Abstract
Conjugation of drugs with antibodies to surface endothelial antigens is a potential strategy for drug delivery to endothelium. We studied antibodies to platelet-endothelial adhesion molecule 1 (PECAM-1, a stably expressed endothelial antigen) as carriers for vascular immunotargeting. Although 125I-labeled anti-PECAM bound to endothelial cells in culture, the antibody was poorly internalized by the cells and accumulated poorly after intravenous administration in mice and rats. However, conjugation of biotinylated anti-PECAM (b-anti-PECAM) with streptavidin (SA) markedly stimulated uptake and internalization of anti-PECAM by endothelial cells and by cells expressing PECAM. In addition, conjugation with streptavidin markedly stimulated uptake of 125I-labeled b-anti-PECAM in perfused rat lungs and in the lungs of intact animals after either intravenous or intraarterial injection. The antioxidant enzyme catalase conjugated with b-anti-PECAM/SA bound to endothelial cells in culture, entered the cells, escaped intracellular degradation, and protected the cells against H2O2-induced injury. Anti-PECAM/SA/125I-catalase accumulated in the lungs after intravenous injection or in the perfused rat lungs and protected these lungs against H2O2-induced injury. Thus, modification of a poor carrier antibody with biotin and SA provides an approach for facilitation of antibody-mediated drug targeting. Anti-PECAM/SA is a promising candidate for vascular immunotargeting of bioactive drugs.
Keywords: lung, bioconjugation, catalase, PECAM-1
Conjugation of drugs with monoclonal antibodies is a potential strategy for site-specific therapy (immunotargeting). Vascular immunotargeting has been proposed using antibodies to endothelial antigens, such as angiotensin-converting enzyme (ACE) (1), thrombomodulin (2), tumor endothelial antigens (3), E-selectin (4), and intercellular adhesion molecule-1 (ICAM-1)(5). Because the lungs contain ≈30% of endothelial cells in the body and receive the entire cardiac blood output, these antibodies accumulate in the lungs, providing targeting to the pulmonary endothelium (1, 2, 5).
None of the antibodies used to date for vascular immunotargeting has proven ideal. They all have relatively limited binding capacities [e.g., 1–3 × 105 binding sites per cell for anti-ACE (6)]. Poor internalization of anti-intercellular adhesion molecule by endothelium precludes intracellular delivery of a drug or genetic material (7). Although endothelium internalizes anti-thrombomodulin, it undergoes rapid cellular degradation (8). Anti-ACE selectively delivers drugs to the pulmonary endothelium and is internalized without rapid degradation (1, 5, 6). However, cytokines and oxidants suppress ACE expression in endothelium and, thus, inflammation may compromise targeting (9).
To develop a more efficient and safer strategy for drug targeting to endothelium, we studied antibodies to platelet-endothelial cell adhesion molecule-1 (PECAM-1, or CD31). PECAM-1 is a transmembrane adhesion molecule expressed at high levels on endothelial cells (>1 million copies per cell) that plays an important role in transendothelial migration of leukocytes (10–12). Unlike ACE, expression levels are not markedly altered by cytokines (11).
We therefore evaluated the targeting profile of anti-PECAM in cell cultures and in animals. Although endothelial cells possessed very high anti-PECAM binding capacity, they did not internalize anti-PECAM. Similarly, although anti-PECAM intensely stained endothelium in the lung tissue, 125I-labeled anti-PECAM accumulated poorly in the lungs.
However, because immunotargeting requires that the drug be coupled to a carrier, we studied the effect of conjugation of biotinylated anti-PECAM (b-anti-PECAM) with streptavidin (SA) on the targeting profile of b-anti-PECAM. In this paper, we report that streptavidin markedly facilitates internalization and pulmonary targeting of anti-PECAM and, therefore, provides a useful mechanism for delivery of drugs to endothelial cells. The effect of SA is not limited to anti-PECAM and endothelial cells. Therefore, SA-mediated conversion of poor carriers to highly internalizable/targetable carriers provides a paradigm for drug targeting strategies.
MATERIALS AND METHODS
Antibodies.
Four PECAM-1 antibodies were used: (i) “Houston”, a polyclonal rabbit IgG against human and rat PECAM-1 (13); (ii) mAb 62, provided by M. Nakada (Centocor) an IgG2 mAb reacting with human and rat PECAM-1 (14); (iii) mAb 4G6, a mouse mAb IgG2b reacting with the most membrane-proximal, sixth Ig-like loop of human PECAM-1 (15); and (iv) mAb 390, a rat mAb reacting with murine PECAM-1/CD31 (15). Soluble purified PECAM was generously provided by P. Newman (Blood Center of Southeastern Wisconsin). Murine mAb 1045 recognizes a chondroitin sulfate-dependent epitope of human thrombomodulin (8).
Biotinylation, Radiolabeling, and Conjugation of Proteins.
Proteins were biotinylated with 6-biotinylaminohexanoic acid N-hydroxysuccinimide ester (BxNHS, Calbiochem) without detectable reduction of functional activity and designated as b-IgG, b-mAb, or b-catalase (Fluka) following procedures described (16). Proteins were labeled with 125I (Amersham Pharmacia) using Iodogen (Pierce). Catalase was conjugated with b-IgG or b-anti-PECAM by using a procedure described previously (16).
