alterations of colonic mucins in response to E. histolytica have not been addressed, changes in the glycosylation patterns of rat mucin have been observed during Nippostrongylus brasiliensis infection (77). These transient alterations in sialylated oligosaccharides have been directly related to parasite expulsion.
2. Host bacterial flora and intestinal motility
Accumulating studies have suggested that gut resident flora appear to be more than just a food source for ameba. The interactions between resident bacteria and E. histolytica may be critical determinants for host defense or parasite virulence.
A number of studies have shown that E. histolytica trophozoites enhanced their virulence (78, 79) and modulated surface antigens (80, 81) in response to co-cultured bacteria. Certain strains of bacteria have been shown to reduce the virulence of the trophozoite in vitro, which is associated with a downregulated level of the Gal/GalNAc lectin light subunit (82). Although the mechanisms by which commensal bacteria could compromise the virulence of E. histolytica remain hypothetical, it was suggested that a glycosidase produced by intestinal bacteria could degrade the adherence lectin, the key virulent factor of E. histolytica (83). Bacteria could also regulate the invasiveness of trophozoites by conditioning the flora profile which favors encystation of E. histolytica; the levels of short-chain fatty acids produced by resident bacterial flora may be involved in signaling encystation (84).
In addition, intestinal motility may also contribute to the host defense against amebic invasion. It was reported that immune-dependent increase in intestinal smooth muscle function might contribute to the eradication of nematode parasites (85); likewise, expulsion of Giardia lamblia in mice was accompanied by small-intestinal hypermotility, which appeared to depend on the adaptive immune response against the parasite (86). Understanding the mechanisms behind the immunomodulation of intestinal motility may also impact on other enteric infections or inflammatory bowel disease, in which motility disturbances often occur.
Conclusions
Exciting progress has been made in understanding the way in which ameba interacts with the host defense mechanisms. Despite these intense investigations, we have only just begun to ask the fundamental and intricate questions. To name but a few: What are the determinants for invasive and noninvasive infection? What is the role of inflammation seen in the initial phases of amebic infection? What are the signals for the initiation of amebic invasion process? With the growing understanding of amebic genomes and virulence mechanisms, future studies should focus on what molecules and cellular processes ameba elaborates to manipulate host defense and determine the outcome of infection. A better understanding of the parasite-host interactions will help the design of effective vaccines delivered in such a way as to induce long-lasting immunity without induction of pathology.
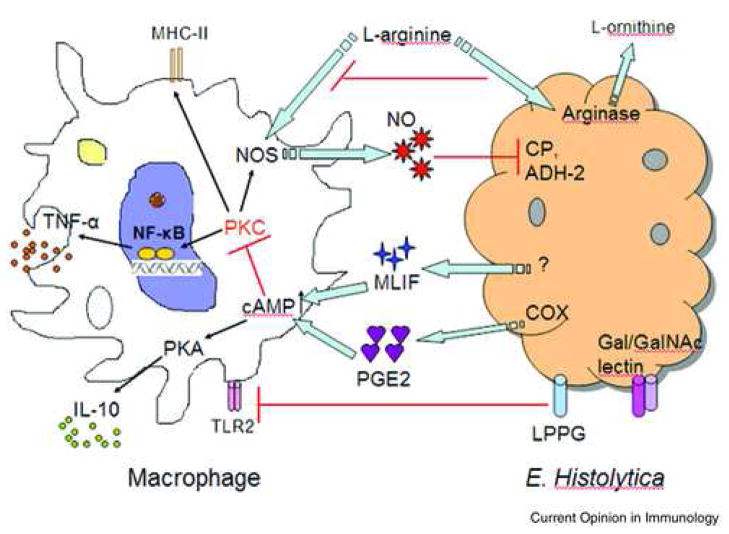
Fig. 1. Modulation of macrophage functions by E. histolytica.
