Abstract
The purpose of this study was to evaluate in an animal model the safety and efficacy of dietary supplementation with high doses of selenium for the mitigation of the type of radiation injury that might be sustained during a nuclear accident or an act of radiological terrorism. Age-matched male rats were exposed to 10 Gy (single dose) of total-body irradiation (TBI) followed by a syngeneic bone marrow transplant, then randomized to standard drinking water or drinking water supplemented with sodium selenite or seleno-L-methionine. At 21 weeks after TBI, most rats on standard drinking water had severe renal failure with a mean blood urea nitrogen (BUN) level of 124 ± 29 mg/dl (geometric mean ± SE) whereas rats on selenium-supplemented drinking water (100 μg/day) had a mean BUN level of 67 ± 12 mg/dl. The mitigating effect of selenium was confirmed by histopathological analyses. None of the animals on high-dose selenium showed signs of selenium toxicity. Our results suggest that dietary supplementation with high-dose selenium may provide a safe, effective and practical way to mitigate radiation injury to kidneys.
INTRODUCTION
Medical countermeasures for acts of radiological terrorism or nuclear accidents require drugs that have a good safety profile, are easy to administer, do not degrade the performance of first responders, are stable under a broad range of storage and shipment conditions, and are effective when given after radiation exposure. We report here on a pilot study in a rat model that evaluated high-dose oral selenium as a means to mitigate radiation injury to the kidneys. In the context of this paper, the terms (1) prevention, (2) mitigation and (3) treatment refer to interventions that are initiated (1) before or during radiation exposure, (2) after radiation exposure but before the onset of symptoms, and (3) after the onset of symptoms, respectively (1).
METHODS AND MATERIALS
Animal Model
WAG/Rij/MCW rats were bred and housed in a moderate-security barrier inside the central animal facility of the Medical College of Wisconsin. The facility is fully accredited by the American Association for the Accreditation of Laboratory Animal Care, and all animal experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee. Animals were free of Mycoplasma pulmonis, Pseudomonas and common rat viruses. Rat chow and drinking water were provided ad libitum.
Male rats (7–9 weeks old) underwent 10 Gy of total-body irradiation (TBI) delivered by a Pantak orthovoltage X-ray machine as a single dose at a dose rate of 1.95 Gy/min. The X-ray machine was operated at 300 kVp with a half-value layer of 1.4 mm copper, and the dose was defined at midline. For dosimetry details, see Cohen et al. (2). The LD50 for WAG/Rij/MCW rats is 6.3 Gy (at 30 days) without a bone marrow transplant and 13.4 Gy (at 7 days) with a bone marrow transplant (3). Therefore, TBI was followed by a syngeneic bone marrow transplant (approximately 6 × 107 nucleated cells per graft) to rescue the rats from otherwise lethal damage to the hematopoietic system. Two to 4 h after TBI, rats were randomized to standard drinking water or selenium-supplemented drinking water.
Selenium Supplements
Sodium selenite and seleno-L-methionine were purchased from Sigma (St. Louis, MO). Supplements were dissolved in distilled water and sterilized by filtration through 0.2-μm filters. Dose calculations were based on the assumption that the average fluid intake was 20 ml per day. To facilitate comparisons between different selenium species, we express all doses as amounts of atomic selenium. Thus, when the target dose was 100 μg of (atomic) selenium per day, the concentrations of sodium selenite (formula weight: 174.94) and seleno-L-methionine (formula weight: 196.11) in supplemented drinking water were 10.95 μg/ml and 12.40 μg/ml, respectively. Rats remained on the same supplement (either sodium selenite or seleno-L-methionine) for the duration of the experiment.
Histopathological Analyses
Approximately 21 weeks after TBI, kidneys were harvested from irradiated rats and age-matched controls, fixed in zinc-formalin, and embedded in paraffin. Four-micrometer sections were prepared and stained with hematoxylin and eosin. Sections were examined with a light microscope at total magnifications of 200× and 400× to obtain a qualitative overall assessment of radiation injury as well as a quantitative assessment of four specific histological changes. The quantitative scoring of histopathological changes was done in a masked fashion by one of the coauthors (EPC) as described previously (4). In brief, each specimen was assigned a total numerical score between 0 and 10 based on the cumulative scores of four types of histopathological changes according to the following scheme (numerical scores in parentheses).
