Abstract
Hormonal regulation of adrenal function occurs primarily through G protein-coupled receptors (GPCR), which may play different roles in fetal vs. adult adrenal glands. In this study, we compared the transcript levels of GPCR between fetal and adult adrenal and found that gonadotropin-releasing hormone receptor (GnRHR), latrophilin 3 receptor, G protein-coupled receptor 37, angiotensin II receptor type 2, latrophilin 2 receptor and melanocortin receptor were expressed at significant higher levels in fetal adrenal. High GnRHR protein expression was also detected in fetal adrenal using immunohistochemical analysis. To define potential ligand sources for fetal adrenal GnRHR, we demonstrated that GnRH1 mRNA was expressed at high levels in the placenta, while fetal adrenal had high expression of GnRH2. In summary, certain GPCR particularly GnRHR were highly expressed in fetal adrenal and the expression of GnRH mRNA in the placenta and the fetal adrenal raises the possibility of endocrine and/or paracrine/autocrine influence on fetal adrenal function. However, the exact function of GnRHR in fetal adrenal remains to be determined.
Keywords: Fetal adrenal, GPCR, GnRHR
1. Introduction
G protein-coupled receptors (GPCR) represent a large family of seven transmembrane proteins responsible for mediating extracellular to intracellular signaling. GPCR play a vital role in many biological processes, including regulation of growth, death and metabolism within target cells (Chakraborty,2001). Given the broad range of functions controlled by GPCR in both physiological and pathologic conditions, they have been the focus of a significant amount of pharmaceutical research with more than 50% of prescription drugs targeting individual GPCR (Jacoby et al.,2006; Kostenis,2006).
The gonadotropin-releasing hormone receptor (GnRHR), also known as the luteinizing hormone-releasing hormone receptor (LHRHR), is a member of the GPCR family. GnRHR protein consists of 328 amino acids (Chi et al.,1993) and is highly expressed on the surface of pituitary gonadotrope cells (La Rosa et al.,2000). Once activated by gonadotropin-release hormone (GnRH) secreted from the hypothalamus, GnRHR can trigger the synthesis and release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are responsible for the regulation of ovarian and testicular function. Aside from its expression in the pituitary, GnRHR has also been detected in many extra-pituitary tissues including lymphocytes, breast, ovary and prostate (Peng et al.,1994; Minaretzis et al.,1995; Kottler et al.,1997; Yin et al.,1998; Chen et al.,1999). Recently, GnRHR was shown to have much higher expression in some adrenal aldosterone-producing tumors compared to normal adrenal or cortisol-producing tumors (Ye et al.,2007).
GnRH, the ligand for GnRHR, is a decapeptide characterized as the physiological regulator of gonadotropin secretion. However, the presence of multiple forms of GnRH in individual vertebrate species and their expression in extra-pituitary tissues indicate that GnRH likely has had multiple functions during the course of evolution (Somoza et al.,2002; Kah et al.,2004). Two GnRH forms are detected in humans, namely GnRH1 and GnRH2 (Chen et al.,1998). While GnRH1 is mainly expressed in hypothalamus, the newly detected GnRH2 gene is found significantly higher in organs outside brain, including kidney, bone marrow and prostate (White et al.,1998; Chen et al.,2002; Parker et al.,2007).
Morphologically and physiologically, the human fetal adrenal glands are remarkable organs that exhibit distinct differences from the adult adrenal. Near the end of gestation, the fetal adrenal weighs as much as the adult adrenal and represents the largest endocrine gland in fetus (Anderson et al.,1971; Shepard et al.,1988; Marecki,1989). The fetal adrenals produce as much as 100 to 200 mg/day of steroid hormones, which is higher than the 30 to 40 mg/day seen in adult adrenals at rest (Bloch and Benirschke,1959; Vermeulen,1976; Rainey et al.,2004; Yildirim et al.,2004). Within the fetal adrenal, steroidogenic function and zonation are different from the adult. The major difference is the presence of a histologically distinct fetal zone (Dhom,1973; Parker et al.,1978), which constitutes 85% of the human fetal adrenal gland during most of the gestation and is the source for steroid precursors that are used by the placenta to produce estrogens. The fetal zone is not present in most mammals but appears to be unique to humans and a few non-human primates (Mesiano and Jaffe,1997; Beuschlein et al.,2002). The outer definitive zone (or neocortex) is believed to give rise to the postnatal adrenal zona glomerulosa. A third transitional zone, between the fetal zone and neocortex, is believed to give rise to the postnatal zona fasciculata (Mesiano and Jaffe,1997). Interestingly, the fetal zone is transient and involutes shortly after birth (Spencer et al.,1999; Bocian-Sobkowska,2000).
Due to the dramatic differences between fetal and adult adrenals during development, studies have focused on defining the unique mechanisms controlling their activities. Because the fetal zone rapidly regresses shortly after birth, there has been much speculation that the fetal zone has the unique ability to respond to placenta-derived hormones that are needed for its maintenance (Riopel et al.,1989). Herein, we compared the expression of GPCR in fetal and adult adrenals, and found GnRHR to be the most differentially expressed GPCR between these two tissues.
2. Materials and methods
2.1. Tissue Collection
Human adrenal, pituitary, hypothalamus and placenta tissues were obtained through the Cooperative Human Tissue Network (Philadelphia, PA), Clontech (Palo Alto, CA) and Tohoku University School of Medicine (Sendai, Japan). These samples came from autopsies performed no more than 6 h after death or in the case of placenta, term deliveries. Fetal adrenal glands (14-19 wk gestation) were obtained from pathological examination following elective pregnancy terminations from Advanced Bioscience Resources with informed consent. For immunohistochemistry studies fetal adrenals were collected at the time of autopsy following spontaneous abortion at Tohoku University Hospital (28-32 wk gestation). The use of these tissues was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas, Texas, Medical College of Georgia (Augusta, GA), and Tohoku University School of Medicine.
2.2. RNA Isolation and Reverse Transcription Reaction
Total RNA was extracted from whole tissues (Chirgwin et al.,1979) and the quantity of the RNA was checked spectroscopically using a Nanodrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). Deoxyribonuclease I (Ambion Inc, Austin, TX)-treated total RNA (2 μg) was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) following manufacturer recommendations and stored at -80°C.
2.3. Microarray analysis
Total RNA isolated from whole human adult and fetal adrenal glands were used on 2 genomic expression arrays as has been previously described (Bassett et al.,2005; Ye et al.,2007). Briefly, RNA was hybridized to an Affymetrix human HG-U133+2 oligonucleotide microarray set containing 54,675 probe sets representing approximately 40,500 independent human genes. The arrays were scanned at high resolution using an Affymetrix GeneChip Scanner 300 from Microarray Core Facility, Medical College of Georgia. Results were analyzed using GeneSpring GX 7.3.1 software (Silicon Genetics, Redwood City, CA) to study GPCR expression. There were 544 independent GPCR included on this Affymetrix array.