The killing of E. hitolytica trophozoites by macrophages is mainly mediated by nitric oxide, derived from L-arginine by nitric oxide synthase (NOS). NO could inhibit amebic cysteine proteinases (CP) and alcohol dehydrogenase 2 (ADH2), the critical enzymes conferring virulence of E. histolytica. The arginase activity was detected in E. histolytica, which putatively converts L-arginine into L-ornithine, in turn limiting NO production by macrophage NOS. Prostaglandin E2 (PGE2) is an immunoregulatory molecule produced by cyclooxygenase (COX) in ameba or ameba-exposed macrophages. By activating cAMP-PKA pathway, PGE2 suppress macrophage effector functions by inhibiting PKC-mediated MHC-II expression and TNF-α production, while favoring IL-10 production. Monocyte Locomotion Inhibitory Factor (MLIF) produced by ameba may suppress macrophage functions in a manner similar to PGE2. Amebic lipophosphoglycan (LPG) might downregulate TLR2 expression on macrophages, thus control the effector mechanisms triggered through TLR2 signaling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.WHO. Amoebiasis, WHO Weekly Epidemiologic Record. 1997;72:97–100. [Google Scholar]
- 2.Haque R, Ali IKM, Sack RB, Ramakrishnan G, Farr BM, Petri WA., Jr Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J Infect Dis. 2001;183:1787–1793. doi: 10.1086/320740. [DOI] [PubMed] [Google Scholar]
- 3*.Haque R, Mondal D, Duggal P, Kabir M, Roy S, Farr BM, Sack RB, Petri WA., Jr Entamoeba histolytica Infection in Children and Protection from Subsequent Amebiasis. Infect Immun. 2006;74:904–909. doi: 10.1128/IAI.74.2.904-909.2006. (Conclusions were drawn from a 4-year prospective study that immunity to amebic diarrhea and colitis exists; protection from amebiasis was associated with a stool anti-CRD IgA response.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caballero-Salcedo A, Viveros-Rogel M, Salvatierra B, Tapia-Conyer R, Sepulveda-Amor J, Gutierrez G, Ortiz-Ortiz L. Seroepidemiology of amebiasis in Mexico. Am J Trop Med Hyg. 1994;50:412–419. doi: 10.4269/ajtmh.1994.50.412. [DOI] [PubMed] [Google Scholar]
- 5.Ravidin JI, Stauffer WM. Entamoeba histolytica (Amebiasis) Infectious Disease and Their Etiologic Agents. 2004;270:3097–3111. [Google Scholar]
- 6.Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nature Immunology. 2002;3:1041 – 1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 7.Chadee K, Meerovitch E. The pathogenesis of experimentally induced amebic liver abscess in the gerbil (Meriones unguiculatus) Am J Pathol. 1984;117:71–80. [PMC free article] [PubMed] [Google Scholar]
- 8.Tsutsumi V, Mena-Lopez R, Anaya-Velazquez F, Martinez-Palomo A. Cellular bases of experimental amebic liver abscess formation. Am J Pathol. 1984;117:81–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Palomo A, Tsutsumi V, Anaya-Velazquez F, Gonzalez-Robles A. Ultrastructure of experimental intestinal invasive amebiasis. Am J Trop Med Hyg. 1989;41:273–279. [PubMed] [Google Scholar]
- 10.Jarillo-Luna RA, Campos-Rodriguez R, Tsutsumi V. Entamoeba histolytica: immunohistochemical study of hepatic amoebiasis in mouse. Neutrophils and nitric oxide as possible factors of resistance. Exp Parasitol. 2002;101:40–56. doi: 10.1016/s0014-4894(02)00021-8. [DOI] [PubMed] [Google Scholar]
- 11.Chadee K, Moreau F, Meerovitch E. Entamoeba histolytica: chemoattractant activity for gerbil neutrophils in vivo and in vitro. Exp Parasitol. 1987;64:12–23. doi: 10.1016/0014-4894(87)90003-8. [DOI] [PubMed] [Google Scholar]
- 12.Salata RA, Ahmed P, Ravdin JI. Chemoattractant activity of Entamoeba histolytica for human polymorphonuclear neutrophils. J Parasitol. 1989;75:644–646. [PubMed] [Google Scholar]
- 13.Yu Y, Chadee K. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology. 1997;112:1536–1547. doi: 10.1016/s0016-5085(97)70035-0. [DOI] [PubMed] [Google Scholar]
