Abstract
Protease-activated receptors such as PAR1 and PAR2 have been implicated in the regulation of endothelial nitric oxide production. We hypothesized that PAR1 and PAR2 distinctly regulate the activity of endothelial nitric oxide synthase through the selective phosphorylation of a positive regulatory site, Ser1179 and a negative regulatory site, Thr497 in bovine aortic endothelial cells. A selective PAR1 ligand, TFLLR, stimulated the phosphorylation of endothelial nitric oxide synthase at Thr497. It had a minimal effect on Ser1179 phosphorylation. In contrast, a selective PAR2 ligand, SLIGRL, stimulated the phosphorylation of Ser1179 with no noticeable effect on Thr497. Thrombin has been shown to transactivate PAR2 through PAR1. Thus, thrombin as well as a peptide mimicking the PAR1 tethered ligand, TRAP, stimulated phosphorylation of both sites. Also, thrombin and SLIGRL, but not TFLLR, stimulated cyclic GMP production. A Gq inhibitor blocked thrombin- and SLIGRL-induced Ser1179 phosphorylation whereas it enhanced thrombin-induced Thr497 phosphorylation. In contrast, a G12/13 inhibitor blocked thrombin- and TFLLR-induced Thr497 phosphorylation whereas it enhanced the Ser1179 phosphorylation. Although a Rho-kinase inhibitor, Y27632, blocked the Thr497 phosphorylation, other inhibitors that targeted Rho-kinase failed to block TFLLR-induced Thr497 phosphorylation. These data suggest that PAR1 and PAR2 distinctly regulate endothelial nitric oxide synthase phosphorylation and activity through G12/13 and Gq, respectively, delineating the novel signaling pathways by which the proteases act on protease-activated receptors to potentially modulate endothelial functions.
Keywords: nitric oxide synthase, endothelial cell, thrombin, protease-activated receptor
Introduction
Thrombin is a multifunctional serine protease that not only mediates the coagulation cascade but also has a wide variety of actions within the endothelium and vascular smooth muscle. The signal transduction and functions of thrombin through the protease-activated receptors (PARs) are strongly implicated in vascular physiology and pathophysiology 1, 2. PARs represent a unique class of G protein-coupled receptors (GPCRs) activated by proteolytic cleavage of their extracellular N-terminal domains. The new N-terminus acts as a “tethered ligand” and activates the receptor itself. Four members of this family have been cloned, of which PAR1 and PAR3 are essentially thrombin receptors, while PAR2 is activated by trypsin or mast cell tryptase, and PAR4 is activated by both thrombin and trypsin 1. However, transactivation of PAR2 by thrombin through PAR1 (the tethered ligand of PAR1 acts as a PAR2 ligand to activate PAR2 if both receptors co-exist) has been demonstrated in cultured human umbilical vein endothelial cells (HUVECs) 3. Activation of PARs stimulate a myriad arrays of signal transduction pathways, which include the activation of distinct heterotrimeric G proteins and tyrosine and serine/threonine kinases 1, 2.
Importantly, multiple PARs seem to regulate the release of nitric oxide (NO) via endothelial NO synthesis (eNOS) 4-6. Although these data suggest a significant link between eNOS and PARs, detailed mechanisms by which PARs regulate eNOS activity have been insufficiently characterized. Recent studies revealed that eNOS needs to be specifically phosphorylated to exert its full activity 7. There are multiple phosphorylation sites on eNOS, however most is known about the functional consequences of phosphorylation of Ser1179 (bovine)/1177 (human) and Thr497 (bovine)/495 (human). When the Ser1179 is phosphorylated, NO production is increased two- to threefold above basal level. In contrast, Thr497 is a negative regulatory site of eNOS associated with decreased enzymatic activity. Several eNOS kinases such as Akt, which phosphorylates Ser1179 or protein kinase C (PKC), which phosphorylates Thr497 have been identified 7.
Here, we have hypothesized that PAR1 and PAR2 distinctly regulate the two phosphorylation sites on eNOS and NO production. We found several lines of evidence indicating the reciprocal regulation of the phosphorylation of these sites on eNOS by the PARs in bovine aortic endothelial cells (BAECs) through different G protein-dependent signal transduction pathways. These data demonstrate novel signal transduction characteristics of PAR1 and PAR2 in endothelial cells representing a diverse physiological and pathophysiological role of PARs in regulating endothelial functions.
Materials and Methods
Reagents
Thrombin from bovine plasma was purchased from Sigma. A PAR1 selective agonist, TFLLR-NH2, a PAR4 selective agonist, AY-NH2, and a peptide mimicking the PAR1 tethered ligand, TRAP, were purchased from Tocris. A PAR2 selective agonist, SLIGRL-NH2, was purchased from Bachem. A Gq inhibitor, YM-254890, was a gift from Astellas Pharma Inc. Pertusis toxin, phorbol 12-myristrate 13-acetate (PMA), and Rho-kinase (ROCK) inhibitors, Y27632 and H-1152, and a PKC inhibitor, GF109203X, were purchased from Calbiochem. Antibodies against Ser1179-phosphorylated eNOS and Thr497-phosphorylated eNOS were purchased from Cell Signaling Technology. Total eNOS antibody was purchased from BD Transduction Laboratories. Antibody for Thr853-phosphorylated myosin phosphatase target subunit-1 (MYPT1) was purchased from Upstate and antibody against total MYPT1 was purchased from Covance.