2.4. Quantitative real-time RT-PCR (qPCR)
Taqman gene expression assays used for GnRH1, GnRH2, GnRHR, G protein-coupled receptor 37 (GPR37), latrophilin 3 (LPHN3), Angiotensin II receptor (AGTR2), cholinergic receptor muscarinic 5 (CHRM5), latrophilin 2 (LPHN2) and melanocortin 2 receptor (MC2R) mRNA detection were obtained from Applied Biosystems. qPCR reactions were performed by the ABI Prism 7000 Sequence Detection System (Applied Biosystems) in a total volume of 20 μl reaction mixture following the reaction parameters recommended by the manufacturer. Relative quantification of mRNA levels between different tissues were determined using the comparative Ct value as described previously (Ye et al.,2007), and 18S rRNA was used as an internal control.
2.5. Protein extraction and protein assay
Cells were lysed in 100 μl 1x Mammalian Protein Extraction Reagent (Pierce Chemical Co., Rockford, IL). The protein content of samples was then determined by the bicinchoninic acid (BCA) protein assay using the micro BCA protocol (Pierce Chemical Co.).
2.6. Immunohistochemical assay
Immunohistochemical analysis for GnRHR was performed employing the streptavidin-biotin amplification method using Vectastain® Universal Quick Kit (Vector Laboratories, Burlingame, CA). Monoclonal mouse anti-human GnRHR antibody (1:100 dilution) was purchased from Novocastra (Newcastle, UK). Antigen retrieval for immunostaining was performed using a method described previously (Nakamura et al.,2007). The antigen-antibody complex was visualized with 3.3’-diaminobenzidine solution [1 mM diaminobenzidine, 50 mM Tris-HCl buffer (pH 7.6), and 0.006 % H2O2] and counterstained with hematoxylin. Human pituitary was used as a positive control and adrenal samples processed in the absence of GnRHR antibody were used as negative controls.
2.7. Cell Culture and Treatment
Human fetal zone cells were isolated from fetal adrenal with collogenase-dispase digestion as described previously (Rehman et al.,2007). Cells were plated at a density of 200,000/ well in 24 well dishes and allowed to attach and grow for 5 days in growth medium (10 % Hyclone cosmic calf serum, 1 % ITS plus, 1 % antibiotic). The day before treatment, cells were changed to working medium (1 % cosmic calf serum, 1 % IT IS plus and 1 % antibiotic) for overnight incubation. The fetal zone cells were then incubated for 24 h either under basal conditions, with ACTH (10 nM) (Organon, Bedford, OH) or with GnRH1 (10 nM) (Sigma, St. Louis, MO) treatment. The media were collected for steroid assays as described below.
2.8. Steroid Assay
The cortisol, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S) content of experimental medium were determined using EIA kits (Diagnostic System Laboratories, Webster, TX). The assays were conducted following the manufacture recommendation and standard curves were prepared in the experimental cell culture medium. The results are shown as fold change over basal condition.
2.9. Statistical Analysis
Results are given as mean ± SD. Individual experiments were repeated at least three times. Paired t-tests with a confidence interval of 95 % were performed using GraphPad Prism 3.0 (GraphPad Software, Inc. San Diego, CA).
3. Results
3.1. Variation in GPCR expression between fetal and adult adrenals
GPCR are involved in a wide variety of physiological processes, including inflammation, neurotransmission, cell proliferation and migration. Given the important roles of GPCR in development, herein we compared the GPCR expression pattern between pooled adult and fetal adrenal RNA samples using microarray analysis (Fig.1). The most differentially expressed GPCR and their relative expression levels are organized in Table 1 and these six genes were further studied by qPCR with at least five different tissue samples per group. Except for CHRM5, qPCR confirmed higher fetal adrenal expression of all the five other transcripts (Fig.2). Among them, GPR37 had the largest difference compared to adult adrenal which had 1 % of that seen in the fetal adrenal. Interestingly, ATGR2 and MC2R were also expressed lower in adult than fetal adrenal.
Fig. 1. Microarray analysis comparing GPCR expression between fetal adrenal (FA) and adult adrenal (AA).
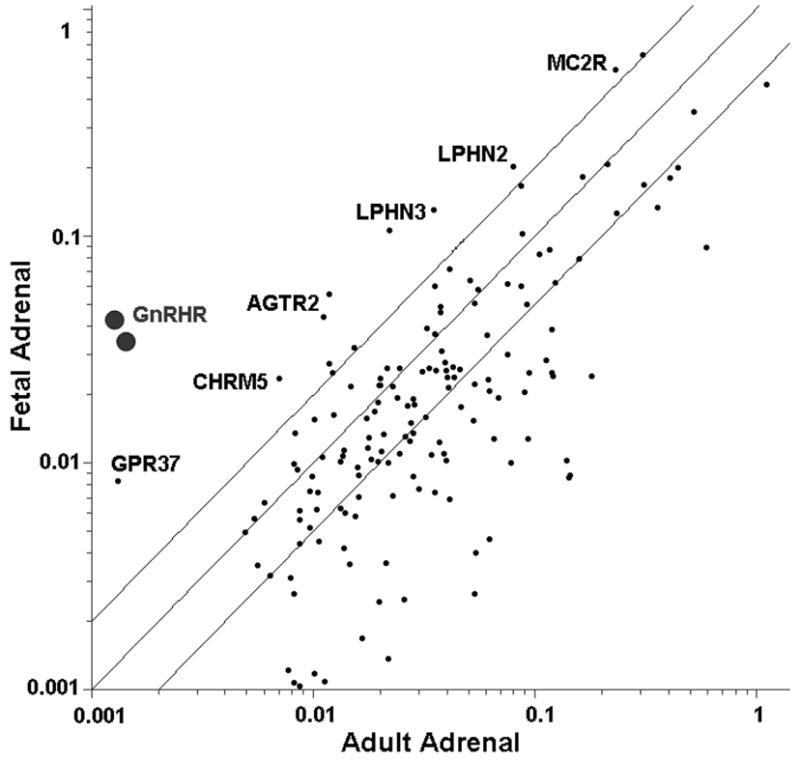
Total RNA from pools of three fetal and three adult adrenals was used for oligonucleotide microarray analysis. The graph represents 165 GPCR that were found to have a signal which indicated that it was present in at least one tissue sample. The transcripts with the highest variation between the FA and AA are labeled.
Table. 1. Differentially expressed GPCR based on microarray analysis.
The common and full name of each gene is given in the first two columns, followed by the fold change between FA and AA on the transcript level. The last column shows the ranking of individual transcripts based on fold difference. Several genes had confirmatory probes on the microarray thus explaining the dual ranking for several of the genes.
| TOP GPCR change FA vs AA | |||
|---|---|---|---|
| Common name | Description | fold | number |
| GNRHR | gonadotropin-releasing hormone receptor | 32.9 | 1,2 |
| GPR37 | G protein coupled receptor 37 | 6.5 | 3 |
| LPHN3 | Latrophilin 3 | 4.8 | 4,7 |
| AGTR2 | Angiotensin II receptor, type 2 | 4.7 | 5, 6 |
| CHRM5 | Cholinergic receptor, muscarinic 5 | 3.4 | 8 |
| LPHN2 | Latrophilin 2 | 2.5 | 9 |
| MC2R | Melanocortin 2 receptor | 2.4 | 10 |
Fig. 2. Quantitative realtime PCR (qPCR) confirmation for the GPCR expressed differently between HFA and AA.