Blood Urea Nitrogen (BUN)
Blood was obtained from the retro-orbital venous plexus under fluran anesthesia and assayed for BUN levels by the enzymatic colorimetric Bertholet method using a commercial assay kit (Sterling Diagnostics, Sterling Heights, MI) according to the manufacturer’s instructions. Data were derived from multiple experiments. For logistical reasons it was not possible to perform all blood draws at exactly the same times. Therefore, the three time windows during which blood draws were performed were 61 to 68 days (arithmetic mean: 63 days), 102 to 110 days (mean: 104 days), and 137 to 145 days (mean: 142 days), respectively. The mean number of days after TBI was used for graphic representations of data.
RESULTS
Acceptance
Rats readily accepted drinking water containing up to 5 μg of selenium (as sodium selenite or seleno-L-methionine) per ml (intended to deliver 100 μg/day). When the concentrations of sodium selenite and seleno-L-methionine were doubled with the intent to deliver a daily dose of 200 μg, the supplemented drinking water was consumed at a reduced rate, and rats had to be returned to the lower dose (100 μg/day) after 2 days to prevent dehydration.
Kidney Function
BUN levels in nonirradiated control rats remained steady (21 ± 1 mg/dl; geometric mean ± SE) during the 21-week observation period (Fig. 1). Irradiated rats on standard drinking water showed moderately elevated BUN levels at the first time (61–68 days after TBI) (Fig. 1). At the third time (137–145 days after TBI), most irradiated rats on standard drinking water had severe renal failure with BUN values in excess of 120 mg/dl or were approaching severe renal failure (Fig. 1). Two irradiated rats maintained near-normal BUN levels for 21 weeks after TBI. Past experience with this animal model predicts that the latter rats would also have developed renal failure if observation times had been extended beyond 21 weeks.
FIG. 1.
Blood urea nitrogen (BUN) levels in normal age-matched control rats on standard drinking water, irradiated (10 Gy TBI) rats on standard drinking water, and irradiated (10 Gy TBI) rats on selenium-supplemented (100 μg/day) drinking water. Data are geometric means ± SE and are expressed as geometric rather than arithmetic means because physiological parameters in groups showing abnormal kidney function are log-normally distributed (5). Selenium supplementation (100 μg/day) in the form of sodium selenite or seleno-L-methionine reduced terminal BUN levels in irradiated rats from 123 ± 25 mg/dl to 67 ± 12 mg/dl with sodium selenite- and seleno-L-methionine-treated subgroups showing similar reductions of BUN levels (65 ± 14 mg/dl and 70 ± 21 mg/dl, respectively). All differences between the three main groups (normal controls, irradiated rats on standard drinking water, and irradiated rats on selenium-supplemented drinking water) were statistically significant at the 5% level (Games-Howell ANOVA post hoc test). Sample sizes were n = 16 for normal controls (all times), n = 15 for irradiated animals on standard drinking water (all times), and n = 12 (first time) and n = 11 (second and third times) for irradiated animals on selenium-supplemented drinking water. Of the animals on supplemented drinking water, three were on 100 μg/day sodium selenite, three on 200 μg/day sodium selenite for 2 days followed by 100 μg/day sodium selenite, three (first time) or two (second and third times) on 100 μg/day seleno-L-methionine, and three on 200 μg/day seleno-L-methionine for 2 days followed by 100 μg/day seleno-L-methionine.
Supplementation with 100 μg/day of selenium in the form of sodium selenite or seleno-L-methionine reduced terminal BUN levels from 123 ± 25 mg/dl to 67 ± 12 mg/dl (significant at 5% level, Games-Howell ANOVA post hoc test) (Fig. 1). BUN levels in rats treated with sodium selenite (65 ± 14 mg/dl) were slightly lower than in rats treated with seleno-L-methionine (70 ± 21 mg/dl), but the difference was not significant (Fig. 1). At the first time (61–68 days), BUN levels in irradiated animals on standard drinking water and irradiated animals on selenium-supplemented drinking water were virtually identical (Fig. 1). At the second time (102–110 days), BUN levels were lower in animals on selenium-supplemented drinking water, but the difference was not statistically significant. The reason for the slow response to selenium supplementation is not known. One possible explanation is that the buildup of selenium levels in tissues in response to dietary supplementation was a slow process, especially during the days immediately after TBI when the gastrointestinal tract had not yet recovered from radiation injury. Supplementation with 50 μg/day failed to reduce terminal BUN levels (130 ± 19 mg/dl) (data not shown), suggesting that a rather high threshold dose of selenium was required to mitigate radiation injury to kidneys.