- 14.Sengelov H. Complement receptors in neutrophils. Annual Review of Immunology. 1995;15:107–131. [PubMed] [Google Scholar]
- 15.Denis M, Chadee K. Human neutrophils activated by interferon-gamma and tumor necrosis factor-alpha kill Entamoeba histolytica trophozoites in vitro. J Leukoc Biol. 1989;46:270–274. doi: 10.1002/jlb.46.3.270. [DOI] [PubMed] [Google Scholar]
- 16.Guerrant RL, Brush J, Ravdin JI, Sullivan JA, Mandell GL. Interaction between Entamoeba histolytica and human polymorphonuclear neutrophils. J Infect Dis. 1981;143:83–93. doi: 10.1093/infdis/143.1.83. [DOI] [PubMed] [Google Scholar]
- 17.Seydel KB, Zhang T, Stanley SL., Jr Neutrophils play a critical role in early resistance to amebic liver abscesses in SCID mice. Infect Immun. 1997;65:3951–3953. doi: 10.1128/iai.65.9.3951-3953.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velazquez C, Shibayama-Salas M, Aguirre-Garcia J, Tsutsumi V, Calderon J. Role of neutrophils in innate resistance to Entamoeba histolytica liver infection in mice. Parasite Immunol. 1998;20:255–262. doi: 10.1046/j.1365-3024.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- 19*.Asgharpour A, Gilchrist C, Baba D, Hamano S, Houpt E. Resistance to intestinal Entamoeba histolytica infection is conferred by innate immunity and Gr-1+ cells. Infect Immun. 2005;73:4522–4529. doi: 10.1128/IAI.73.8.4522-4529.2005. (Using different amebic colitis mouse models, this study show that there are discrete mechanisms conferring innate resistance to E. histolytica depending on the host background. Administration of a neutrophil-depleting monoclonal antibody exacerbated cecal pathology, indicating a protective role of neutrophils in intestinal amebiasis) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivero-Navaa L, Aguirre-Garcíab J, Shibayama-Salasc M, Hernández-Pandod R, Tsutsumic V, Calderón J. Entamoeba histolytica: acute granulomatous intestinal lesions in normal and neutrophil-depleted mice. Experimental Parasitology. 2002;101:183–192. doi: 10.1016/s0014-4894(02)00106-6. [DOI] [PubMed] [Google Scholar]
- 21.Salata RA, Ravdin JI. The interaction of human neutrophils and Entamoeba histolytica increases cytopathogenicity for liver cell monolayers. J Infect Dis. 1986;154:19–26. doi: 10.1093/infdis/154.1.19. [DOI] [PubMed] [Google Scholar]
- 22.Arbo A, Hoefsloot M, Ramirez A, Ignacio-Santos J. Entamoeba histolytica inhibits the respiratory burst of polymorphonuclear leukocytes. Arch Invest Med (Mex) 1990;21(Suppl 1):57–61. [PubMed] [Google Scholar]
- 23.Bruchhaus I, Richter S, Tannich E. Recombinant expression and biochemical characterization of an NADPH:flavin oxidoreductase from. Entamoeba histolytica Biochem J. 1998;330:1217–1221. doi: 10.1042/bj3301217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sim S, Yong TS, Park SJ, Im K, Kong Y, Ryu JS, Min DY, Shin MH. NADPH oxidase-derived reactive oxygen species-mediated activation of ERK1/2 is required for apoptosis of human neutrophils induced by Entamoeba histolytica. J Immunol. 2005;174:4279–4288. doi: 10.4049/jimmunol.174.7.4279. [DOI] [PubMed] [Google Scholar]
- 25.Bruchhaus I, Richter S, Tannich E. Removal of hydrogen peroxide by the 29kDa protein of Entamoeba histolytica. Biochem J. 1997;326:785–789. doi: 10.1042/bj3260785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi MH, Sajed D, Poole L, Hirata K, Herdman S, Torian BE, Reed SL. An unusual surface peroxiredoxin protects invasive Entamoeba histolytica from oxidant attack. Mol Biochem Parasitol. 2005;143:80–89. doi: 10.1016/j.molbiopara.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 27*.Davis PH, Zhang X, Guo J, Townsend RR, Stanley SL., Jr Comparative proteomic analysis of two Entamoeba histolytica strains with different virulence phenotypes identifies peroxiredoxin as an important component of amoebic virulence. Mol Microbiol. 