Cell Culture
BAECs were purchased from Cambrex and grown in DMEM containing 10% fetal bovine serum, penicillin and streptomycin as previously described 8. BAECs were subcultured using Versene (0.53 mmol/L EDTA in phosphate-buffered saline) to avoid trypsin exposure. HUVECs were a gift from Dr. Yi Wu (Temple University School of Medicine) 9. Cells from passage 4-12 were grown to about 90% confluency and serum-starved for 48 h before the experiments.
Adenovirus Infection
Generation and characterization of replication-deficient adenoviruses encoding a dominant-negative mutant of Rho (dnRho), myc-N19-RhoA, and myc-p115RGS were described in detail elsewhere 10, 11. Adenovirus construct encoding GRK2/βARK-ct was generated by Dr. Andrea Eckhart from Gene Transfer Vector Core (Thomas Jefferson University). An adenovirus vector encoding GFP was used as a control for other adenovirus vectors which also encode GFP independently of their respective inserts. The adenovirus titers were determined by Adeno-X™ Rapid Titer Kit (BD Biosciences). Subconfluent BAECs were infected with adenovirus for 2 days as previously described 8.
Immunoblotting
Cell lysates were subjected to SDS-PAGE gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane as previously described 12. The membranes were then exposed to primary antibodies overnight at 4°C. After incubation with the peroxidase linked secondary antibody for 1 h at room temperature, immunoreactive proteins were visualized by ECL reagent (Pierce).
Intracellular cyclic GMP Assay
BAECs were incubated with agonists at 37°C for 20 min in the presence of 0.5 mmol/L methylisobutylxanthine and intracellular cyclic GMP (cGMP) was determined by an enzyme immunoassay kit (Cayman Chemical) 8.
Intracellular Ca2+ ([Ca2+]i) Measurements
[Ca2+]i was measured as described previously using fura 2 as an indicator 13. In brief, BAECs subcultured on coverslips were loaded with 3 μM fura 2-AM. The fura 2 fluorescence was measured at a frequency of 1 Hz and [Ca2+]i-values were then obtained as described 13.
Statistical Analysis
The data shown are either from 3 or 4 independent experiments (the total n number is given in the figure legends) and presented as mean±SEM. The data were analyzed using one-way ANOVA followed by a post-hoc modified t-test or a two-way ANOVA. P<0.05 was considered significant.
Results
eNOS Phosphorylation by Thrombin in BAECs
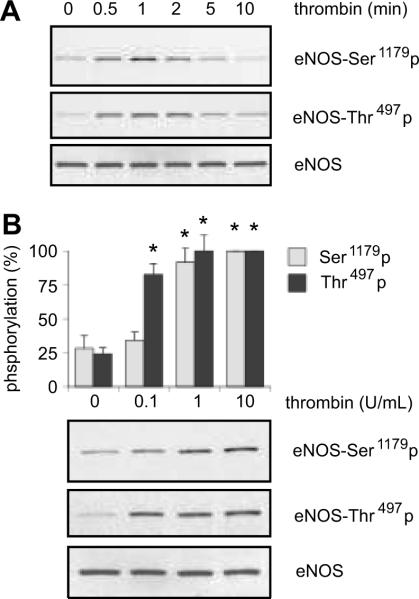
In cultured BAECs, we have examined whether thrombin stimulates phosphorylation of eNOS at Ser1179, a catalytically positive regulatory site, and at Thr497, a catalytically negative regulatory site 7. As shown in Figure 1A, thrombin (10 U/mL) stimulated eNOS phosphorylation at Ser1179, which began at 0.5 min, peaked at 1 min, and phosphorylation declined thereafter. Also, thrombin stimulated eNOS phosphorylation at Thr497 from 0.5 to 2 min. Figure 1B shows the concentration dependence of thrombin-induced phosphorylation of eNOS in BAECs. eNOS phosphorylation at Ser1179 was detectable with 1U/mL of thrombin, and the maximum phosphorylation was observed at a concentration of 10 U/mL. In contrast, thrombin-stimulated phosphorylation at Thr497 occurred at an even lower concentration of thrombin (0.1 U/mL) in BAECs. Thus, these results suggest the possibility that thrombin mediates the phosphorylation of each eNOS site through distinct PARs or G proteins in BAECs.
Figure 1.