Binding, Internalization, and Degradation of 125I-Labeled Anti-PECAM in Cell Culture.
The following cell lines were used: (i) human umbilical vein endothelial cells (HUVEC); (ii) EAhy926 cells (immortalized PECAM-expressing transformed hybrid cells), kindly provided by C. Edgel (University of North Carolina, Chapel Hill, NC) (17); and (iii) human mesothelioma REN cells or REN cells transfected with human PECAM-1 cDNA (REN/PECAM cells) (14). 125I-labeled antibodies or conjugates were incubated with washed cells in M199 medium containing 0.2% BSA at 4°C or 37°C. After washing with M199, cells were incubated for 15 min with 50 mM glycine/100 mM NaCl, pH 2.5 to release surface-associated antibody. There was no detectable cell detachment or plasma membrane damage (determined by leakage of 51Cr) immediately after treatment with glycine buffer. After glycine elution, cells were lysed with 0.1% Triton X-100. Cellular uptake was calculated as a sum of 125I in the glycine eluates (surface-associated label) and the cell lysates (internalized label). Percent internalization was calculated as % = (total uptake−surface associated) × 100/(total uptake). To determine degradation of the 125I-labeled proteins and detachment of 125I from the proteins, cell lysates were incubated for 1 h at 4°C with 10% trichloroacetic acid and centrifuged, and 125I in the pellet and in the supernatants was determined. Percent degradation was calculated as % = (supernatant 125I) × 100/(supernatant 125I + pellet 125I) (6, 8).
Electron Microscopy of the Endothelial Uptake of b-Anti-PECAM/SA/b-Ferritin Complex.
Confluent HUVEC (passage 2–3) grown on gelatin-coated 60 mm Petri dishes were incubated with b-anti-PECAM/SA/b-ferritin or b-IgG/SA/b-ferritin for 60 or 120 min at 37°C in M199 medium supplemented with 10% fetal bovine serum. After washing, cells were incubated for 10 min with ice-cold 2% paraformaldehyde/0.5% glutaraldehyde in 0.1M cacodylate buffer (pH 7.4) and processed for routine transmission electron microscopy.
Experiments in Isolated Perfused Rat Lungs.
Preparation of isolated perfused lungs (IPL) was performed in anesthetized Sprague–Dawley male rats (170–200 g) as described (5, 6). IPL were ventilated with a humidified gas mixture containing 5% CO2 and 95% air and perfused via the pulmonary artery with 45 ml of recirculating filtered Krebs–Ringer buffer (KRB) (pH 7.4), containing 10 mM glucose and 3% fatty acid-free BSA at 37°C. 125I-labeled proteins were perfused in IPL for 1 hr followed by 5 min with perfusion with KRB/BSA to eliminate nonbound material. 125I uptake in the lungs was measured in a γ counter and expressed as a % of injected dose per gram (ID/g) (5). Catalase protection experiments were performed as described (5) by perfusing IPL for 1 hr with 45 ml of KRB/BSA containing 100 μg of catalase conjugated with either mouse IgG (IgG/Cat) or anti-PECAM mAb 62 (anti-PECAM/Cat). Control lungs were perfused with KRB/BSA. The nonbound material was eliminated by nonrecirculating perfusion of KRB/BSA for 5 min, and lungs were again perfused in a recirculating manner for 1 hr with KRB/BSA containing 5 mM H2O2. Lungs were rinsed with saline and gently blotted with a filter paper, and wet weight was determined. Thereafter lungs were dried and weighed to determine wet/dry lung weight coefficient.
In Vivo Injection of Radiolabeled Antibodies and Conjugates.
Biodistribution of 125I-labeled antibodies and conjugates in intact animals was studied as described (1). One hour after injection of 125I-labeled preparations into the tail vein in anesthetized Sprague–Dawley rats or BALB/c male mice, animals were sacrificed, internal organs were harvested and rinsed with saline, and the 125I in tissues and in blood was determined in a γ counter.
RESULTS
Streptavidin Facilitates Intracellular Uptake of b-Anti-PECAM-1 Antibodies.
HUVEC, EAhy926, and REN/PECAM cells expressed PECAM-1 predominantly at the intercellular junctions (Fig. 1A) and possessed a very high binding capacity for 125I-anti-PECAM. In HUVEC, Scatchard analysis revealed that Kd was 10 nM for mAb 62 (Bmax = 1.5 × 106 per cell) and 250–350 nM for the polyclonal Ab Houston (Bmax = 5–8 × 106 per cell). Neither biotinylation nor conjugation with SA altered the Kd of [125I]anti-PECAM binding to HUVEC or REN/PECAM cells at 4°C (data not shown).
Figure 1.