2006;61:1523–1532. doi: 10.1111/j.1365-2958.2006.05344.x. (In this work, by using the comparative proteomics, the authors defined differential levels of two virulent proteins linked to resistance of host oxidative defense in E. histolytica HM-1:IMSS and E. histolytica Rahman. Overexpression of peroxiredoxin in Rahman trophozoites caused greater inflammatory responses and damage to human colonic xenografts, as well as higher resistance to killing by H2O2, suggesting a potential role for peroxiredoxin in E. histolytica virulence.) [DOI] [PubMed] [Google Scholar]
- 28.Salata RA, Martinez-Palomo A, Murray HW, Conales L, Trevino N, Segovia E, Murphy CF, Ravdin JI. Patients treated for amebic liver abscess develop cell mediated immune responses effective in vitro against Entamoeba histolytica. J Immunol. 1986;136:2633–2639. [PubMed] [Google Scholar]
- 29.Lin JY, Seguin R, Keller K, Chadee K. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect Immun. 1994;62:1534–1541. doi: 10.1128/iai.62.5.1534-1541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghadirian E, Denis M. Entamoeba histolytica extract and interferon-gamma activation of macrophage-mediated amoebicidal function. Immunobiology. 1992;185:1–10. doi: 10.1016/S0171-2985(11)80312-8. [DOI] [PubMed] [Google Scholar]
- 31*.Maldonado-Bernal C, Kirschning CJ, Rosenstein Y, Rocha LM, Rios-Sarabia N, Espinosa-Cantellano M, Becker I, Estrada I, Salazar-Gonzalez RM, Lopez-Macias C, Wagner H, Sanchez J, Isibasi A. The innate immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by toll-like receptors 2 and 4. Parasite Immunol. 2005;27:127–137. doi: 10.1111/j.1365-3024.2005.00754.x. (This work demonstrated that E. histolytica surface component LPPG could induce innate immune responses via TLR2 and TLR4 mediated signalings. Macrophages derived from TLR-deficient mice showed impaired responses to LPPG). [DOI] [PubMed] [Google Scholar]
- 32.Kammanadiminti SJ, Mann BJ, Dutil L, Chadee K. Regulation of Toll-like receptor-2 expression by the Gal-lectin of Entamoeba histolytica. FASEB J. 2004;18:155–167. doi: 10.1096/fj.03-0578fje. [DOI] [PubMed] [Google Scholar]
- 33.Siman-Tov R, Ankri S. Nitric oxide inhibits cysteine proteinases and alcohol dehydrogenase 2 of. Entamoeba histolytica, Parasitol Res. 2003;89:146–149. doi: 10.1007/s00436-002-0716-2. [DOI] [PubMed] [Google Scholar]
- 34.Seydel KB, Smith SJ, Stanley SL., Jr Innate immunity to amebic liver abscess is dependent on gamma interferon and nitric oxide in a murine model of disease. Infect Immun. 2000;68:400–402. doi: 10.1128/iai.68.1.400-402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JY, Keller K, Chadee K. E. histolytica proteins modulate the respiratory burst potential by murine macrophages. Immunology. 1993;78:291–297. [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Keller K, Chadee K. Entamoeba histolytica modulates the nitric oxide synthase gene and nitric oxide production by macrophages for cytotoxicity against amoebae and tumour cells. Immunology. 1994;83:601–610. [PMC free article] [PubMed] [Google Scholar]
- 37.Elnekave K, Siman-Tov R, Ankris S. Consumption of L-arginine mediated by Entamoeba histolytica L-arginase (EhArg) inhibits amoebicidal activity and nitric oxide production by activated macrophages. Parasite Immunol. 2003;25:597–608. doi: 10.1111/j.0141-9838.2004.00669.x. [DOI] [PubMed] [Google Scholar]