The effects of thrombin stimulation on eNOS phosphorylation at Ser1179 and Thr497 were investigated in BAECs. The cells were stimulated with 10 U/mL of thrombin for the indicated periods (A) or with indicated concentrations of thrombin for 1 min (B), and phosphorylation of eNOS at each site was determined using immunoblot analysis. The bar graphs show densitometric analyses of the phosphorylation of eNOS at each site (mean ± SEM, *p < 0.05 compared with the basal control, n=3).
eNOS Phosphorylation and Activation by Selective PAR Agonists in BAECs
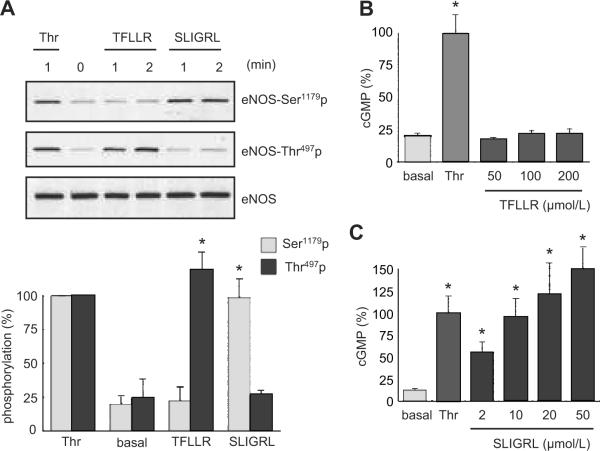
To examine the participation of PARs in the regulation of NO production, we stimulated BAECs with a selective PAR1 agonist, TFLLR, or a selective PAR2 agonist, SLIGRL, and evaluated eNOS phosphorylation. As shown in Figure 2A, TFLLR stimulated eNOS phosphorylation at Thr497 at 1 to 2 min, whereas it had no effect on the Ser1179 phosphorylation. On the other hand, SLIGRL stimulated eNOS phosphorylation at Ser1179 but not Thr497 at 1 to 2 min. In line with a theory that thrombin activates PAR2 via the intermolecular transactivation of PAR2 3, a peptide mimicking the PAR1 tethered ligand, TRAP, stimulated both phosphorylation sites in a concentration dependent manner (10-100 μmol/L). However, a PAR4 agonist, AY-NH2 (up to 200 μmol/L), had no effect on either phosphorylation site (data not shown). The distinct responses by the PAR1 and PAR2 agonists were also confirmed in HUVECs and bovine pulmonary artery endothelial cells (data not shown). These results suggest that PAR1 and PAR2 have distinct roles in regulating eNOS phosphorylation at least in these types of endothelial cells.
Figure 2.
Distinct phosphorylation responses of eNOS stimulated by PAR1 and PAR2. A. The effects of a PAR1 or PAR2 agonist on eNOS phosphorylation. BAECs were stimulated with a PAR1 agonist, TFLLR (50 μmol/L), a PAR2 agonist, SLIGRL (50 μmol/L) for 2 min, or thrombin (10 U/mL) for the indicated periods. The bar graphs show densitometric analyses of the phosphorylation of eNOS at each site (mean ± SEM, bands scanned are n=3, each, *p < 0.05 compared with the basal control). Results were expressed as percentage increase in which the maximum response to thrombin (10 U/mL) was defined as 100%, because the basal signals were more varied depending on film exposure than the stimulated signals. In B and C, the cells were stimulated with thrombin (10 U/mL), a PAR1 agonist, TFLLR (B), or a PAR2 agonist, SLIGRL (C) for 20 min at the indicated concentrations, and intracellular cGMP production was determined. The data shown are mean ± SEM, (*p < 0.05 compared with the basal control, cGMP values shown are n=4, each).
To assess eNOS activation by the PAR agonists, intracellular cGMP production was measured as a marker of NO production after stimulation of BAECs by thrombin or PAR agonists. Although thrombin stimulated intracellular cGMP production (basal 3.55±0.36 pmol/well vs. thrombin 17.50±2.53 pmol/well), a PAR1 agonist, TFLLR, at concentrations from 50 to 200 μmol/L did not stimulate cGMP production (Figure 2B). In contrast, a PAR2 agonist, SLIGRL, stimulated intracellular cGMP production in a concentration-dependent manner from 2 to 50 μmol/L (Figure 2C). Also, AY-NH2, a PAR4 agonist, did not increase in cGMP production (data not shown). We confirmed that PAR2 stimulated cGMP through NO production because L-NAME treatment completely blocked PAR2-induced cGMP production (data not shown). We have previously shown that L-NAME inhibited cGMP production by thrombin 9. These data indicate a preferential role of PAR2 in eNOS activation in BAECs.