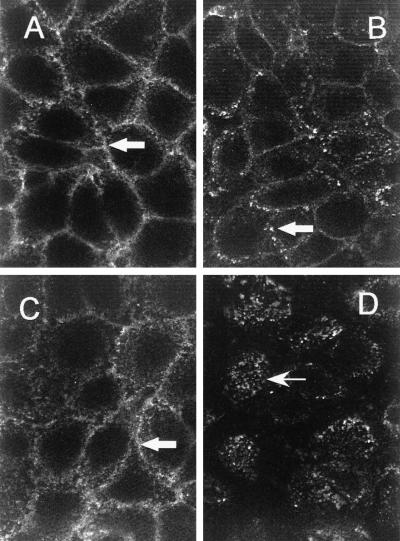
SA facilitates internalization of biotinylated anti-PECAM. EAhy926 cells were incubated for 90 min with either b-Ab Houston (A and B) or b-Ab Houston conjugated with streptavidin (C and D) at 4°C (A and C) or at 37°C (B and D). After washing, fixation, and permeabilization, cells were stained with fluorescein isothiocyanate-labeled antibody against rabbit IgG. Note granular intracellular fluorescence in the cells incubated with b-anti-PECAM/SA (D, arrow); in A, B, and C the fluorescent signal is localized on the cellular surface at cell–cell borders (arrows).
Despite high binding levels, a number of lines of evidence showed that PECAM-expressing cells internalized very small amounts (<10%) of bound anti-PECAM. First, binding of 125I-anti-PECAM to cells at 37°C did not exceed that at 4°C. For example, in REN/PECAM cells, uptake of 125I-mAb 62 was 6.9 ± 0.4 ng per well at 4°C vs. 6.0 ± 0.1 ng per well at 37°C. Second, glycine buffer eluted 81 ± 12% of 125I from cells after 90 min of incubation with 125I-mAb 62 at 37°C, a value almost identical to 125I eluted after 90 min of incubation with 125I-mAb 62 at 4°C (84 ± 15%). Third, anti-PECAM mAb incubated with cells for 90 min at either 37°C or 4°C showed clear localization to the plasma membrane with minimal cytoplasmic staining (Fig. 1 A and B). Poor internalization was seen with all PECAM antibodies in cell types tested. Biotinylation did not alter internalization of anti-PECAM by PECAM-expressing cells (data not shown).
In contrast, SA markedly stimulated the uptake at 37°C of b-125I-mAb 62 by REN/PECAM cells (from 42 ± 2 to 102 ± 11 ng per well) and by HUVEC (from 16.2 ± 0.5 to 114.1 ± 5.3 ng per well) and stimulated the uptake of b-125I-Ab Houston by HUVEC (data not shown). SA had no effect on the uptake of b-125I-IgG by cells (e.g., 0.62 ± 0.1 and 0.61 ± 0.1 ng per well for b-125I-IgG and b-125I-IgG/SA in EAhy926 cells).
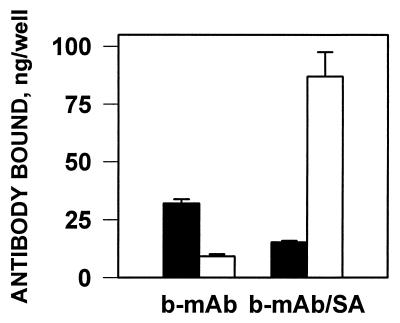
SA also markedly facilitated b-125I-anti-PECAM internalization by the target cells. In REN/PECAM cells, only 13 ± 10% of cell-bound b-125I-mAb 62/SA was accessible for elution by glycine buffer (Fig. 2), indicating that 85–90% of antibody was internalized. SA caused 85–95% internalization of b-125I-mAb 62 by HUVEC, and 80–95% internalization of b-125I-mAb 4G6 and b-125I-Ab Houston in all PECAM-expressing cells. SA had no effect on internalization of nonbiotinylated anti-PECAM, nor did b-125I-IgG (data not shown).
Figure 2.
SA stimulates the cellular uptake of biotinylated 125I-anti-PECAM. REN/PECAM cells were incubated for 90 min at 37°C with 125I-b-mAb 62 or 125I-b-mAb 62/SA. After washing, 125I in the surface fraction (filled bars) and cell lysates (open bars) in the cells was determined (mean ± SD, n = 3).
Fig. 1 shows confocal microscope images of EAhy926 cells incubated for 90 min with b-Ab Houston and b-Ab Houston/SA conjugate at 4°C or 37°C. Staining of the fixed permeabilized cells with fluorescein isothiocyanate-labeled secondary antibody revealed surface (cell–cell) localization of both b-anti-PECAM and b-anti-PECAM/SA at 4°C. Although cellular distribution of b-anti-PECAM at 37°C did not differ markedly from that at 4°C, b-anti-PECAM/SA accumulated intracellularly at 37°C. Similar results were reproduced in HUVEC and in REN/PECAM cells with b-mAb 62 and Ab Houston (data not shown).