- 38.Se′guin R, Keller K, Chadee K. Entamoeba histolytica stimulates the unstable transcription of c-fos and tumor necrosis factor-a messenger RNA by protein kinase C signal transduction in macrophages. Immunology. 1995;86:49–57. [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W, Chadee K. Entamoeba histolytica suppresses gamma interferon-induced macrophage class II major histocompatibility complex Ia molecule and I-A beta mRNA expression by a prostaglandin E2-dependent mechanism. Infect Immun. 1995;63:1089–1094. doi: 10.1128/iai.63.3.1089-1094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dey I, Keller K, Belley A, Chadee K. Identification and characterization of a cyclooxygenase-like enzyme from Entamoeba histolytica. PNAS. 2003;100:13561–13566. doi: 10.1073/pnas.1835863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Gutierrez-Alarcon A, Moguel-Torres M, Mata-Leyva O, Cuellar-Nevarez G, Siqueiros-Cendon T, Erosa G, Ramos-Martinez E, Talamas-Rohana P, Sanchez-Ramirez B. Entamoeba histolytica: inflammatory process during amoebic liver abscess formation involves cyclooxygenase-2 expression in macrophages and trophozoites. Exp Parasitol. 2006;114:154–159. doi: 10.1016/j.exppara.2006.03.008. (In an ALA hamster model, COX-2 expression was detected in PMN and macrophages as well as E. histolytica trophozoites by in situ hybridization and RT-PCR, suggesting the involvement of COX-2 in the inflammatory process during amebic infection). [DOI] [PubMed] [Google Scholar]
- 42.Rico G, Leandro E, Rojas S, Gimenez JA, Kretschmer RR. The monocyte locomotion inhibitory factor produced by Entamoeba histolytica inhibits induced nitric oxide production in human leukocytes. Parasitol Res. 2003;90:264–267. doi: 10.1007/s00436-002-0780-7. [DOI] [PubMed] [Google Scholar]
- 43.Utrera-Barillas D, Velazquez JR, Enciso A, Cruz SM, Rico G, Curiel-Quesada E, Teran LM, Kretschmer RR. An anti-inflammatory oligopeptide produced by Entamoeba histolytica down-regulates the expression of pro-inflammatory chemokines. Parasite Immunol. 2003;25:475–482. doi: 10.1111/j.1365-3024.2003.00657.x. [DOI] [PubMed] [Google Scholar]
- 44.Rojas-Dotor S, Rico G, Perez J, Velazquez J, Silva R, Morales E, Kretschmer R. Cytokine expression in CD4(+) cells exposed to the monocyte locomotion inhibitory factor produced by Entamoeba histolytica. Parasitol Res. 2006;98:493–495. doi: 10.1007/s00436-005-0090-y. [DOI] [PubMed] [Google Scholar]
- 45**.Lotter H, Jacobs T, Gaworski I, Tannich E. Sexual dimorphism in the control of amebic liver abscess in a mouse model of disease. Infect Immun. 2006;74:118–124. doi: 10.1128/IAI.74.1.118-124.2006. (This study demonstrated that female B6 mice were more resistant to development of ALA than male mice. This resistance was associated with higher IFN-γ production contributing to faster clearance of E. histolytica trophozoites from the liver. Using NKT knock out mice, they present evidence that a source of early IFN-γ production is natural killer T (NKT) cells). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim KH, Shin CO, Im K. Natural killer cell activity in mice infected with free-living amoeba with reference to their pathogenicity. Korean J Parasitol. 1993;31:239–248. doi: 10.3347/kjp.1993.31.3.239. [DOI] [PubMed] [Google Scholar]
- 47**.Velin D, Bachmann D, Bouzourene H, Michetti P. Mast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse model. Gastroenterology. 2005;129:142–155. doi: 10.1053/j.gastro.2005.04.010. (This study demonstrated the potential role of mast cells in conferring the vaccine-mediated protection from Helicobacter infection. Following vaccination, increased mast cell population was detected in draining lymph nodes, associated with upregulated mast cell proteases expression in stomach. Mast cell-deficient mice (W/W v mice) were not protected from H felis colonization after vaccination, while the mice were recovered the ability to clear infection after reconstitution with mast cells. These experiments show that mast cells are critical mediators of anti- Helicobacter vaccination). [DOI] [PubMed] [Google Scholar]