PAR Activation of Gq is Required for Phosphorylation of eNOS at Ser1179 but not Thr497
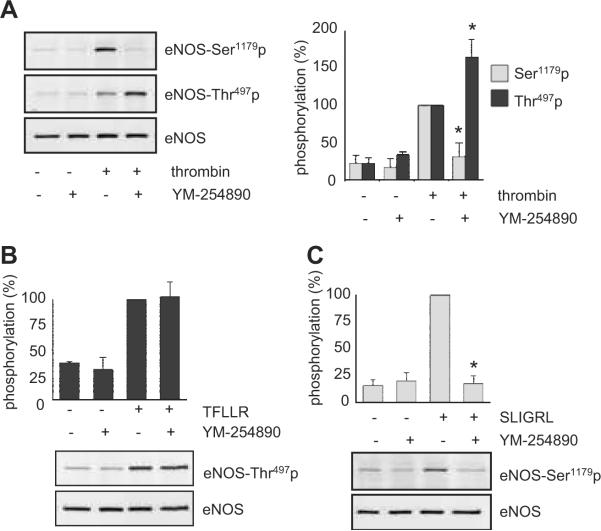
PARs are known to couple to multiple G proteins including Gq, Gi and G12/13 1. Thus, the difference in eNOS phosphorylation profile by PARs may be due to distinct G proteins coupling to PAR1 and PAR2 in endothelial cells. We have confirmed our recent observation that a selective Gq inhibitor, YM-254890 14, markedly inhibited thrombin-induced eNOS Ser1179 phosphorylation 9, whereas it enhanced the thrombin-induced phosphorylation of eNOS at Thr497 (Figure 3A). The maximum inhibition was observed with 100-1000 nmol/L (Figure 3A and Figure S1A, please see http://hyper.ahajournals.org.). However, neither pertusis toxin (100 ng/mL, 24 h), a Gi inhibitor, nor infection of adenovirus (100 moi, 48 h) encoding GRK2-ct, a Gβγ inhibitor, blocked thrombin-induced eNOS phosphorylation at Ser1179 or Thr497 (data not shown). YM-254890 also markedly inhibited PAR2 (SLIGRL)-induced eNOS Ser1179 phosphorylation, whereas it had no inhibitory effect on PAR1 (TFLLR)-induced eNOS Thr497 phosphorylation (Figure 3B and 3C). YM-254890 also inhibited PAR2-induced cGMP production (Figure S1B) as it did in BAECs stimulated with thrombin 9.
Figure 3.
Gq is required for eNOS Ser1179 phosphorylation stimulated by PAR2 in BAECs. In A-C, the cells were pretreated with or without 1 μmol/L YM-254890 for 10 min, and stimulated with or without thrombin (10 U/mL) for 1 min (A), a PAR1 agonist, TFLLR (50 μmol/L) for 2 min (B), or a PAR2 agonist, SLIGRL (50 μmol/L) for 2 min (C). eNOS phosphorylation was evaluated by immunoblot analysis. The bar graphs show densitometric analyses of the phosphorylation of eNOS at each site (mean ± SEM, *p < 0.05 compared with the stimulated control, bands scanned are n=3, each).
Gq activation by PARs leads to phospholipase C-dependent rapid and transient intracellular Ca2+ elevation 1. We have also demonstrated that thrombin-induced eNOS Ser1179 phosphorylation requires intracellular Ca2+ elevation 9. To test whether Gq coupling of PARs is distinct in BAECs, the effects of the PAR agonists on intracellular Ca2+ concentrations were examined. Both TFLLR and SLIGRL significantly elevated intracellular Ca2+ concentrations, which were completely inhibited by 1 μmol/L YM-254890 (data not shown). These data suggest that Gq coupling and subsequent intracellular Ca2+ elevation are required but not sufficient enough for eNOS Ser1179 phosphorylation as well as its activation.