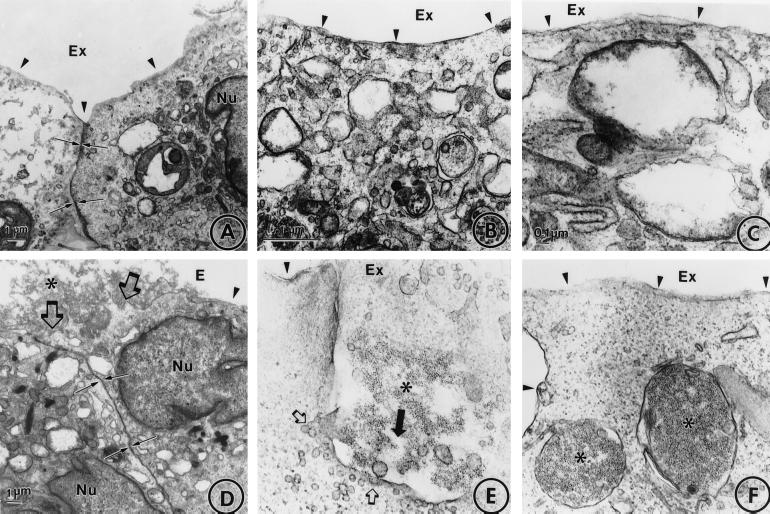
Fig. 3 shows transmission electron microscopic images of the cellular uptake of b-ferritin conjugated with b-mAb 62/SA or with b-IgG/SA. After 1 hr of incubation at 37°C, anti-PECAM/SA/ferritin was localized predominantly in the sites of intercellular contacts (Fig. 3D) and in the invaginations of the plasma membrane packed with the conjugate (Fig. 3E). After 2 hr of incubation at 37°C, the conjugate was found predominantly in large intracellular vacuoles (Fig. 3F), whereas the plasma membrane appeared to be cleared of the conjugate. Fig. 3A–C show that there was no detectable uptake of IgG/SA/ferritin.
Figure 3.
Uptake of b-ferritin conjugated with b-mAb 62/SA by endothelial cells. HUVEC were incubated with b-IgG/SA/b-ferritin (A–C) or b-mAb 62/SA/b-ferritin (D–F) for 60 min (A, B, D, and E) or 120 min (C and F) at 37°C, fixed, and processed for electron microscopy. ∗, ferritin; arrowheads, the plasma membrane; Nu, nucleus; Ex, extracellular space; small arrows in A and D, the intracellular contact; large open arrows in D, cell-associated ferritin conjugate; large closed arrow in E, direction of the conjugate uptake; small open arrows in E, microvesicles associated with large invagination of the plasma membrane.
Streptavidin did not alter the rate of b-anti-PECAM degradation by cells. For example, only 1.9 ± 0.7% or 0.7 ± 0.2% of trichloroacetic acid-soluble 125I was detected in cell lysates after incubation of b-125I-mAb 62 or b-125I-mAb 62/SA with REN/PECAM cells for 90 min at 37°C. Trichloroacetic acid-soluble 125I in the cell medium after incubation did not exceed 2% in either case. Similar results were obtained in HUVEC (data not shown). Thus, the rate of cellular degradation of b-125I-mAb 62/SA was slow, despite its effective internalization.
The Effect of SA on Internalization of Biotinylated Antibodies Is Generalizable and Does Not Depend on the Intracellular Domain of PECAM or on the RGD Domain of SA.
We also studied the effect of SA on internalization of b-mAb 1045, an antibody against thrombomodulin that is poorly internalized by HUVEC (8). Like our results with PECAM-1, we found that SA stimulated internalization from 20.5 ± 8.3% for nonconjugated b-125I-mAb 1045 to 92 ± 7% for b-125I-mAb 1045/SA. This result implies that the effect of SA is not specific for PECAM-1.
To determine whether the PECAM-1 intracellular domain was involved in the internalization of anti-PECAM/SA, we compared the uptake and internalization of b-125I-mAb 62/SA by REN cells transfected with full-length PECAM-1 or a truncated mutant form of PECAM-1 lacking the intracellular domain. Internalization of 125I-anti-PECAM/SA was equally effective in both cell types (≈95%), indicating that the intracellular domain of PECAM-1 is not important for the SA-facilitated uptake of anti-PECAM.
We compared the effects of SA, a neutral tetrameric biotin-binding protein possessing an RGD-like sequence (RYD), and neutravidin, a neutral derivative of the tetrameric biotin-binding protein avidin that lacks its RYD domain. Both proteins stimulated uptake and binding of 125I-anti-PECAM to a similar extent (data not shown), thus indicating that the RYD domain of SA is not important for the SA-facilitated intracellular delivery of anti-PECAM.
To determine whether binding/uptake of anti-PECAM/SA conjugate activated nonspecific fluid-phase uptake of proteins, we incubated REN/PECAM cells with 125I-albumin in the presence or absence of nonlabeled anti-PECAM/SA for 90 min at 37°C. In control cells, cellular uptake of 125I-albumin was 104 ± 19 pg per well, with internalization of 69 ± 20% of 125I. Neither anti-PECAM/SA nor IgG/SA stimulated cellular uptake (96 ± 18 and 89 ± 6 pg per well) or internalization of 125I-albumin (44 ± 9% and 62 ± 17%).
SA Facilitates Targeting of Biotinylated Anti-PECAM to the Pulmonary Vascular Endothelium.