- 48.Maurer M, Lopez Kostka S, Siebenhaar F, Moelle K, Metz M, Knop J, von Stebut E. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006;20:2460–2467. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- 49.Furuta T, Kikuchi T, Iwakura Y, Watanabe N. Protective roles of mast cells and mast cell-derived TNF in murine malaria. J Immunol. 2006;177:3294–3302. doi: 10.4049/jimmunol.177.5.3294. [DOI] [PubMed] [Google Scholar]
- 50.Wershil BK, Furuta GT, Wang AS, Galli SJ. Mast cell-dependent neutrophil and mononuclear cell recruitment in immunoglobulin E-induced gastric reactions in mice. Gastroenterology. 1996;110:1482–1487. doi: 10.1053/gast.1996.v110.pm8613053. [DOI] [PubMed] [Google Scholar]
- 51.Houpt ER, Glembocki DJ, Obrig TG, Moskaluk CA, Lockhart LA, Wright RL, Seaner RM, Keepers TR, Wilkins TD, Petri WA., Jr The mouse model of amebic colitis reveals mouse strain susceptibility to infection and exacerbation of disease by CD4+ T cells. J Immunol. 2002;169:4496–4503. doi: 10.4049/jimmunol.169.8.4496. [DOI] [PubMed] [Google Scholar]
- 52.Reed SL, Gigli I. Lysis of complement-sensitive Entamoeba histolytica by activated terminal complement components. J Clin Invest. 1990;86:1815–1822. doi: 10.1172/JCI114911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reed SL, Ember JA, Herdman DS, DiScipio RG, Hugli TE, Gigli I. The extracellular neutral cysteine proteinase of Entamoeba histolytica degrades anaphylatoxins C3a and C5a. J Immunol. 1995;155:266–274. [PubMed] [Google Scholar]
- 54.Braga LL, Ninomiya H, McCoy JJ, Eacker S, Wiedmer T, Pham C, Wood S, Sims PJ, Petri WA., Jr Inhibition of the complement membrane attack complex by the galactose-specific adhesion of Entamoeba histolytica. J Clin Invest. 1992;90:1131–1137. doi: 10.1172/JCI115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seydel KB, Li E, Zhang Z, Stanley SL., Jr Epithelial cell-initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology. 1998;115:1446–1453. doi: 10.1016/s0016-5085(98)70023-x. [DOI] [PubMed] [Google Scholar]
- 56.Seydel KB, Li E, Swanson PE, Stanley SL., Jr Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eckmann L, Reed SL, Smith JR, Kagnoff MF. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1 alpha. J Clin Invest. 1995;96:1269–1279. doi: 10.1172/JCI118161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58**.Kammanadiminti SJ, Chadee K. Suppression of NF-κB Activation by Entamoeba histolytica in intestinal epithelial cells is mediated by heat shock protein 27. J Biol Chem. 2006;281:26112–26120. doi: 10.1074/jbc.M601988200. (Novel observations were reported in this paper: Amebic antigen could induce stress response in macrophage-conditioned epithelial cells, characterized by heat shock protein upregulation. The protective effect was mediated by Hsp-27, which suppresses NF-κB activation by virtue of its association with IκB kinase (IKK) complex in intestinal epithelial cells). [DOI] [PubMed] [Google Scholar]
- 59.Musch MW, Petrof EO, Kojima K, Ren H, McKay DM, Chang EB. Bacterial superantigen-treated intestinal epithelial cells upregulate heat shock proteins 25 and 72 and are resistant to oxidant cytotoxicity. Infect Immun. 2004;72:3187–3194. doi: 10.1128/IAI.72.6.3187-3194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60**.Hamano S, Asgharpour A, Stroup SE, Wynn TA, Leiter EH, Houpt E. Resistance of C57BL/6 mice to amoebiasis is mediated by nonhemopoietic cells but requires hemopoietic IL-10 production. J Immunol. 2006;177:1208–1213. doi: 10.4049/jimmunol.177.2.1208. (Using bone marrow chimera and IL-10 knock out model, the authors proposed that B6 resistance to amebic colitis occurs at the nonhemopoietic level, yet the protective capacity is maintained via hemopoietic IL-10. A novel two-step protection model was proposed in this paper that initial protection was mediated by nonhemopoietic cells (e.g., epithelium) under homeostatic status; and inflammatory response provide the second-phase protection in individuals with underlying nonhemopoietic predisposition to amoebic establishment). [DOI] [PubMed] [Google Scholar]