G12/13 and a Y27632 Sensitive Kinase but not ROCK are Involved in eNOS Phosphorylation at Thr497 by PAR1
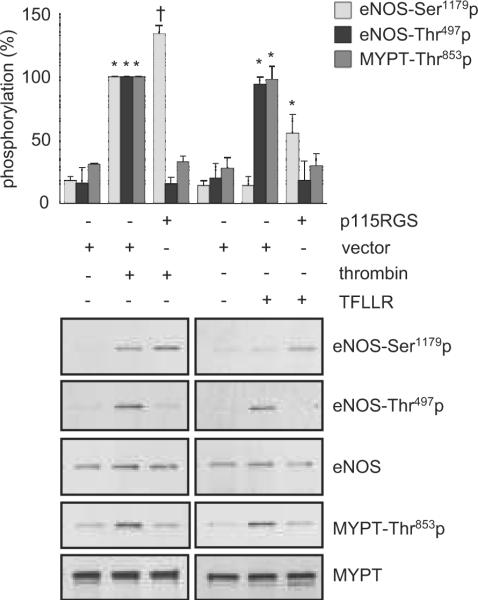
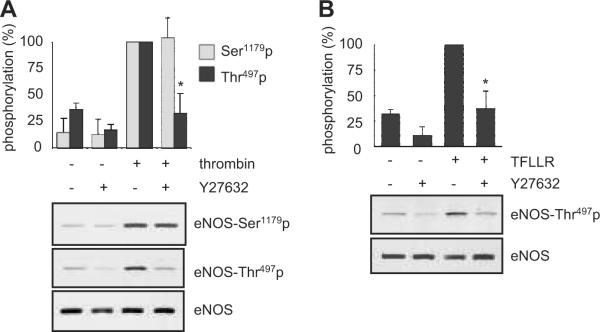
PAR1 has been shown to couple to G12/13 leading to its downstream Rho and ROCK activation in endothelial cells 15. As shown in Figure 4, a specific G12/13 inhibitor, p115RGS, markedly inhibited thrombin- or TFLLR (PAR1)-induced eNOS Thr497 phosphorylation. It also inhibited thrombin- or TFLLR-induced ROCK activation as judged by phosphorylation of a ROCK substrate, MYPT1, at Thr853. Inhibition of G12/13 leads to stimulation of Ser1179 phosphorylation by TFLLR or thrombin. p115RGS by itself had no obvious effect on either site of eNOS phosphorylation (Figure S2A). A ROCK inhibitor, Y27632, markedly inhibited thrombin- or PAR1 (TFLLR)-induced eNOS Thr497 phosphorylation, whereas this inhibitor minimally affected eNOS Ser1179 phosphorylation stimulated by thrombin (Figure 5A) or the PAR2 agonist (data not shown). Y27632 also inhibited TFLLR (PAR1)-induced eNOS Thr497 phosphorylation (Figure 5B). However, Y27632 did not modulate cGMP production induced by thrombin in BAECs (Figure S2B).
Figure 4.
Requirement of G12/13 for eNOS Thr497 phosphorylation stimulated by PAR1 in BAECs. The cells infected with adenovirus encoding p115RGS, a G12/13 inhibitor, or control vector were stimulated with thrombin (10 U/mL) for 1 min or a PAR1 agonist TFLLR (50 μmol/L) for 2 min, and phosphorylation of eNOS and a ROCK substrate, MYPT, was evaluated. The bar graphs show densitometric analyses of the phosphorylation of eNOS and MYPT (mean ± SEM, *p < 0.05 compared with the basal control, †p < 0.05 compared with the stimulated control, bands scanned are n=3, each).
Figure 5.
Requirement of a Y27632-sensitive kinase for eNOS Thr497 phosphorylation stimulated by PAR1 in BAECs. In A and B, the cells were pretreated with Y27632 (10 μmol/L) for 30 min and stimulated with thrombin (10 U/mL) for 1 min (A) or a PAR1 agonist TFLLR (50 μmol/L) for 2 min (B). Phosphorylation of eNOS was evaluated. The bar graphs show densitometric analyses of the phosphorylation of eNOS at each site (mean ± SEM, *p < 0.05 compared with the stimulated control, bands scanned are n=3, each).
The specificity of Y27632 as a ROCK inhibitor has been questioned 16. H-1152, which has a better selectivity for ROCK than Y27632 17, inhibited TFLLR-induced MYPT1 Thr853 phosphorylation but not eNOS Thr497 phosphorylation (Figure S3A). Also, dnRho markedly inhibited TFLLR-induced MYPT Thr853 phosphorylation but not eNOS Thr497 phosphorylation (Figure S3B). In addition, PKC did not contribute to the PAR1-induced eNOS Thr497 phosphorylation because it was insensitive to the PKC inhibitor GF109203X, whereas this inhibitor blocked the phorbol ester-induced eNOS Thr497 phosphorylation in BAECs (Figure S3C). Taken together, these data suggest that PAR1-induced phosphorylation of eNOS at Thr497 requires G12/13 and subsequent activation of a Y27632-sensitive eNOS Thr497 kinase that is distinct from ROCK or PKC.
Discussion
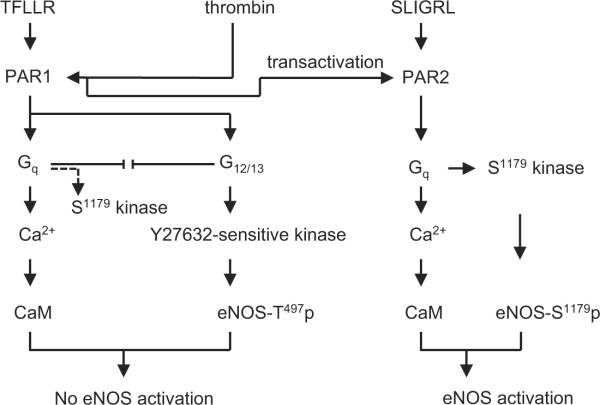
The major findings of the present study are 1) stimulation of PAR2 in BAECs results in eNOS Ser1179 phosphorylation and subsequent NO production, 2) whereas stimulation of PAR1 solely stimulates eNOS Thr497 phosphorylation and does not lead to NO production, and 3) the downstream mechanisms utilized involve Gq for Ser1179 phosphorylation by PAR2, and G12/13 and a previously un-identified Y27632-sensitive eNOS kinase for Thr497 phosphorylation by PAR1 (Figure 6). These data suggest distinct physiological and pathophysiological roles of PAR1 and PAR2 in regulating eNOS activity, representing novel mechanisms of PAR signal transduction in endothelial cells.