In rat lungs stained with anti-PECAM-1 mAb 62, we found intense staining of endothelium in the large and small vessels (data not shown), in good agreement with our previous results with the polyclonal antibody Houston (12). We next determined the pulmonary uptake of 125I-anti-PECAM in rat IPL. Although pulmonary uptake of 125I-mAb 62 in the IPL was 5-fold higher than that of control 125I-IgG (2.3 ± 0.2 vs. 0.5 ± 0.2% of ID/g), uptake of 125I-anti-PECAM in the IPL was an order of magnitude lower than that of 125I-anti-ACE (21 ± 3% ID/g). In intact rat experiments, 125I-mAb 62 displayed no specific pulmonary targeting after injection (3.6 ± 1.1 vs. 4.3 ± 0.7% ID/g for 125I-IgG). Thus, despite high levels of PECAM expression in the pulmonary vasculature, anti-PECAM displayed poor targeting potential.
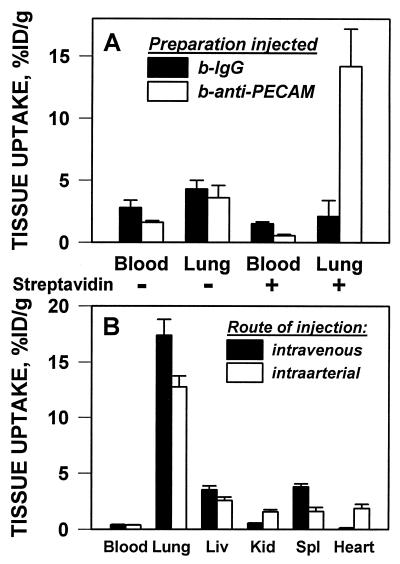
Because SA stimulated cellular binding and internalization of b-anti-PECAM in cell culture, we studied the pulmonary uptake of b-125I-anti-PECAM/SA in intact animals. In contrast to the low uptake of unconjugated b-125I-mAb 62 (3.6 ± 1.1% ID/g), pulmonary uptake was 14.2 ± 3.0% ID/g 1 hr after i.v. injection of b-125I-mAb 62/SA in rats (Fig. 4A). Facilitation of the targeting of b-anti-PECAM was specific, because SA reduced pulmonary uptake of b-125I-IgG (2.1 ± 1.1% for b-125I-IgG/SA vs. 4.3 ± 0.7% ID/g for b-125I-IgG). SA conjugation reduced the blood levels of both b-IgG and b-mAb 62 in rats. Thus, the lung/blood ratio of b-125I-mAb 62/SA was 25.9 ± 4.8, vs. 1.3 ± 0.6 for b-125I-IgG/SA. The stimulation of pulmonary uptake was reproduced in mice by using the rat anti-mouse PECAM b-mAb 390: the pulmonary uptake of b-125I-mAb 390/SA was 30 ± 6% ID/g vs. 4.9 ± 0.4% ID/g for b-125I-IgG/SA. SA reduced the pulmonary level of b-125I-IgG by 50% in mice, thus confirming the specificity of anti-PECAM/SA targeting.
Figure 4.
Pulmonary targeting of b-125I-anti-PECAM/SA in vivo. (A) Rats were injected via tail vein with 125I-b-IgG (filled bars) or 125I-b-anti-PECAM (open bars) either conjugated with streptavidin (+) or without streptavidin (−). (B) Rats were injected with b-125I-anti-PECAM/SA via the tail vein (filled bars) or via the left ventricle (open bars). One hour after injection, rats were sacrificed, and 125I in the tissues was determined (mean ± SD, n = 5).
To exclude the role of blood in the pulmonary targeting, we tested the uptake of b-anti-PECAM/SA in IPL perfused with blood-free buffer. SA caused 20-fold stimulation of the pulmonary uptake of b-125I-mAb 62 (39 ± 3.1% ID/g) and b-125I-Ab Houston (53.1 ± 9.35 ID/g) with no effect on b-125I-IgG (0.6+0.1% ID/g) or nonbiotinylated 125I-anti-PECAM (2.3+0.3% ID/g). Both in IPL and in intact rats, neutravidin stimulated pulmonary uptake of b-125I-mAb 62 to the same extent as SA (data not shown).
Fig. 4B shows the distribution of b-125I-mAb 62/SA after intravenous or intraarterial injection in rats. Both routes of administration provided high pulmonary uptake of the conjugate, implying that pulmonary targeting is not caused by mechanical retention of anti-PECAM/SA in capillaries. Because the injected dose (less than 1 μg/kg) was below saturation, a first passage phenomenon contributed to the conjugate distribution in the organs. Thus, intraarterially injected b-125I-mAb 62/SA displayed higher cardiac and renal uptake and lower pulmonary, hepatic, and splenic uptake.
Anti-PECAM/SA/b-Catalase Accumulates Intracellularly in Endothelial Cells and Protects from H2O2-Mediated Injury in Cell Culture.