- 61.Kaur U, Sharma AK, Sharma M, Vohra H. Distribution of Entamoeba histolytica Gal/GalNAc lectin-specific antibody response in an endemic area. Scandinavian Journal of Immunology. 2004;60:524–528. doi: 10.1111/j.0300-9475.2004.01512.x. [DOI] [PubMed] [Google Scholar]
- 62.Pillai DR, Wan PS, Yan YC, Ravdin JI, Kain KC. The cysteine-rich region of the Entamoeba histolytica adherence lectin (170-kilodalton subunit) is sufficient for high-affinity Gal/GalNac-specific binding in vitro. Infect Immun. 1999;67:3836–3841. doi: 10.1128/iai.67.8.3836-3841.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelsall BL, Jackson TG, Pearson RD, Ravdin JI. Secretory immunoglobulin A antibodies to the galactose inhibitable adherence protein in the saliva of patients with amoebic liver disease. Am J Trop Med Hyg. 1994;51:454–459. [PubMed] [Google Scholar]
- 64.Haque R, Duggal P, Ali IM, Hossain MB, Mondal D, Sack RB, Farr BM, Beaty TH, Petri WA., Jr Innate and acquired resistance to amebiasis in bangladeshi children. J Infect Dis. 2002;186:547–552. doi: 10.1086/341566. [DOI] [PubMed] [Google Scholar]
- 65.Huston CD, Petri WA., Jr Host-pathogen interaction in amebiasis and progress in vaccine development. Eur J Clin Microbiol Infect Dis. 1998;17:601–614. doi: 10.1007/BF01708342. Review. [DOI] [PubMed] [Google Scholar]
- 66.Petri WA, Ravdin JI. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect Immun. 1991;59:97–101. doi: 10.1128/iai.59.1.97-101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Houpt E, Barroso L, Lockhart L, Wright R, Cramer C, Lyerly D, Petri WA. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine. 2004;22:611–617. doi: 10.1016/j.vaccine.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Haque R, Mondal D, Shu J, Roy S, Kabir M, Davis AN, Duggal P, Petri WA., Jr IFN-g production by peripheral blood mononuclear cells is associated with childhood resistance to amebiasis . AJTMH. :#06–0528. In press. [PubMed] [Google Scholar]
- 69.Ghardirian E, Meerovitch E. Effect of immunosuppression on the size and metastasis of amoebic liver abscesses in hamsters. Parasite Immunol. 1981;3:329–333. doi: 10.1111/j.1365-3024.1981.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 70.Ghardirian E, Meerovitch E. Effect of splenectomy on the size of amoebic liver abscesses and metastic foci in hamsters. Infect Immun. 1981;31:571–576. doi: 10.1128/iai.31.2.571-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Talamás-Rohana P, Schlie-Guzmán MA, Hernández-Ramírez VI, Rosales-Encina JL. T-cell suppression and selective in vivo activation of TH2 subpopulation by Entamoeba histolytica 220-kilodalton lectin. Infect Immun. 1995;63:3953–3958. doi: 10.1128/iai.63.10.3953-3958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell D, Chadee K. Interleukin (IL)-2, IL-4, and tumor necrosis factor-alpha responses during Entamoeba histolytica liver abscess development in gerbils. J Infect Dis. 1997;175:1176–83. doi: 10.1086/520355. [DOI] [PubMed] [Google Scholar]
- 73.Moncada DM, Kammanadiminti SJ, Chadee K. Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol. 2003;19:305–311. doi: 10.1016/s1471-4922(03)00122-3. Review. [DOI] [PubMed] [Google Scholar]
- 74.Belley A, Keller K, GottKe M, Chadee K. Intestinal mucins in colonization and host defense against pathogens. Am J Trop Med Hyg. 1999;60(4 suppl):10–15. doi: 10.4269/ajtmh.1999.60.10. [DOI] [PubMed] [Google Scholar]