Figure 6.
Proposed signal transduction cascade of PAR1 and PAR2 in mediating the regulation of eNOS phosphoryation in endothelial cells. Note that upon thrombin stimulation of PAR1, there may be a competition between Gq and G12/13, which favors Gq activation upon inhibition of G12/13 and vise versa. In addition, G12/13 inhibition might unmask the PAR1 coupling to eNOS Ser1179 phosphorylation via Gq.
In line with a theory of PAR2 transactivation by PAR1 proposed in HUVECs 3, our data suggest that both PAR1 and PAR2 mediate eNOS regulation by thrombin in BAECs. It is likely that thrombin at low concentration induces Thr497 phosphorylation through its high affinity receptor, PAR1, whereas at a higher concentration, thrombin further transactivates PAR2 through the PAR1 tethered ligand leading to Ser1179 phosphorylation and subsequent eNOS activation. This is further supported by the findings with a PAR1 tethered peptide used in the present study. Although a marked difference of G protein coupling affinity of PAR1 upon stimulation with thrombin or an agonistic peptide (the peptide prefers Gq coupling more than G12/13) has been demonstrated 15, it appears not to be applicable to our findings. This is because no difference was observed between thrombin- or TFLLR- induced elevation of intracellular Ca2+ (Gq-dependent signal) or stimulation of MYPT phosphorylation (G12/13-dependent signal) in BAECs.
We have observed that the Gq inhibitor YM-254890 enhanced thrombin-induced eNOS phosphorylation at Thr497. This is most likely due to competition of PAR1 coupling between Gq and G12/13, which leads to the enhanced G12/13 signal transduction upon inhibition of Gq. The presence of such competition is further supported by the enhanced Ser1179 phosphorylation by TFLLR or thrombin when G12/13 activities were inhibited with p115RGS. Thus, G12/13 inhibition might unmask the PAR1 coupling to eNOS Ser1179 phosphorylation via Gq as illustrated in Figure 6. However, it remains unclear why the phenomenon was not observed upon TFLLR stimulation with the Gq inhibitor. It is possible that TFLLR binding to the PAR1 may not be sufficient enough to change the competition which favors G12/13 activation upon inhibition of Gq. In addition, TFLLR may not fully mimic the conformational change of the receptor induced by thrombin which causes cleavage of the receptor.
In the present study, we found that Gq is critical in PAR2-induced eNOS Ser1179 phosphorylation and subsequent enzymatic activation, as was recently shown in BAECs stimulated with thrombin or angiotensin II 8, 9. Although thrombin-induced NO production has been shown to be markedly inhibited by Ca2+ chelators 9, the Gq-mediated Ca2+/CaM-dependent eNOS activation mechanism alone may be insufficient to stimulate NO production in BAECs. In this regard, we have previously demonstrated that thrombin-induced eNOS Ser1179 phosphorylation is mediated through a non-Akt kinase acting downstream of Ca2+, and that the Ser1179 phosphorylation is functionally indispensable for NO production stimulated by thrombin 9.
We have demonstrated the specificity of the Gq inhibitor, YM-254890, at the concentrations of 1-10 μmol/L in COS7 cells and vascular smooth muscle cells 13, 14. Recently, the specificity was also verified in BAECs pretreated 30 min with 30 nmol/L of YM-254890. This concentration appeared to be sufficient to inhibit intracellular Ca2+ elevation by bradykinin. It also inhibited NO production induced by thrombin but not by ionomycin in BAECs 18. However, the concentrations greater than 50 nmol/L were required for the inhibition of eNOS Ser1179 phosphorylation by thrombin (Figure S1A) or ERK phosphorylation by angiotensin II 14, which is most likely due to the shorter treatment timw of 10 min.
Thors et al. proposed that Ser1179 phosphorylation of eNOS stimulated by thrombin is mediated through the Ca2+-dependent activation of an eNOS Ser1179-kinase, AMPK, by using a nonselective AMPK inhibitor in HUVECs 19. However, adenovirus transduction of dnAMPK did not prevent thrombin-induced eNOS Ser1179 phosphorylation in HUVECs 20. In addition, the species differences in eNOS regulation by PARs 18 and the involvement of thrombomodulin in thrombin-induced eNOS phosphorylation have been reported 21. Therefore, further investigation is needed to identify the Ser1179 kinase as well as its exact upstream signal transduction utilized by PAR2.