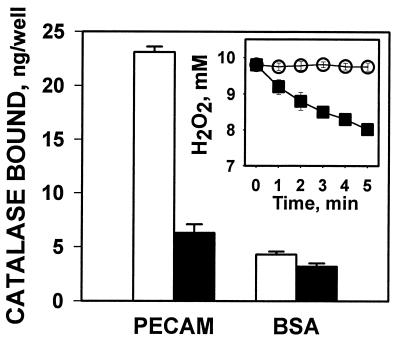
To evaluate b-anti-PECAM/SA as a carrier for endothelial targeting of drugs, we conjugated it with b-catalase and studied the uptake and activity of b-anti-PECAM/SA/b-catalase in vitro. The b-mAb 62/SA/b-catalase, but not b-IgG/SA/b-catalase, specifically bound in the PECAM-coated wells (Fig. 5) and degraded H2O2 in the wells (Fig. 5 Inset). Whereas b-IgG/SA/b-125I-catalase did not bind to HUVEC (1.3 ± 0.1 ng per well), both b-mAb 62/SA or b-Ab Houston/SA provided high-level binding of b-125I-catalase (67.2 ± 2.9 and 184.5 ± 0.3 ng per well). HUVEC internalized >90% of b-anti-PECAM/SA/b-125I-catalase, whereas degradation of internalized catalase did not exceed 5%. The antibody-conjugated catalase was not degraded and then secreted, because only 5.6 ± 1.2% of TCA-soluble 125I was detected in the cell medium after 90 min of incubation of b-mAb 62/SA/b-125I-catalase at 37°C in HUVEC. In REN/PECAM cells, the intracellular uptake of anti-PECAM/125I-catalase was 195 ± 8 ng per well (vs. 3.5 ± 0.5 ng per well for IgG/catalase), with 95% internalization of the cell-bound 125I-catalase. In EAhy926 cells, internalization of b-Ab Houston/SA/b-125I-catalase was 78.1 ± 3.3%.
Figure 5.
Functional activity of b-anti-PECAM/SA/b-catalase complex. Ten micrograms of b-mAb 62/SA/b-125I-catalase (open bars) or b-IgG/SA/b-125I-catalase (filled bars) were incubated for 1 hr in wells coated with PECAM-1 or BSA. After washing, 125I in the wells was determined. (Inset) Wells coated with BSA (○) or PECAM (■) were incubated with 20 μg of b-mAb 62/SA/b-catalase for 1 hr. After washing, 10 mM H2O2 was added to the wells and its decay was determined.
To determine whether the conjugate could protect cultured cells from H2O2-induced injury, HUVEC were labeled with 51Cr, incubated for 1 hr at 37o with b-mAb 62/SA/b-catalase or b-IgG/SA/b-catalase, washed, and exposed to 2 mM H2O2 for 6 hr. H2O2 induced 14.5 ± 1.3% and 15.1 ± 1.3% release of 51Cr from HUVEC treated with either b-mAb 62/SA/b-catalase or b-Ab Houston/SA/b-catalase, vs. 56.1 ± 5.7% of 51Cr released from cells treated with b-IgG/SA/b-catalase. In control cells, H2O2 caused 73 ± 33% release of 51Cr. After subtraction of the level of spontaneous 51Cr release from control cells not treated with H2O2 (10.9 ± 0.6%), anti-PECAM/catalase afforded a 91% reduction of 51Cr release. Thus, anti-PECAM/SA/b-catalase protected cells against oxidative injury by H2O2, whereas b-IgG/SA/b-catalase provided no significant protection.
Anti-PECAM/SA/b-Catalase Accumulates in the Lungs and Protects from H2O2-Mediated Injury in the Lung.
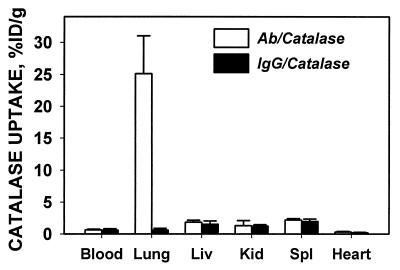
To test the ability of anti-PECAM/SA to deliver an active drug to the pulmonary endothelium, b-anti-PECAM/SA/b-125I-catalase or b-IgG/SA/b-125I-catalase were injected intravenously into intact animals. In rats, b-mAb 62/SA/b-125I-catalase specifically accumulated in rat lungs after intravenous injection, with lung/blood ratio in rats 39.8 ± 4.1 for b-mAb 62/SA/b-125I-catalase vs. 1.1 ± 0.2 for b-IgG/SA/b-125I-catalase (Fig. 6). Similar results were seen in mice, with lung/blood ratio equal to 7.5 ± 1.1 for b-mAb 390/SA/b-125I-catalase vs. 0.6 ± 0.1 for b-IgG/SA/b-125I-catalase.
Figure 6.
Pulmonary targeting of b-anti-PECAM/SA/b-125I-catalase in rats. Ten micrograms of b-anti-PECAM/SA/b-125I-catalase (open bars) or b-IgG/SA/b-125I-catalase (filled bars) was injected in the tail vein of anesthetized rats. One hour later, rats were sacrificed, and 125I in the tissues was determined (mean ± SD, n = 3–5).
Finally, based on these results, we examined the ability of b-mAb 62/SA/b-catalase to protect the lung against intravascular oxidative insult. In the first experiment, we determined that the uptake of b-mAb 62/SA/b-125I-catalase and b-IgG/SA/b-125I-catalase in IPL was 37.3 ± 4.4% vs. 2.1 ± 0.2% ID/g (1-hr perfusion). In the second experiment, we used perfusion of 5 mM H2O2 in IPL, an intervention that causes lung injury causing elevation of the lung wet/dry ratio, reflecting lung edema (5). IPL were first perfused for 1 hr with 100 μg of b-mAb 62/SA/b-catalase, b-IgG/SA/b-catalase, or buffer alone. After elimination of nonbound material, lungs were further perfused with 5 mM H2O2 for 60 min. In IPL treated with b-IgG/SA/b-catalase, the wet/dry weight ratio (8.1 ± 0.7) was markedly higher (P < 0.001) than that in the control lungs not treated with H2O2 (5.1 ± 0.2), indicating lack of protection against H2O2. In contrast, in IPL treated with b-mAb 62/SA/b-catalase, the wet/dry weight ratio remained normal (5.5 ± 0.1), thus indicating protection of the lung against H2O2-induced oxidative vascular injury.