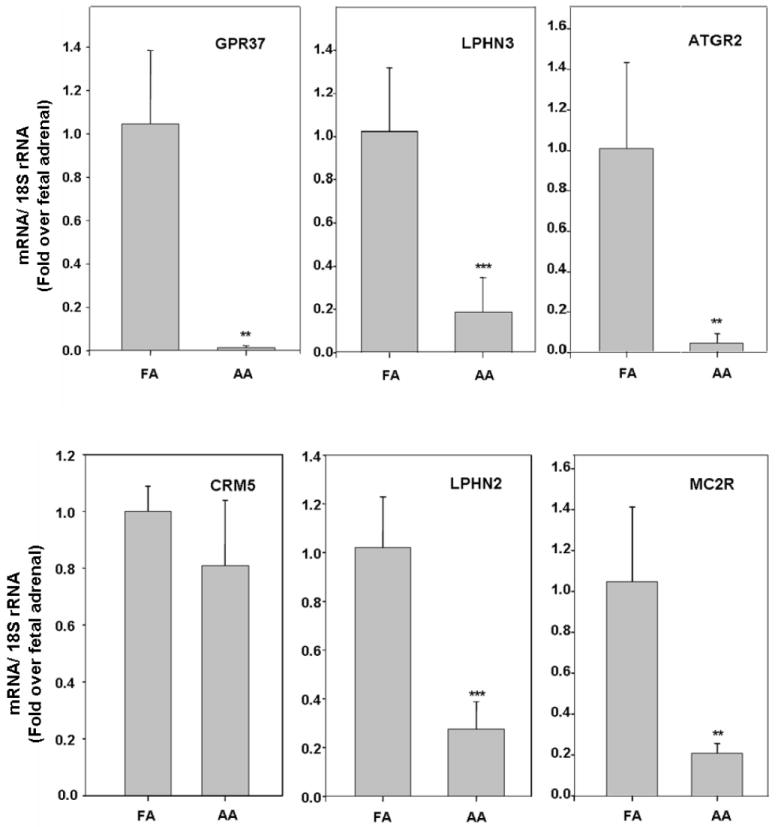
qPCR was performed on adult and fetal adrenal RNA to detect the mRNA expression level of the last six genes listed in Table 1. The results were normalized to 18S rRNA. Data are expressed as fold over fetal adrenal and the mean ± SD of values from at least five different tissue samples each group (**, P<0.01; ***, P< 0.001).
3.2. Expression of GnRHR mRNA in fetal adrenal
To better define the level of expression of GnRHR in fetal adrenal, we compared expression levels in adult and fetal adrenals, adult pituitary (positive control) and placenta. When comparing mRNA levels with qPCR, pituitary gland had the highest level of GnRHR expression. Human fetal adrenal expressed about 20 % of the level of GnRHR seen for pituitary, while modest levels of GnRHR transcript were detected in adult adrenal (about 1.9 % of that seen in fetal adrenal). In addition, GnRHR was not detectable in term placental samples (Fig.3).
Fig. 3. GnRHR mRNA expression in different tissues.
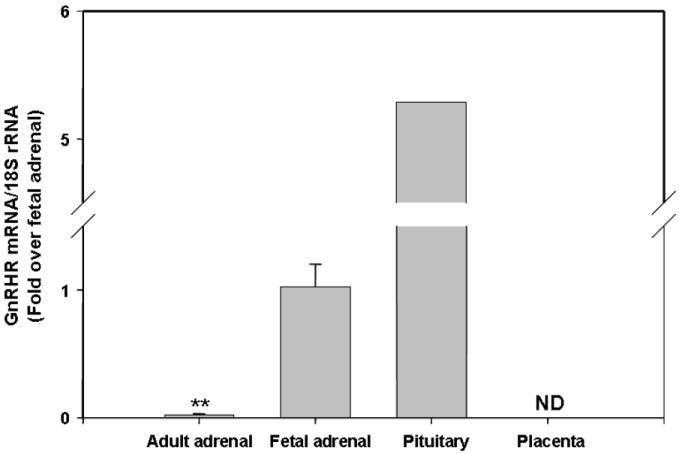
Quantitative PCR was performed on fetal and adult adrenals, pituitary and placenta for GnRHR mRNA. The results were normalized to 18S rRNA as internal control. Data are expressed as fold over fetal adrenal level and are the mean ± SD of values of at least five different tissue samples (**, P<0.01).
3.3. Expression of GnRHR protein in fetal adrenal
Immunohistochemical analysis was performed to determine if GnRHR protein was also expressed in fetal adrenal with human pituitary used as a positive control (Fig. 4A). GnRHR expression was observed in some but not all cells of the pituitary, likely corresponding to the number of gonadotrophs present in the tissue. As noted earlier, the human fetal adrenal has a unique morphology as can be observed following H&E staining (Fig. 4B). The two major regions, the neocortex and fetal zone were easily differentiated. Immunohistochemical analysis of GnRHR suggested a distinct pattern of staining in fetal adrenal compared to the pituitary. GnRHR staining was detected in most fetal adrenal cells, but with variable expression levels. In addition, GnRHR immunoreactivity was also observed in the neocortex zone/ transitional zones (Fig. 4C).
Fig. 4. Immunohistochemistry characterization of GnRHR expression in fetal adrenal.
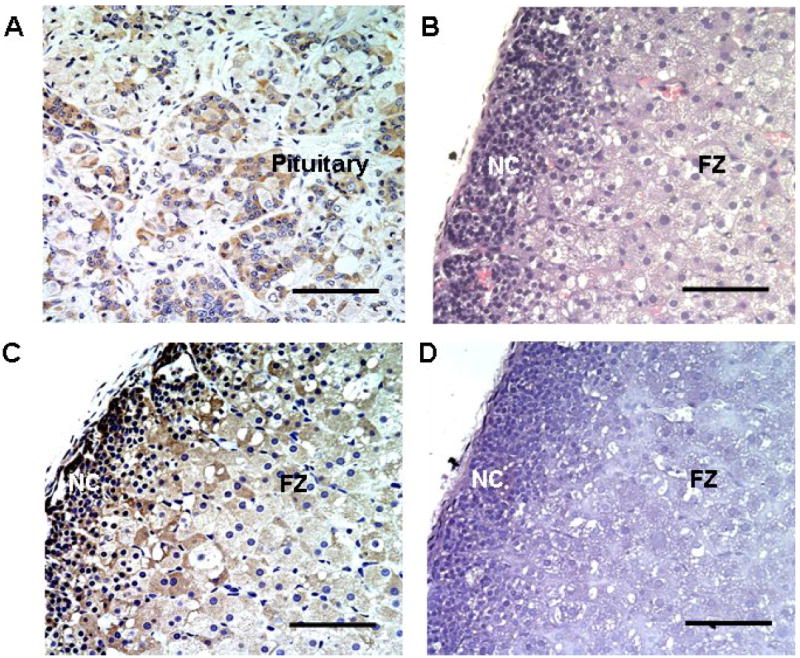
Panel A. Positive control for GnRHR detection using human pituitary. Panel B. H&E staining of HFA showing both neocortex (NC) and fetal zone (FZ). Panel C. GnRHR staining in fetal adrenal gland. GnRHR protein (brown signal) was detectable in most of the neocortex and fetal zone cells. Panel D. Fetal adrenal processed for immunohistochemistry without inclusion of the GnRHR antibody (Negative control). All results are representative of staining from at least three fetal adrenals. Scale bar represents 10 μM in real size.