Little was known about the regulation of eNOS Thr497 by GPCRs. We found that a Rho-kinase/ROCK inhibitor, Y27632, inhibited PAR1-induced eNOS Thr497 phosphorylation. Rho has been reported to inhibit NO production in arteries 22 and to inhibit eNOS activation through inhibition of Akt in endothelial cells 23. In this regard, a recent study proposed ROCK as the eNOS Thr497 kinase activated by thrombin 24. Although this study demonstrated that ROCK was able to phosphorylate eNOS in vitro, only Y27632 was used to block the phosphorylation in vivo. Our data using dnRho and a more selective ROCK inhibitor rather suggest the presence of a Y27632-sensitive novel eNOS Thr497 kinase distinct from the Rho/ROCK.
A negative regulatory role of the eNOS Thr497 phosphorylation has been reported 25, however, we could not observe enhancement of cGMP production upon inhibition of the phosphorylation with Y27632 in the present study. A mutational experiment of eNOS Thr497 to mimic constitutive phosphorylation (Asp495 in human eNOS) with in vitro measurement of the enzymatic activity suggests that eNOS Thr497 phosphorylation reduces Ca2+ sensitivity of the enzyme 25. However, expression and stimulation of an eNOS Thr497 phosphorylation mutant to mimic de-phosphorylation (Ala497) expressed in COS7 cells did not enhance NO production over that of wild type, whereas a mutation in Asp497 inhibited NO production 26. Taken together with our Ca2+ stimulation data by PAR agonists, it is likely that eNOS phosphorylation at Thr497 blocks eNOS activity against Ca2+/CaM but is insufficient to block the activity with a concurrent phosphorylation of Ser1179.
Although we have observed similar regulation of eNOS by PARs in BAECs and HUVECs in the present study, the expression ratio of PARs may be different in endothelial cells from distinct species and/or vascular beds as may be that of G proteins as well. Moreover, expression of PARs in endothelial cells is under the regulation of distinct extracellular conditions such as inflammation 27. PAR1 mRNA was upregulated in rat aorta associated with angiotensin II-induced hypertension 28. However, exact roles of PARs in vascular tonus regulation still remain unclear. Therefore, additional experiments in various in vivo settings will be necessary to better generalize our findings in certain vascular pathophysiology such as in hypertension.
Perspectives
Selective manipulation of Gq or G12/13 in vascular smooth muscle cells revealed the importance of these G proteins in the etiology of high blood pressure 29. The close link between endothelial G13 and PAR1 has been demonstrated 30. Moreover, positive regulation of eNOS expression by G12 has been reported 31. Further detailed research specifically on eNOS phosphorylation regulation by PARs will shed light on critical mechanisms by which multiple GPCRs expressed in the endothelium potentially regulate endothelial dysfunction associated with cardiovascular diseases.
Acknowledgments
We thank Kyoko Hinoki for her technical assistance.
Sources of Funding: This work was supported by National Institute of Health Grant, HL076770 (S.E.), by American Heart Association Established Investigator Award, 0740042N (S.E.), and by W. W. Smith Charitable Trust Grant, H0605 (S.E.).
Footnotes
Disclosures: None.
References
- 1.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 2.Minami T, Sugiyama A, Wu SQ, Abid R, Kodama T, Aird WC. Thrombin and phenotypic modulation of the endothelium. Arterioscler Thromb Vasc Biol. 2004;24:41–53. doi: 10.1161/01.ATV.0000099880.09014.7D. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien PJ, Prevost N, Molino M, Hollinger MK, Woolkalis MJ, Woulfe DS, Brass LF. Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J Biol Chem. 2000;275:13502–13509. doi: 10.1074/jbc.275.18.13502. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton JR, Moffatt JD, Frauman AG, Cocks TM. Protease-activated receptor (PAR) 1 but not PAR2 or PAR4 mediates endothelium-dependent relaxation to thrombin and trypsin in human pulmonary arteries. J Cardiovasc Pharmacol. 2001;38:108–119. doi: 10.1097/00005344-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Robin J, Kharbanda R, McLean P, Campbell R, Vallance P. Protease-activated receptor 2-mediated vasodilatation in humans in vivo: role of nitric oxide and prostanoids. Circulation. 2003;107:954–959. doi: 10.1161/01.cir.0000050620.37260.75. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton JR, Frauman AG, Cocks TM. Increased expression of protease-activated receptor-2 (PAR2) and PAR4 in human coronary artery by inflammatory stimuli unveils endothelium-dependent relaxations to PAR2 and PAR4 agonists. Circ Res. 2001;89:92–98. doi: 10.1161/hh1301.092661. [DOI] [PubMed] [Google Scholar]
- 7.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Eguchi K, Ohtsu H, Higuchi S, Dhobale S, Frank GD, Motley ED, Eguchi S. Activation of endothelial nitric oxide synthase by the angiotensin II type 1 receptor. Endocrinology. 2006;147:5914–5920. doi: 10.1210/en.2006-0834. [DOI] [PubMed] [Google Scholar]