DISCUSSION
The two major goals of most drug delivery strategies are recognition of specific cells in the body and effective intracellular delivery. Our results demonstrate that SA conjugation to biotinylated antibodies can convert antibodies with relatively poor targeting potential to very effective carriers for vascular immunotargeting.
SA, avidin, and its derivative, neutravidin, are tetrameric 60- to 66-kDa proteins possessing four high-affinity binding sites for biotin or biotinylated compounds (18). The SA-biotin crosslinking pair is useful for chemical conjugation of biomolecules. SA is nontoxic and does not cause harmful reactions in laboratory animals or in humans (19). SA-biotin crosslinkers have recently been used in vivo for targeting of drugs, toxins, radiolabels, and genetic material (19–21). It has been noted that biotinylation may reduce the affinity of antibodies (22) and that SA accelerates elimination of biotinylated antibodies from the bloodstream (23) and may hinder interaction of biotinylated enzymes with their substrates (16). In the present study, we did not observe changes in the biological behavior of b-anti-PECAM with regard to binding to immobilized antigen or uptake by PECAM-expressing cells. However, we found that SA caused dramatic stimulation of cellular binding, internalization, and pulmonary targeting of biotinylated antibodies.
The pulmonary targeting cannot be explained by mechanical retention of large complexes in the pulmonary capillaries because: (i) b-IgG/SA conjugates do not accumulate in the lungs; (ii) SA stimulates specific uptake of b-anti-PECAM in cell cultures; and (iii) both intravenous and intraarterial administration of b-anti-PECAM/SA provide pulmonary targeting. Thus, facilitation of the cellular uptake (i.e., binding and internalization) of b-anti-PECAM by SA is likely the mechanism for the pulmonary targeting. Because PECAM is expressed by normal endothelium in various vascular areas, an anti-PECAM/SA carrier may be useful for drug delivery to endothelium in various organs, depending on the route and mode of administration.
Importantly, conjugates escaped massive intracellular degradation, thus allowing the successful intracellular delivery of an active antioxidant enzyme, catalase, in our cell culture models. Our animal studies confirm the potential therapeutic applicability of this approach, although the final (intra)cellular destination of the conjugates in animal models needs to be addressed more precisely. The strategy of targeting of enzymes such as catalase or superoxide dismutase may be useful for the cell-specific augmentation of antioxidant defense in the pulmonary endothelium and protection against oxidative insults such as the adult respiratory distress syndrome or other disease conditions associated with or manifested by oxidative endothelial injury (24).
The exact mechanisms of how SA affects the targeting, cellular uptake, and intracellular trafficking of biotinylated anti-PECAM have yet to be determined. Neither the cytoplasmic domain of PECAM nor an RGD-like domain on SA plays an important role in cellular uptake of b-anti-PECAM/SA. Preliminary studies suggest that the size of the b-anti-PECAM/SA complex is an important determinant for the cellular uptake, and we favor a mechanism that involves a phagocyte-like uptake of complexes in large vacuoles (see Fig. 3F) in which proteins seem to be protected, at least initially, from rapid degradation.
The targeting strategy we have described here will likely be applicable to a wide range of therapeutic or experimental compounds and agents. For example, in addition to delivery of antioxidant enzymes, we have found that conjugation of biotinylated glucose oxidase to anti-PECAM/SA provides specific targeting of active biotinylated glucose oxidase to PECAM-1-expressing cells resulting in intracellular generation of H2O2 and severe oxidative stress in endothelial cells in culture, in perfused rat lungs, and in intact mice. Another potential use of the strategy described in the present paper could be vascular gene therapy. In preliminary experiments, we have found that b-anti-PECAM/SA/DNA conjugates specifically bind to REN/PECAM cells and result in 90% internalization of radiolabeled DNA, allowing transfection of the target cells.
In the wider context of general strategies for drug delivery, our results suggest that SA conjugation of an otherwise poorly internalizable/targetable antibody could convert it to a highly internalizable/targetable carrier. This observation thus serves as a paradigm for a novel drug targeting strategy. Although we have focused on the anti-PECAM antibodies in this study, SA-mediated internalization of biotinylated mAb 1045 against thrombomodulin indicates that this effect is not limited to PECAM antibodies. Moreover, effective internalization of anti-PECAM/SA by nonendothelial cells transfected with PECAM (REN mesothelioma cells) implies that the strategy described in the present paper may be applicable to a wide variety of target cells that could include tumor cells or HIV-infected cells.