3.4. Effects of GnRH1 treatment on fetal zone cells in monolayer culture
Although fetal zone cells mainly produce DHEA and DHEA-S under physiological condition, studies have shown that these cells can also produce cortisol when placed in culture (Fujieda et al.,1981; Taga et al.,1981). Our experiments confirmed that 24 h treatment with ACTH increased both DHEA/DHEA-S (5 fold) and cortisol (7.5 fold) production of fetal zone cells. However, 24 h treatment with GnRH1 (10 nM) did not cause any significant changes in steroids production (Fig. 5A). Similar effects were detected with GnRH2 (10 nM) treatment (Data not shown). To better understand the lack of GnRH activity in fetal zone cells, we examined the expression of GnRHR in cultured cells. Interestingly, the expression of GnRHR was reduced dramatically compared to levels seen in the fetal adrenal (Fig. 5B). This may explain loss of GnRH activity in cultured fetal zone cells.
Fig. 5. ACTH and GnRH steroidogenic effects on fetal zone monolayer cultures.
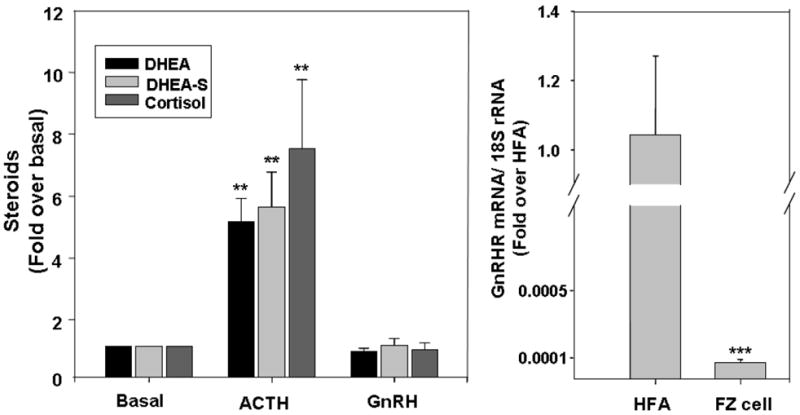
Panel A. Fetal zone cells were plated for 5 day before experiments. Cells were treated with ACTH (10 nM) or GnRH (10 nM) in 1 % serum for 24 h, and medium was collected for steroid assays. Data points are the mean ± SD of values from at least three independent experiments that are expressed as the fold change over basal (**, P < 0.01, compared to basal level). Panel B. Reduction of GnRHR mRNA expression in fetal zone cells after culturing. qPCR on GnRHR transcript was performed on cDNA from fetal adrenal tissues (HFA) or fetal zone cells in culture (FZ). The results were normalized to 18S rRNA as internal control. Data are expressed as fold over human fetal adrenal tissue level and are the mean ± SD of values from at least three different independent experiments (***, P<0.001).
3.5. Expression of GnRH1 and GnRH2 mRNA in fetal adrenal
Because GnRH does not normally circulate in the post-natal circulation, we examined its expression in several fetal tissues to determine if ligands would be available to regulate fetal adrenal activity in an endocrine, autocrine or paracrine pattern. The mRNA levels of GnRH1 and GnRH2 were studied using qPCR (Fig. 6). In adult hypothalamus, which was used as a positive control, GnRH1 was expressed at 8.3 fold above that seen in the fetal adrenal. Interestingly, placental GnRH1 mRNA expression was similar to that seen in the adult hypothalamus, suggesting that this organ could provide GnRH1 to the fetal circulation. Intriguingly, fetal adrenal was the only organ examined that had expression of GnRH2, with levels that were at least 15 fold higher than either the adult adrenal and pituitary or placenta (Fig. 6). This suggests that GnRH2 could act as an autocrine/paracrine hormone in the fetal adrenal.
Fig.6. GnRH1 and GnRH2 mRNA expression levels in different tissues.
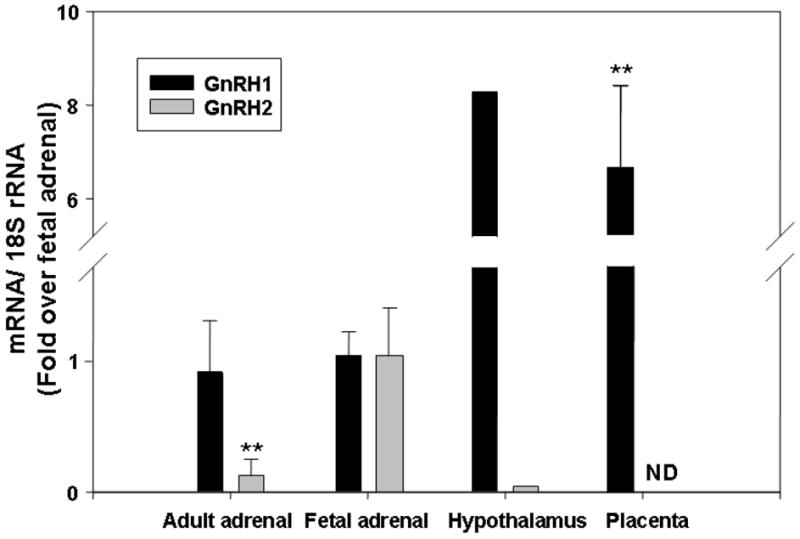
Quantitative PCR was performed to detect GnRH1 and GnRH2 transcript expression in FA, AA, hypothalamus and placenta. All the results were normalized to 18S rRNA as internal control. Data are expressed as fold over fetal adrenal levels and are presented as the mean ± SD of values from at least three different samples (**, P<0.01).