Since sample sizes were small (see the legend to Fig. 1 for detailed information on sample sizes), our statistical analysis treated all rats receiving selenium supplementation at 100 μg/day as a single group regardless of the selenium species used. Rats that were intended to get 200 μg/day were returned to the lower dose of 100 μg/day after 2 days because of reduced fluid intake; they were also included in the analysis (Figs. 1, 3 and 4) because the reduced volume consumed during the first 2 days was equivalent to about 100 μg/day.
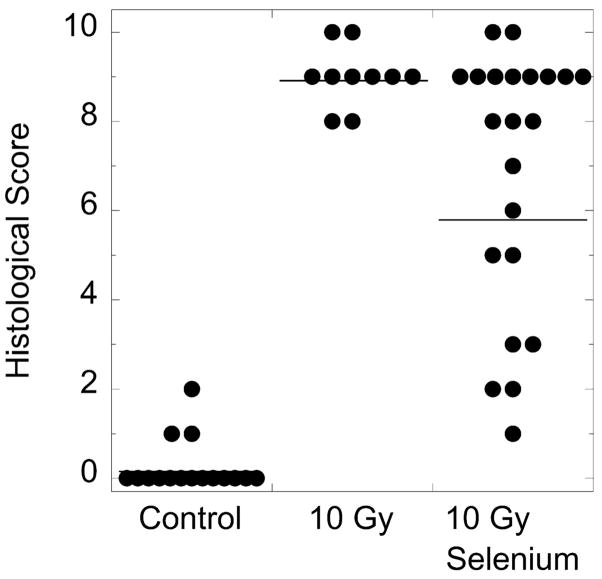
FIG. 3.
Distribution of histological scores for kidneys from age-matched nonirradiated control rats, irradiated (10 Gy TBI) rats on standard drinking water, and irradiated (10 Gy TBI) rats on selenium-supplemented drinking water. Selenium supplementation (100 μg/day as sodium selenite or seleno-L-methionine) reduced the mean score from 8.98 ± 0.21 (geometric mean ± SE) to 5.88 ± 0.62. Horizontal bars indicate geometric means. All differences between groups were statistically significant at the 5% level (Games-Howell ANOVA post hoc test). The mean histology scores of kidneys from seleno-L-methionine- and sodium selenite-treated subgroups were similar, 5.02 ± 1.06 and 6.88 ± 0.69, respectively.
FIG. 4.
Correlation between blood urea nitrogen (BUN) values and histological scores. Solid squares and solid triangles show data derived from animals that were intended to receive 200 μg of selenium per day but had to be returned to the lower dose of 100 μg/day after 2 days because of reduced fluid intake. Numbers in parentheses indicate sample sizes (number of evaluated slides). Two randomly selected slides were evaluated per animal. Coefficient of correlation (Spearman): r = 0.9.
Kidney Histology
Histopathological analyses of irradiated rats on standard drinking water performed at 21 weeks after TBI showed bilateral glomerular degeneration, loss of capillaries and early sclerosis, mild to moderate interstitial inflammation, focal collections of hemosiderin-containing macrophages, and epithelial vacuolization and increased mitotic activity with focal dilation and thyroidization in tubules. The term “thyroidization” describes dilated tubules that are filled with pink hyaline casts, which gives end-stage kidneys the microscopic appearance of thyroid tissue.
In rats on selenium-supplemented drinking water, radiation-induced histological abnormalities were reduced or absent. For example, in the representative set shown in Fig. 2, the kidney from the irradiated rat on standard drinking water showed prominent glomerular mesangiolysis and interstitial fibrosis, whereas the kidney from the irradiated rat on seleno-L-methionine-supplemented drinking water showed no interstitial fibrosis and only minimal mesangiolysis.
FIG. 2.