Acknowledgments
We thank Dr. M. Nakada (Centocor) for supplying antibody mAb 62 and Dr. P. Newman (Blood Center of Southwest Wisconsin) for the gift of purified soluble PECAM-1. This work was supported by the American Heart Association Established Investigator Grant (Grant 9640204 to V.R.M.) and Specialized Center of Research in Acute Lung Injury from National Institutes of Health Heart, Lung, and Blood Institute (HL 60290 Project 4 to V.R.M. and S.M.A.).
ABBREVIATIONS
- SA
streptavidin
- PECAM
platelet-endothelial cell adhesion molecule
- b-anti-PECAM
biotinylated anti-PECAM
- ACE
angiotensin-converting enzyme
- HUVEC
human umbilical vein endothelial cells
- IPL
isolated perfused lungs
- ID/g
injected dose per gram
- KRB
Krebs–Ringer buffer
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Danilov S, Muzykantov V, Martynov A, Atochina E, Sakharov I, Trakht I, Smirnov V. Lab Invest. 1991;64:118–124. [PubMed] [Google Scholar]
- 2.Maruyama K, Kennel S, Huang L. Proc Natl Acad Sci USA. 1990;87:5744–5748. doi: 10.1073/pnas.87.15.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrows F, Thorpe P. Pharmacol Ther. 1994;64:155–174. doi: 10.1016/0163-7258(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 4.Spragg D, Alford D, Greferath R, Larsen C, Lee K, Gurthner G, Cybulsky M, Tosi F, Nicolau C, Gimbrone M. Proc Natl Acad Sci USA. 1997;94:8795–8800. doi: 10.1073/pnas.94.16.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atochina E, Balyasnikova I, Danilov S, Granger D, Fisher A, Muzykantov V. Am J Physiol. 1998;19:L806–L817. doi: 10.1152/ajplung.1998.275.4.L806. [DOI] [PubMed] [Google Scholar]
- 6.Muzykantov V, Atochina E, Kuo A, Barnathan E, Notarfrancesco K, Shuman H, Dodia C, Fisher A. Am J Physiol. 1996;270:L704–L713. doi: 10.1152/ajplung.1996.270.5.L704. [DOI] [PubMed] [Google Scholar]
- 7.Almenar-Queralt A, Duperray A, Miles L, Felez J, Altieri D. Am J Pathol. 1995;147:1278–1288. [PMC free article] [PubMed] [Google Scholar]
- 8.Muzykantov V, Balyasnikova I, Joshi A, Fisher A, Smirnov M, Esmon N, Esmon C. Drug Delivery. 1998;5:197–206. doi: 10.3109/10717549809052035. [DOI] [PubMed] [Google Scholar]
- 9.Muzykantov V, Puchnina E, Atochina E, Hiemish H, Slinkin M, Meertzuk F, Danilov S. J Nucl Med. 1991;32:453–460. [PubMed] [Google Scholar]
- 10.Newman P. J Clin Invest. 1997;99:3–7. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLisser H, Baldwin H, Albelda S. Trends Cardiovasc Med. 1997;151:671–677. doi: 10.1016/S1050-1738(97)00049-2. [DOI] [PubMed] [Google Scholar]
- 12.Vaporician A, DeLisser H, Yan H, Mendiguren I, Thom S, Jones M, Ward P, Albelda S. Science. 1993;262:1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- 13.Albelda S, Muller W, Buck C, Newman P. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurubhagavatula I, Amrani Y, Pratico D, Ruberg F, Albelda S, Panettieri R. J Clin Invest. 1998;101:212–222. doi: 10.1172/JCI269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan H-C, Pilewski J, Zhang Q, DeLisser H, Romer L, Albelda S. Cell Adhes Comm. 1995;3:45–66. doi: 10.3109/15419069509081277. [DOI] [PubMed] [Google Scholar]
- 16.Muzykantov V, Atochina E, Ischiropoulos H, Danilov S, Fisher A. Proc Natl Acad Sci USA. 1996;93:5213–5218. doi: 10.1073/pnas.93.11.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgell C, McDonald C, Graham J. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilchek M, Bayer E. Anal Biochem. 1988;171:1–32. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]
- 19.Paganelli G, Belloni C, Magnani P. Eur J Nucl Med. 1992;19:322–329. doi: 10.1007/BF00177053. [DOI] [PubMed] [Google Scholar]
- 20.Bickel U, Toshikawa T, Landaw E, Faull K, Pardridge W. Proc Natl Acad Sci USA. 1993;90:2618–2622. doi: 10.1073/pnas.90.7.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hnatovich D, Virzi F, Ruskovski M. J Nucl Med. 1987;28:1294–1302. [PubMed] [Google Scholar]
- 22.Muzykantov V, Gavriluk V, Reinecke A, Atochina E, Kuo A, Barnathan E, Fisher A. Anal Biochem. 1995;226:279–287. doi: 10.1006/abio.1995.1226. [DOI] [PubMed] [Google Scholar]
- 23.Klibanov A, Martynov A, Slinkin M, Sakharov I, Smirnov M, Muzykantov V, Danilov S, Torchilin V. J Nucl Med. 1988;29:1951–1958. [PubMed] [Google Scholar]
- 24.Louie S, Halliwell B, Cross C. Adv Pharmacol. 1997;38:457–490. doi: 10.1016/s1054-3589(08)60995-3. [DOI] [PubMed] [Google Scholar]