4. Discussion
The fetal adrenal differs from the adult adrenal both physiologically and morphologically. It is characterized by rapid growth and high steroidogenic activity, but the molecular mechanisms underlying these phenomenons remain unclear. In our current study, we focused on the analysis of the GPCR family, which includes many of the receptors involved in the regulation of adrenal, ovarian and gonadal functions. In particular, the type 1 angiotensin II receptor (ATGR1) and MC2R which represent the key receptor regulators of the adult adrenal are also GPCR family members. Numerous studies have shown that a wide variety of GPCR ligands can regulate normal and pathologic adrenal cell steroid production (Catt et al.,1987; N’Diaye et al.,1998; Breault et al.,2000; Lacroix et al.,2000; Gimpl and Fahrenholz,2001; Christopoulos et al.,2004; Nakamura et al.,2007; Romero et al.,2007; Vuorenoja et al.,2007). In addition, some GPCR vary in expression between the adrenocortical zones as well as in adrenal tumors, suggesting the involvement of GPCR in adrenal zonation and tumorigenesis. In this study, we compared the GPCR expression profiles between the fetal and adult adrenals in order to define new candidate regulators of the human fetal adrenal glands. Six GPCR were found to have significant higher levels in fetal vs. adult adrenal glands: GPR37, latrophilin receptors 2 and 3, AGTR2, MCR2, and GnRHR.
GPR37 is an orphan GPCR highly expressed in mammalian brain oligodendrocytes, Purkinje cells, and neurons belonging to the CA3 hippocampal region and to the substantia nigra pars compacta (Marazziti et al.,1998). GPR37 is the substrate of parkin and it has been suggested that the cellular accumulation of an insoluble form of this receptor can result in cell death and thereby contribute to Parkinson’s disease (Imai et al.,2007). Recently, the neuropeptide head activator has been identified as a potential high affinity ligand for GPR37 (Rezgaoui et al.,2006). However, this receptor has not been previously studied in any endocrine tissues. Here we demonstrated a clear difference in the expression of GPR37 between fetal and adult adrenal. Because of the association of this receptor with neuronal tissue, it will be important to determine if fetal adrenal GPR37 are present within the steroidogenic or the developing medullary cells within this gland.
LPHN2 and LPHN3 are two closely related homologs to latrophilin1 with similar domain structure (Ichtchenko et al.,1999). They belong to the subfamily of Ca2+-independent receptors with typical structural components of both GPCR and cell adhesion protein (Krasnoperov et al.,1997; Bittner et al.,1998). While LPHN3 is mainly present in brain, LPHN2 is expressed ubiquitously with the highest levels seen in placenta, kidney, spleen, ovary, heart and lung (Ichtchenko et al.,1999; Matsushita et al.,1999). As for ligands of this GPCR subfamily, α-latrotoxin, a neurotoxic protein from black widow spider, can bind to LPHN2 and LPHN1 with different affinities (Petrenko et al.,1990; Ichtchenko et al.,1999; Rahman et al.,1999). But until now no endogenous ligand has been discovered. Our results indicate that both LPHN2 and 3 are highly expressed in the adrenal gland and the mRNA level is significantly higher in fetal vs. adult adrenal. Given the fact that the placenta secretes a variety of GPCR ligands, the placenta could be a potential source of endogenous ligands for those two receptors.
AGTR2 was first identified in rat adrenal with the help of specific antagonists (Chiu et al.,1989; Whitebread et al.,1989). While ATGR1 predominantly exists in zona glomerulosa and mediates most of the recognized effects of Ang II, the AGTR2 receptor is mainly present in medulla (Balla et al.,1991; Timmermans et al.,1992; Inagami et al.,1995). However, studies conducted by Gallo-Payet and colleges showed that AGTR2 is expressed in the fetal adrenal throughout gestation, while the AGTR1 receptor does not appear until 16 wk and only exists at the periphery of the gland (Breault et al.,1996). The exact function of AGTR2 in the adrenal remains unclear, but results from bovine adrenal cells in culture suggest that cortisol production can be increased through AGTR2 by mechanisms involving protein kinase C as well as calcium or potassium channels (Defaye et al.,1995). In this study, we confirmed the high mRNA level of AGTR2 in the fetal adrenal but further studies are needed to define the role of AGTR2 in adrenal development and steroidogenesis.
MC2R is a GPCR almost exclusively expressed in the adrenal cortex (Vamvakopoulos et al.,1993; Chhajlani,1996). Once activated, MC2R signals through the cAMP-PKA pathway (Forti et al.,2006; Li et al.,2008) and can acutely regulate aldosterone production in zona glomerulosa and cortisol production in zona fasciculata. In contrast to the adult adrenal, the midgestation fetal adrenal responds to ACTH mainly through the production of DHEA-S (Di Blasio and Jaffe,1988; Mesiano and Jaffe,1997). Research conducted in baboon has shown that while the expression level of MC2R is very low in the first trimester, it peaks in midgestation and starts to decline thereafter. But contrary to the declining trend of the whole fetal adrenal, the newly emerging definitive zone seems to express higher levels of MC2R (Albrecht et al.,1996; Aberdeen et al.,1997). Here by applying microarray and qPCR techniques, we showed that human fetal adrenal expressed higher levels of MC2R than the adult adrenal. This may help explain the higher sensitivity to ACTH seen in the fetal adrenal.
It is well established that pituitary GnRHR regulates the production of gonadotropins and thus controls reproductive function. GnRHR gene and its ligands (GnRH1 and GnRH2) have also been detected outside the hypothalamus and pituitary. A growing body of literature supports an extrapituitary role for GnRHR (Harrison et al.,2004; Cheng and Leung,2005; Metallinou et al.,2007). GnRHR mRNA expression is seen in lymphocytes (Chen et al.,1999), breast (Kottler et al.,1997), ovary (Minaretzis et al.,1995; Choi et al.,2006) and prostate (Gnanapragasam et al.,2005; Finch et al.,2008). In addition, there appears to be high expression level of GnRHR in some cancers (Finch et al.,2004; Gnanapragasam et al.,2005; Kim et al.,2006; Sedgley et al.,2006). This is the first report to demonstrate fetal adrenal expression of GnRHR although a previous report showed expression in adrenal tumors (Ye et al.,2007). We were unable to define the role of GnRH in the regulation of fetal adrenal cells due to the fact that isolated fetal adrenal cells rapidly lost GnRHR mRNA expression. We would speculate that this loss of mRNA related to the lack of GnRH effects we observed in fetal adrenal cell culture. However, another possible explanation for the lack of GnRH effect on fetal adrenal cells could be that the receptor expressed in the fetal adrenal is inactive or lacks the appropriate coupling system to steroid production.
Interestingly, GnRHR, GnRH1 and GnRH2 are all expressed in human placenta. While placental expression of GnRH1 is high throughout gestation, GnRH2 mRNA decreases remarkably with gestational age (Lin et al.,1995; Chou et al.,2004). Our term placental qPCR data confirmed the presence of high levels of GnRH1, but no GnRH2. Previous studies have also shown high level of GnRH1 in the fetal circulation and the placenta is the likely source for this hormone (Aksel and Tyrey,1977; Gilmore et al.,1978; Siler-Khodr and Khodr,1978). On the other hand, the fetal adrenal itself expressed high levels of GnRH2, whose cellular site of expression requires to be determined by further analysis. Taken together, these data indicate that within the fetus there is the potential for GnRH regulation of the fetal adrenal, by ligands produced in the placenta and released into the circulation and/or by paracrine/autocrine action of ligands produced in the fetal adrenal.