Representative examples of hematoxylin and eosin-stained sections of kidneys from an age-matched nonirradiated control rat on standard drinking water (top row), an irradiated (10 Gy TBI) rat on standard drinking water (middle row), and an irradiated (10 Gy TBI) rat on seleno-L-methionine-supplemented drinking water (bottom row). Magnification: 200× (left column) and 400× (right column). Corresponding terminal blood urea nitrogen (BUN) values and histological scores are shown for each set.
The mitigating effect of high-dose dietary selenium on radiation injury to kidneys was confirmed quantitatively (4) (Figs. 2 and 3). While kidneys from irradiated rats on standard drinking water had a mean histology score of 8.98 ± 0.21 (geometric mean ± SE), kidneys from irradiated rats on selenium-supplemented drinking water had a mean score of 5.88 ± 0.62. The difference was significant at the 5% level (Games-Howell ANOVA post hoc test). Scores of kidneys from rats on seleno-L-methionine (5.02 ± 1.06) and rats on sodium selenite (6.88 ± 0.69) were not significantly different (Fig. 3). Numerical histology scores correlated well with BUN values (r = 0.9, Spearman) (Fig. 4).
As expected, BUN values painted a more optimistic picture of the mitigating effect of selenium supplementation than the numerical histology scores. In radiation-damaged kidneys, the less-damaged nephrons probably compensated for the reduced performance of the more severely damaged nephrons. Furthermore, it should be noted that unlike BUN values, histology scores are not always able to distinguish between different degrees of major radiation injury. For example, if the kidney from an irradiated rat on standard drinking water shows 10 sclerosed glomeruli per 20 glomeruli examined, the kidney is assigned 3 points, the maximum score for this particular histological abnormality. If the kidney from an irradiated rat on selenium-supplemented drinking water shows only half as many (5) sclerosed glomeruli, a change that would be immediately obvious to the pathologist making a global assessment of the section, the kidney would still be assigned a numerical score of 3, because only a reduction to ≤4 sclerosed glomeruli would result in a lower score. In other words, when dealing with different degrees of major radiation injury, the numerical scoring system may underestimate mitigating effects.
Toxicity
None of the animals on high-dose selenium showed gross evidence of selenium toxicity such as hair loss, skin lesions, brittle claws, diarrhea, weight loss or neurological symptoms such as hind limb paresis. Furthermore, histopathological examinations of liver, kidney, heart and lung did not find any abnormalities indicative of selenium toxicity (e.g. liver necrosis, Kupffer cell proliferation, vacuolar degeneration of hepatocytes, pericarditis, myocarditis, or tubular necrosis of the kidney).
DISCUSSION
Selenium is an essential trace mineral. Its best understood physiological function is its role as an integral part of selenoproteins (6). Selenoproteins play key roles in antioxidant defense mechanisms (7). However, there is growing evidence that selenium also has biological effects that are not mediated by selenoproteins (8–13). Among the latter are processes that prevent DNA damage (9, 10), promote DNA repair (11, 12), or suppress apoptosis through inhibition of ASK1/JNK and activation of P13-K/Akt pathways (13). The role of selenium as a radioprotective agent is well established (14, 15). More recently, selenium has also been used successfully to treat radiation-induced lymphedema and radiochemotherapy-induced mucositis (16–18). A rat study designed to simulate a Chernobyl-like exposure to both internal and external radiation showed that dietary supplementation with selenium initiated after radiation exposure reduced the incidence of radiation-induced tumors (19).
Our pilot study shows that dietary supplementation with high-dose selenium initiated shortly after radiation exposure significantly mitigates radiation injury to kidneys. The dose of supplemental selenium required to mitigate radiation injury was high in comparison to the typical dietary intake of rats (1–4 μg/day) but was well tolerated. Our experiments were routinely terminated 21 weeks after TBI, but there is no reason to suspect that supplementation for longer periods would not be feasible. In fact, Knizhnikov et al. (19) maintained selenium supplementation at daily doses of up to 100 μg for the natural life span of the animals without noticing any adverse effects. On the contrary, animals on high-selenium diets lived significantly longer than animals on the standard diet. It is also possible that future experiments will show that extensions of selenium supplementation beyond 21 weeks are neither necessary nor useful, and that adequate and lasting mitigation of radiation injury can be achieved with supplementation lasting less than 21 weeks.