- 75*.Moncada D, Keller K, Ankri S, Mirelman D, Chadee K. Antisense inhibition of Entamoeba histolytica cysteine proteases inhibits colonic mucus degradation. Gastroenterology. 2006;130:721–730. doi: 10.1053/j.gastro.2005.11.012. (This work mainly demonstrated that cysteine protease–deficient amebae generated by antisense inhibition of EhCP5 were ineffective at degrading radioactive cysteine-labeled colonic mucin compared to wild-type amoebae by >60%. These findings unravel a central role for E histolytica CPs as key virulence factors in disrupting an intact mucus barrier in the pathogenesis of intestinal amebiasis.) [DOI] [PubMed] [Google Scholar]
- 76.Tse SK, Chadee K. Biochemical characterisation of rat colonic mucins secreted in response to Entamoeba histolytica. Infect Immun. 1992;60:1603–1612. doi: 10.1128/iai.60.4.1603-1612.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karlsson NG, Olson FJ, Jovall PA, Andersch Y, Enerback L, Hansson GC. Identification of transient glycosylation alterations of sialylated mucin oligosaccharides during infection by the rat intestinal parasite Nippostrongylus brasiliensis. Biochem J. 2000;350:805–814. [PMC free article] [PubMed] [Google Scholar]
- 78.Anaya-Velazquez F, Padilla-Vaca F. Effect of intestinal bacteria on the virulence of Entamoeba histolytica. Arch Med Res. 1992;23:183–185. [PubMed] [Google Scholar]
- 79.Bracha R, Mirelman D. Virulence of Entamoeba histolytica trophozoites. Effects of bacteria, microaerobic conditions, and metronidazole. J Exp Med. 1984;160:353–368. doi: 10.1084/jem.160.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhattacharya A, Ghildyal R, Prasad J, Bhattacharya S, Diamond LS. Modulation of a surface antigen of Entamoeba histolytica in response to bacteria. Infect Immun. 1992;60:1711–1713. doi: 10.1128/iai.60.4.1711-1713.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhattacharya A, Anand MT, Jayshree P, Yadav N, Bhattacharya S. Molecular changes in Entamoeba histolytica in response to bacteria. J Eukaryot Microbiol. 1998;45:28S–33S. doi: 10.1111/j.1550-7408.1998.tb04521.x. [DOI] [PubMed] [Google Scholar]
- 82.Padilla-Vaca F, Ankri S, Bracha R, Koole LA, Mirelman D. Down regulation of Entamoeba histolytica virulence by monoxenic cultivation with Escherichia coli O55 is related to a decrease in expression of the light (35-kilodalton) subunit of the Gal/GalNAc lectin. Infect Immun. 1999;67:2096–2102. doi: 10.1128/iai.67.5.2096-2102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Variyam EP. Luminal bacteria and proteases together decrease adherence of Entamoeba histolytica trophozoites to Chinese hamster ovary epithelial cells: a novel host defence against an enteric pathogen. Gut. 1996;39:521–527. doi: 10.1136/gut.39.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84*.Byers J, Faigle W, Eichinger D. Colonic short-chain fatty acids inhibit encystation of Entamoeba invadens. Cell Microbiol. 2005;7:269–279. doi: 10.1111/j.1462-5822.2004.00457.x. (This work revealed that short-chain fatty acids (SCFAs) in the mucus layer overlying the colonic epithelium did not affect multiplication of trophozoite-stage Entameba, but did reversibly inhibit in vitro cyst formation in a concentration-dependent manner, through a mechanism that may involve histone modification.) [DOI] [PubMed] [Google Scholar]
- 85.Vallance BA, Collins SM. The effect of nematode infection upon intestinal smooth muscle function. Parasite Immunol. 1998;20:249–253. doi: 10.1046/j.1365-3024.1998.00155.x. [DOI] [PubMed] [Google Scholar]
- 86.Andersen YS, Gillin FD, Eckmann L. Adaptive immunity-dependent intestinal hypermotility contributes to host defense against Giardia spp. Infect Immun. 2006;74:2473–2476. doi: 10.1128/IAI.74.4.2473-2476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]