In summary, we have shown that fetal and adult adrenal glands exhibit similar expression patterns for most GPCR, however six receptors were found to have significantly higher levels of expression in fetal than adult adrenal. Most intriguing was the elevated expression of the GnRHR, which is known to have a role in the regulation of the hypothalamic-pituitary-gonadal axis. The increased expression of GnRHR was coupled to high expression levels of its ligand GnRH within the human placenta and fetal adrenal. While further studies are needed, these findings suggest potential for GnRH regulation of fetal adrenal function.
Acknowledgments
This work was supported by grant from National Institutes of Health T32 HD07190 and R01 HD11149 to WER.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberdeen GW, Babischkin JS, Davies WA, Pepe GJ, Albrecht ED. Decline in adrenocorticotropin receptor messenger ribonucleic acid expression in the baboon fetal adrenocortical zone in the second half of pregnancy. Endocrinology. 1997;138:1634–1641. doi: 10.1210/endo.138.4.5079. [DOI] [PubMed] [Google Scholar]
- Aksel S, Tyrey L. Luteinizing hormone-releasing hormone in the human fetal brain. Fertil Steril. 1977;28:1067–1071. [PubMed] [Google Scholar]
- Albrecht ED, Aberdeen GW, Babischkin JS, Tilly JL, Pepe GJ. Biphasic developmental expression of adrenocorticotropin receptor messenger ribonucleic acid levels in the baboon fetal adrenal gland. Endocrinology. 1996;137:1292–1298. doi: 10.1210/endo.137.4.8625902. [DOI] [PubMed] [Google Scholar]
- Anderson AB, Laurence KM, Davies K, Campbell H, Turnbull AC. Fetal adrenal weight and the cause of premature delivery in human pregnancy. J Obstet Gynaecol Br Commonw. 1971;78:481–488. doi: 10.1111/j.1471-0528.1971.tb00305.x. [DOI] [PubMed] [Google Scholar]
- Balla T, Baukal AJ, Eng S, Catt KJ. Angiotensin II receptor subtypes and biological responses in the adrenal cortex and medulla. Mol Pharmacol. 1991;40:401–406. [PubMed] [Google Scholar]
- Bassett MH, Mayhew B, Rehman K, White PC, Mantero F, Arnaldi G, Stewart PM, Bujalska I, Rainey WE. Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab. 2005;90:5446–5455. doi: 10.1210/jc.2005-0836. [DOI] [PubMed] [Google Scholar]
- Beuschlein F, Keegan CE, Bavers DL, Mutch C, Hutz JE, Shah S, Ulrich-Lai YM, Engeland WC, Jeffs B, Jameson JL, Hammer GD. SF-1, DAX-1, and acd: molecular determinants of adrenocortical growth and steroidogenesis. Endocr Res. 2002;28:597–607. doi: 10.1081/erc-120016972. [DOI] [PubMed] [Google Scholar]
- Bittner MA, Krasnoperov VG, Stuenkel EL, Petrenko AG, Holz RW. A Ca2+-independent receptor for alpha-latrotoxin, CIRL, mediates effects on secretion via multiple mechanisms. J Neurosci. 1998;18:2914–2922. doi: 10.1523/JNEUROSCI.18-08-02914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E, Benirschke K. Synthesis in vitro of steroids by human fetal adrenal gland slices. J Biol Chem. 1959;234:1085–1089. [PubMed] [Google Scholar]
- Bocian-Sobkowska J. Morphometric study of the human suprarenal gland in the first postnatal year. Folia Morphol (Warsz) 2000;58:275–284. [PubMed] [Google Scholar]
- Breault L, Chamoux E, Lehoux JG, Gallo-Payet N. Localization of G protein alpha-subunits in the human fetal adrenal gland. Endocrinology. 2000;141:4334–4341. doi: 10.1210/endo.141.12.7834. [DOI] [PubMed] [Google Scholar]
- Breault L, Lehoux JG, Gallo-Payet N. The angiotensin AT2 receptor is present in the human fetal adrenal gland throughout the second trimester of gestation. J Clin Endocrinol Metab. 1996;81:3914–3922. doi: 10.1210/jcem.81.11.8923838. [DOI] [PubMed] [Google Scholar]
- Catt KJ, Carson MC, Hausdorff WP, Leach-Harper CM, Baukal AJ, Guillemette G, Balla T, Aguilera G. Angiotensin II receptors and mechanisms of action in adrenal glomerulosa cells. J Steroid Biochem. 1987;27:915–927. doi: 10.1016/0022-4731(87)90168-3. [DOI] [PubMed] [Google Scholar]
- Chakraborty P. G-protein-mediated signaling and its control in macrophages and mammalian cells. Crit Rev Microbiol. 2001;27:1–8. doi: 10.1080/20014091096666. [DOI] [PubMed] [Google Scholar]
- Chen A, Kaganovsky E, Rahimipour S, Ben-Aroya N, Okon E, Koch Y. Two forms of gonadotropin-releasing hormone (GnRH) are expressed in human breast tissue and overexpressed in breast cancer: a putative mechanism for the antiproliferative effect of GnRH by down-regulation of acidic ribosomal phosphoproteins P1 and P2. Cancer Res. 2002;62:1036–1044. [PubMed] [Google Scholar]
- Chen A, Yahalom D, Ben-Aroya N, Kaganovsky E, Okon E, Koch Y. A second isoform of gonadotropin-releasing hormone is present in the brain of human and rodents. FEBS Lett. 1998;435:199–203. doi: 10.1016/s0014-5793(98)01064-3. [DOI] [PubMed] [Google Scholar]
- Chen HF, Jeung EB, Stephenson M, Leung PC. Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor gamma-chain messenger ribonucleic acids that are regulated by GnRH in vitro. J Clin Endocrinol Metab. 1999;84:743–750. doi: 10.1210/jcem.84.2.5440. [DOI] [PubMed] [Google Scholar]
- Cheng CK, Leung PC. Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr Rev. 2005;26:283–306. doi: 10.1210/er.2003-0039. [DOI] [PubMed] [Google Scholar]
- Chhajlani V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int. 1996;38:73–80. [PubMed] [Google Scholar]
- Chi L, Zhou W, Prikhozhan A, Flanagan C, Davidson JS, Golembo M, Illing N, Millar RP, Sealfon SC. Cloning and characterization of the human GnRH receptor. Mol Cell Endocrinol. 1993;91:R1–6. doi: 10.1016/0303-7207(93)90278-r. [DOI] [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu AT, Herblin WF, McCall DE, Ardecky RJ, Carini DJ, Duncia JV, Pease LJ, Wong PC, Wexler RR, Johnson AL, et al. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;165:196–203. doi: 10.1016/0006-291x(89)91054-1. [DOI] [PubMed] [Google Scholar]