For a rat, a selenium dose of 100 μg/day is higher than what is thought to be necessary to saturate selenoproteins. Both the high dose requirement and the pronounced threshold effect argue that the observed mitigation of radiation injury probably involved mechanisms that went beyond the induction of selenoproteins.
This study focused on radiation injury to the kidney because an initial survey of radiation injury to kidney, lung, heart, liver, large intestine and small intestine had shown that under the chosen experimental conditions, the kidney was the most severely damaged organ yet was the most responsive to intervention with high-dose selenium. Histopathological examinations of liver and heart showed only mild to moderate levels of radiation injury, which made it difficult to demonstrate and quantify a mitigating effect of selenium. Large and small intestines showed no histopathological evidence of radiation injury at 21 weeks after TBI, presumably because of extensive replacement of damaged cells. Lungs showed histopathological evidence of significant radiation injury (extensive perivascular edema, focal zones of intra-alveolar edema, and scattered large granulated mast cells in edematous stroma surrounding vessels), yet based on histopathological criteria, selenium supplementation at 100 μg/day had only a modest mitigating effect. However, unlike radiation injury to kidneys, which resulted invariably in severe renal failure, radiation damage to lungs did not cause clinical symptoms under the chosen experimental conditions. Therefore, the lack of a more pronounced mitigating effect on radiation injury to lungs had no major adverse effect on health or survival.
Tissues are known to compete hierarchically for dietary selenium, and selenoproteins are known to be differentially expressed in tissues (7, 20). Kidneys compete very effectively for dietary selenium, which could explain why radiation injury in kidneys was more amenable to mitigation by selenium than radiation injury in lungs. Further dose escalations may be sufficient to significantly improve the mitigating effect of selenium on radiation injury to lungs and other tissues. Based on ongoing pilot experiments, rats can be trained to overcome their aversion to the taste of concentrated selenium solutions, and doses of up to 500 μg/day appear feasible at least for certain selenium species.
Radiation nephropathy similar to that seen in our rat model of radiation injury is a frequent and serious complication in recipients of hematopoietic stem cell grafts who receive TBI as part of the conditioning regimen (21). Between 10 and 20% of bone marrow transplant patients develop chronic renal failure, and their prognosis is worse than the prognosis of other patients with end-stage renal disease (22). Selenium-based interventions may thus have clinical applications that go beyond the mitigation of radiation injury sustained during nuclear accidents or acts of radiological terrorism.
Acknowledgments
We thank Lynn M. Gruman and Vikram Gopal for technical assistance. This project was supported by a pilot grant from the Center for Medical Countermeasures Against Radiological Terrorism (U19-AI067734) and the MACC Fund.
References
- 1.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Zaharevitz D. Models for evaluating agents intended for the pro-phylaxis, mitigation and treatment of radiation injuries. Report of an NCI workshop, December 3–4, 2003. Radiat Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 2.Cohen EP, Fish BL, Sharma M, Li XA, Moulder JE. The role of the angiotensin II type-2 receptor in radiation nephropathy. Transl Res. 2007;150:106–115. doi: 10.1016/j.trsl.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moulder JE, Fish BL. Late toxicity of total body irradiation with bone marrow transplantation in a rat model. Int J Radiat Oncol Biol Phys. 1989;16:1501–1509. doi: 10.1016/0360-3016(89)90955-3. [DOI] [PubMed] [Google Scholar]
- 4.Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ACE inhibitors. Int J Radiat Oncol Biol Phys. 1993;27:93–99. doi: 10.1016/0360-3016(93)90425-u. [DOI] [PubMed] [Google Scholar]