- 9.Motley ED, Eguchi K, Patterson MM, Palmer PD, Suzuki H, Eguchi S. Mechanism of endothelial nitric oxide synthase phosphorylation and activation by thrombin. Hypertension. 2007;49:577–583. doi: 10.1161/01.HYP.0000255954.80025.34. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, Nakashima H, Woolfolk EA, Motley ED, Eguchi S. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1831–1836. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
- 11.Stemmle LN, Fields TA, Casey PJ. The regulator of G protein signaling domain of axin selectively interacts with Galpha12 but not Galpha13. Mol Pharmacol. 2006;70:1461–1468. doi: 10.1124/mol.106.023705. [DOI] [PubMed] [Google Scholar]
- 12.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calciumdependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 13.Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, Frank GD, Inagami T, Higashiyama S, Thomas WG, Eckhart AD, Dempsey PJ, Eguchi S. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J Biol Chem. 2005;280:26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsu H, Higuchi S, Shirai H, Eguchi K, Suzuki H, Hinoki A, Brailoiu E, Eckhart AD, Frank GD, Eguchi S. Central role of Gq in the hypertrophic signal transduction of angiotensin II in vascular smooth muscle cells. Endocrinology. 2008;149:3569–3575. doi: 10.1210/en.2007-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE. Functional Selectivity of G Protein Signaling by Agonist Peptides and Thrombin for the Protease-activated Receptor-1. J Biol Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 16.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikenoya M, Hidaka H, Hosoya T, Suzuki M, Yamamoto N, Sasaki Y. Inhibition of rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J Neurochem. 2002;81:9–16. doi: 10.1046/j.1471-4159.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 18.Hirano K, Nomoto N, Hirano M, Momota F, Hanada A, Kanaide H. Distinct Ca2+ requirement for NO production between proteinase-activated receptor 1 and 4 (PAR1 and PAR4) in vascular endothelial cells. J Pharmacol Exp Ther. 2007;322:668–677. doi: 10.1124/jpet.107.121038. [DOI] [PubMed] [Google Scholar]
- 19.Thors B, Halldorsson H, Thorgeirsson G. Thrombin and histamine stimulate endothelial nitric-oxide synthase phosphorylation at Ser1177 via an AMPK mediated pathway independent of PI3K-Akt. FEBS Lett. 2004;573:175–180. doi: 10.1016/j.febslet.2004.07.078. [DOI] [PubMed] [Google Scholar]
- 20.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulindependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933–5945. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David-Dufilho M, Millanvoye-Van Brussel E, Topal G, Walch L, Brunet A, Rendu F. Endothelial thrombomodulin induces Ca2+ signals and nitric oxide synthesis through epidermal growth factor receptor kinase and calmodulin kinase II. J Biol Chem. 2005;280:35999–36006. doi: 10.1074/jbc.M506374200. [DOI] [PubMed] [Google Scholar]
- 22.Shiga N, Hirano K, Hirano M, Nishimura J, Nawata H, Kanaide H. Long-term inhibition of RhoA attenuates vascular contractility by enhancing endothelial NO production in an intact rabbit mesenteric artery. Circ Res. 2005;96:1014–1021. doi: 10.1161/01.RES.0000165483.34603.91. [DOI] [PubMed] [Google Scholar]
- 23.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimoto M, Nakayama M, Goto TM, Amano M, Komori K, Kaibuchi K. Rho-kinase phosphorylates eNOS at threonine 495 in endothelial cells. Biochem Biophys Res Commun. 2007;361:462–467. doi: 10.1016/j.bbrc.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 26.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr., Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem. 2003;278:44719–44726. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 27.Hirano K, Kanaide H. Role of protease-activated receptors in the vascular system. J Atheroscler Thromb. 2003;10:211–225. doi: 10.5551/jat.10.211. [DOI] [PubMed] [Google Scholar]
- 28.Capers Qt, Laursen JB, Fukui T, Rajagopalan S, Mori I, Lou P, Freeman BA, Berrington WR, Griendling KK, Harrison DG, Runge MS, Alexander RW, Taylor WR. Vascular thrombin receptor regulation in hypertensive rats. Circ Res. 1997;80:838–844. doi: 10.1161/01.res.80.6.838. [DOI] [PubMed] [Google Scholar]
- 29.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 30.Ruppel KM, Willison D, Kataoka H, Wang A, Zheng YW, Cornelissen I, Yin L, Xu SM, Coughlin SR. Essential role for Galpha13 in endothelial cells during embryonic development. Proc Natl Acad Sci U S A. 2005;102:8281–8286. doi: 10.1073/pnas.0503326102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreeva AV, Vaiskunaite R, Kutuzov MA, Profirovic J, Skidgel RA, Voyno-Yasenetskaya T. Novel mechanisms of G protein-dependent regulation of endothelial nitric-oxide synthase. Mol Pharmacol. 2006;69:975–982. doi: 10.1124/mol.105.018846. [DOI] [PubMed] [Google Scholar]