- Choi JH, Gilks CB, Auersperg N, Leung PC. Immunolocalization of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and type I GnRH receptor during follicular development in the human ovary. J Clin Endocrinol Metab. 2006;91:4562–4570. doi: 10.1210/jc.2006-1147. [DOI] [PubMed] [Google Scholar]
- Chou CS, Beristain AG, MacCalman CD, Leung PC. Cellular localization of gonadotropin-releasing hormone (GnRH) I and GnRH II in first-trimester human placenta and decidua. J Clin Endocrinol Metab. 2004;89:1459–1466. doi: 10.1210/jc.2003-031636. [DOI] [PubMed] [Google Scholar]
- Christopoulos S, Bourdeau I, Lacroix A. Aberrant expression of hormone receptors in adrenal Cushing’s syndrome. Pituitary. 2004;7:225–235. doi: 10.1007/s11102-005-1083-7. [DOI] [PubMed] [Google Scholar]
- Defaye G, Lecomte S, Chambaz EM, Bottari SP. Stimulation of cortisol production through angiotensin AT2 receptors in bovine fasciculata cells. Endocr Res. 1995;21:183–187. doi: 10.3109/07435809509030433. [DOI] [PubMed] [Google Scholar]
- Dhom G. The prepuberal and puberal growth of the adrenal (adrenarche) Beitr Pathol. 1973;150:357–377. doi: 10.1016/s0005-8165(73)80086-1. [DOI] [PubMed] [Google Scholar]
- Di Blasio AM, Jaffe RB. Adrenocorticotropic hormone does not induce desensitization in human adrenal cells during fetal life. Biol Reprod. 1988;39:617–621. doi: 10.1095/biolreprod39.3.617. [DOI] [PubMed] [Google Scholar]
- Finch AR, Green L, Hislop JN, Kelly E, McArdle CA. Signaling and antiproliferative effects of type I and II gonadotropin-releasing hormone receptors in breast cancer cells. J Clin Endocrinol Metab. 2004;89:1823–1832. doi: 10.1210/jc.2003-030787. [DOI] [PubMed] [Google Scholar]
- Finch AR, Sedgley KR, Caunt CJ, McArdle CA. Plasma membrane expression of GnRH receptors: regulation by antagonists in breast, prostate, and gonadotrope cell lines. J Endocrinol. 2008;196:353–367. doi: 10.1677/JOE-07-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti FL, Dias MH, Armelin HA. ACTH receptor: ectopic expression, activity and signaling. Mol Cell Biochem. 2006;293:147–160. doi: 10.1007/s11010-006-9237-0. [DOI] [PubMed] [Google Scholar]
- Fujieda K, Faiman C, Reyes FI, Winter JS. The control of steroidogenesis by human fetal adrenal cells in tissue culture. I. Responses to adrenocorticotropin. J Clin Endocrinol Metab. 1981;53:34–38. doi: 10.1210/jcem-53-1-34. [DOI] [PubMed] [Google Scholar]
- Gilmore DP, Dobbie HG, McNeilly AS, Mortimer CH. Presence and activity of LH-RH in the mid-term human fetus. J Reprod Fertil. 1978;52:355–359. doi: 10.1530/jrf.0.0520355. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gnanapragasam VJ, Darby S, Khan MM, Lock WG, Robson CN, Leung HY. Evidence that prostate gonadotropin-releasing hormone receptors mediate an anti-tumourigenic response to analogue therapy in hormone refractory prostate cancer. J Pathol. 2005;206:205–213. doi: 10.1002/path.1767. [DOI] [PubMed] [Google Scholar]
- Harrison GS, Wierman ME, Nett TM, Glode LM. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr Relat Cancer. 2004;11:725–748. doi: 10.1677/erc.1.00777. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Bittner MA, Krasnoperov V, Little AR, Chepurny O, Holz RW, Petrenko AG. A novel ubiquitously expressed alpha-latrotoxin receptor is a member of the CIRL family of G-protein-coupled receptors. J Biol Chem. 1999;274:5491–5498. doi: 10.1074/jbc.274.9.5491. [DOI] [PubMed] [Google Scholar]
- Imai Y, Inoue H, Kataoka A, Hua-Qin W, Masuda M, Ikeda T, Tsukita K, Soda M, Kodama T, Fuwa T, Honda Y, Kaneko S, Matsumoto S, Wakamatsu K, Ito S, Miura M, Aosaki T, Itohara S, Takahashi R. Pael receptor is involved in dopamine metabolism in the nigrostriatal system. Neurosci Res. 2007;59:413–425. doi: 10.1016/j.neures.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Inagami T, Yamano Y, Bardhan S, Chaki S, Guo DF, Ohyama K, Kambayashi Y, Takahashi K, Ichiki T, Tsuzuki S, et al. Cloning, expression and regulation of angiotensin II receptors. Adv Exp Med Biol. 1995;377:311–317. doi: 10.1007/978-1-4899-0952-7_21. [DOI] [PubMed] [Google Scholar]
- Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupled receptor target family. ChemMedChem. 2006;1:761–782. doi: 10.1002/cmdc.200600134. [DOI] [PubMed] [Google Scholar]
- Kah O, Lethimonier C, Lareyre JJ. Gonadotrophin-releasing hormone (GnRH) in the animal kingdom. J Soc Biol. 2004;198:53–60. [PubMed] [Google Scholar]
- Kim KY, Choi KC, Auersperg N, Leung PC. Mechanism of gonadotropin-releasing hormone (GnRH)-I and -II-induced cell growth inhibition in ovarian cancer cells: role of the GnRH-I receptor and protein kinase C pathway. Endocr Relat Cancer. 2006;13:211–220. doi: 10.1677/erc.1.01033. [DOI] [PubMed] [Google Scholar]
- Kostenis E. G proteins in drug screening: from analysis of receptor-G protein specificity to manipulation of GPCR-mediated signalling pathways. Curr Pharm Des. 2006;12:1703–1715. doi: 10.2174/138161206776873734. [DOI] [PubMed] [Google Scholar]
- Kottler ML, Starzec A, Carre MC, Lagarde JP, Martin A, Counis R. The genes for gonadotropin-releasing hormone and its receptor are expressed in human breast with fibrocystic disease and cancer. Int J Cancer. 1997;71:595–599. doi: 10.1002/(sici)1097-0215(19970516)71:4<595::aid-ijc14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, Bittner MA, Beavis R, Kuang Y, Salnikow KV, Chepurny OG, Little AR, Plotnikov AN, Wu D, Holz RW, Petrenko AG. alpha-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron. 1997;18:925–937. doi: 10.1016/s0896-6273(00)80332-3. [DOI] [PubMed] [Google Scholar]
- La Rosa S, Celato N, Uccella S, Capella C. Detection of gonadotropin-releasing hormone receptor in normal human pituitary cells and pituitary adenomas using immunohistochemistry. Virchows Arch. 2000;437:264–269. doi: 10.1007/s004280000247. [DOI] [PubMed] [Google Scholar]