- 5.Cohen EP, Fish BL, Moulder JE. The renin-angiotensin system in experimental radiation nephropathy. J Lab Clin Med. 2002;139:251–257. doi: 10.1067/mlc.2002.122279. [DOI] [PubMed] [Google Scholar]
- 6.Flohe L, Gunzler WA, Schock HH. Glutathione peroxidase: a selenoenzyme. FEBS Lett. 1973;32:132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- 7.Hatfield DL, Berry MJ, Gladyshev VN, editors. Its Molecular Biology and Role in Human Health. Springer; New York: 2006. Selenium. [Google Scholar]
- 8.Traynor NJ, McKenzie RC, Beckett GJ, Gibbs NL. Selenomethionine inhibits ultraviolet radiation-induced p53 transactivation. Photodermatol Photoimmunol Photomed. 2006;22:297–303. doi: 10.1111/j.1600-0781.2006.00256.x. [DOI] [PubMed] [Google Scholar]
- 9.Kowalska E, Narod SA, Huzarski T, Zajaczek S, Huzarska J, Gorski B, Lubinski J. Increased rates of chromosome breakage in BRCA1 carriers are normalized by oral selenium supplementation. Cancer Epidemiol Biomarkers Prev. 2005;14:1302–1306. doi: 10.1158/1055-9965.EPI-03-0448. [DOI] [PubMed] [Google Scholar]
- 10.Fischer JL, Lancia JK, Mathur A, Smith ML. Selenium protection from DNA damage involves a Rfl1/p53/Brca1 protein complex. Anticancer Res. 2006;26:899–904. [PubMed] [Google Scholar]
- 11.Blessing H, Kraus S, Heindl P, Bal W, Hartwig A. Interaction of selenium compounds with zinc finger proteins involved in DNA repair. Eur J Biochem. 2004;271:3190–3199. doi: 10.1111/j.1432-1033.2004.04251.x. [DOI] [PubMed] [Google Scholar]
- 12.Fischer JL, Mihelc EM, Pollok KE, Smith ML. Chemo-therapeutic selectivity conferred by selenium: a role for p53-dependent DNA repair. Mol Cancer Ther. 2007;6:355–361. doi: 10.1158/1535-7163.MCT-06-0472. [DOI] [PubMed] [Google Scholar]
- 13.Yoon SO, Kim MM, Park SJ, Kim D, Chung J, Chung AS. Selenite suppresses hydrogen peroxide-induced cell apoptosis through inhibition of ASK1/JNK and activation of P13-K/Akt pathways. FASEB J. 2002;16:111–113. doi: 10.1096/fj.01-0398fje. [DOI] [PubMed] [Google Scholar]
- 14.Weiss JF, Srinivasan V, Kumar KS, Landauer MR. Radio-protection by metals: selenium. Adv Space Res. 1992;12:223–231. doi: 10.1016/0273-1177(92)90112-b. [DOI] [PubMed] [Google Scholar]
- 15.Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann NY Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 16.Büntzel J, Weinaug R, Glatzel M, Fröhlich D, Micke O, Schüller P, Küttner K. Sodium selenite in the treatment of interstitial post-irradiation edema of the head and neck area. Trace Elem Electrolytes. 2002;191:33–37. [Google Scholar]
- 17.Micke O, Bruns F, Mücke R, Schafer U, Glatzel M, DeVries AF, Schonekaes K, Kisters K, Büntzel J. Selenium in the treatment of radiation-associated secondary lymphedema. Int J Radiat Oncol Biol Phys. 2003;56:40–49. doi: 10.1016/s0360-3016(02)04390-0. [DOI] [PubMed] [Google Scholar]
- 18.Fraunholz J, Jüling-Phlit L, Böttcher H. Influence of selenium on acute mucositis in radiochemotherapy of head and neck tumors. Trace Elem Electrolytes. 2001;18:98–99. [Google Scholar]
- 19.Knizhnikov VA, Shandala NK, Komleva VA, Knyashev VA, Tutelyan VA. The effect of dietary levels of selenium on radiation resistance and radiation-induced carcinogenesis. Nutr Res. 1996;16:505–516. [Google Scholar]
- 20.Yeh JY, Ou BR, Gu QP, Whanger PD. Influence of gender on selenoprotein W, glutathione peroxidase and selenium in tissues of rats. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:151–155. doi: 10.1016/s0305-0491(97)00298-8. [DOI] [PubMed] [Google Scholar]
- 21.Cohen EP, Drobyski WR, Moulder JE. Significant increase in end-stage renal disease after hematopoietic stem cell transplantation. Bone Marrow Transpl. 2007;39:571–572. doi: 10.1038/sj.bmt.1705643. [DOI] [PubMed] [Google Scholar]
- 22.Cohen EP, Piering WF, Kabler-Babbitt C, Moulder JE. End-stage renal disease (ESRD) after bone marrow transplantation: Poor survival compared to other causes of ESRD. Nephron. 1998;78:408–412. doi: 10.1159/000045085. [DOI] [PubMed] [Google Scholar]