- Lacroix A, N’Diaye N, Mircescu H, Tremblay J, Hamet P. The diversity of abnormal hormone receptors in adrenal Cushing’s syndrome allows novel pharmacological therapies. Braz J Med Biol Res. 2000;33:1201–1209. doi: 10.1590/s0100-879x2000001000010. [DOI] [PubMed] [Google Scholar]
- Li LA, Xia D, Wei S, Hartung J, Zhao RQ. Characterization of adrenal ACTH signaling pathway and steroidogenic enzymes in Erhualian and Pietrain pigs with different plasma cortisol levels. Steroids. 2008;73:806–814. doi: 10.1016/j.steroids.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Lin LS, Roberts VJ, Yen SS. Expression of human gonadotropin-releasing hormone receptor gene in the placenta and its functional relationship to human chorionic gonadotropin secretion. J Clin Endocrinol Metab. 1995;80:580–585. doi: 10.1210/jcem.80.2.7852524. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Gallo A, Golini E, Matteoni R, Tocchini-Valentini GP. Molecular cloning and chromosomal localization of the mouse Gpr37 gene encoding an orphan G-protein-coupled peptide receptor expressed in brain and testis. Genomics. 1998;53:315–324. doi: 10.1006/geno.1998.5433. [DOI] [PubMed] [Google Scholar]
- Marecki B. Sexual dimorphism of the weight of internal organs in fetal ontogenesis. Anthropol Anz. 1989;47:175–184. [PubMed] [Google Scholar]
- Matsushita H, Lelianova VG, Ushkaryov YA. The latrophilin family: multiply spliced G protein-coupled receptors with differential tissue distribution. FEBS Lett. 1999;443:348–352. doi: 10.1016/s0014-5793(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- Metallinou C, Asimakopoulos B, Schroer A, Nikolettos N. Gonadotropin-releasing hormone in the ovary. Reprod Sci. 2007;14:737–749. doi: 10.1177/1933719107310707. [DOI] [PubMed] [Google Scholar]
- Minaretzis D, Jakubowski M, Mortola JF, Pavlou SN. Gonadotropin-releasing hormone receptor gene expression in human ovary and granulosa-lutein cells. J Clin Endocrinol Metab. 1995;80:430–434. doi: 10.1210/jcem.80.2.7852501. [DOI] [PubMed] [Google Scholar]
- N’Diaye N, Tremblay J, Hamet P, De Herder WW, Lacroix A. Adrenocortical overexpression of gastric inhibitory polypeptide receptor underlies food-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83:2781–2785. doi: 10.1210/jcem.83.8.5038. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Aoki S, Xing Y, Sasano H, Rainey WE. Metastin stimulates aldosterone synthesis in human adrenal cells. Reprod Sci. 2007;14:836–845. doi: 10.1177/1933719107307823. [DOI] [PubMed] [Google Scholar]
- Parker JD, Malik M, Catherino WH. Human myometrium and leiomyomas express gonadotropin-releasing hormone 2 and gonadotropin-releasing hormone 2 receptor. Fertil Steril. 2007;88:39–46. doi: 10.1016/j.fertnstert.2006.11.098. [DOI] [PubMed] [Google Scholar]
- Parker LN, Sack J, Fisher DA, Odell WD. The adrenarche: prolactin, gonadotropins, adrenal androgens, and cortisol. J Clin Endocrinol Metab. 1978;46:396–401. doi: 10.1210/jcem-46-3-396. [DOI] [PubMed] [Google Scholar]
- Peng C, Fan NC, Ligier M, Vaananen J, Leung PC. Expression and regulation of gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acids in human granulosa-luteal cells. Endocrinology. 1994;135:1740–1746. doi: 10.1210/endo.135.5.7956897. [DOI] [PubMed] [Google Scholar]
- Petrenko AG, Kovalenko VA, Shamotienko OG, Surkova IN, Tarasyuk TA, Ushkaryov Yu A, Grishin EV. Isolation and properties of the alpha-latrotoxin receptor. Embo J. 1990;9:2023–2027. doi: 10.1002/j.1460-2075.1990.tb08331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA, Ashton AC, Meunier FA, Davletov BA, Dolly JO, Ushkaryov YA. Norepinephrine exocytosis stimulated by alpha-latrotoxin requires both external and stored Ca2+ and is mediated by latrophilin, G proteins and phospholipase C. Philos Trans R Soc Lond B Biol Sci. 1999;354:379–386. doi: 10.1098/rstb.1999.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WE, Rehman KS, Carr BR. The human fetal adrenal: making adrenal androgens for placental estrogens. Semin Reprod Med. 2004;22:327–336. doi: 10.1055/s-2004-861549. [DOI] [PubMed] [Google Scholar]
- Rehman KS, Sirianni R, Parker CR, Jr, Rainey WE, Carr BR. The regulation of adrenocorticotrophic hormone receptor by corticotropin-releasing hormone in human fetal adrenal definitive/transitional zone cells. Reprod Sci. 2007;14:578–587. doi: 10.1177/1933719107307908. [DOI] [PubMed] [Google Scholar]
- Rezgaoui M, Susens U, Ignatov A, Gelderblom M, Glassmeier G, Franke I, Urny J, Imai Y, Takahashi R, Schaller HC. The neuropeptide head activator is a high-affinity ligand for the orphan G-protein-coupled receptor GPR37. J Cell Sci. 2006;119:542–549. doi: 10.1242/jcs.02766. [DOI] [PubMed] [Google Scholar]
- Riopel L, Branchaud CL, Goodyer CG, Zweig M, Lipowski L, Adkar V, Lefebvre Y. Effect of placental factors on growth and function of the human fetal adrenal in vitro. Biol Reprod. 1989;41:779–789. doi: 10.1095/biolreprod41.5.779. [DOI] [PubMed] [Google Scholar]
- Romero DG, Zhou MY, Yanes LL, Plonczynski MW, Washington TR, Gomez-Sanchez CE, Gomez-Sanchez EP. Regulators of G-protein signaling 4 in adrenal gland: localization, regulation, and role in aldosterone secretion. J Endocrinol. 2007;194:429–440. doi: 10.1677/JOE-07-0153. [DOI] [PubMed] [Google Scholar]
- Sedgley KR, Finch AR, Caunt CJ, McArdle CA. Intracellular gonadotropin-releasing hormone receptors in breast cancer and gonadotrope lineage cells. J Endocrinol. 2006;191:625–636. doi: 10.1677/joe.1.07067. [DOI] [PubMed] [Google Scholar]
- Shepard TH, Shi M, Fellingham GW, Fujinaga M, FitzSimmons JM, Fantel AG, Barr M. Organ weight standards for human fetuses. Pediatr Pathol. 1988;8:513–524. doi: 10.3109/15513818809022307. [DOI] [PubMed] [Google Scholar]
- Siler-Khodr TM, Khodr GS. Content of luteinizing hormone-releasing factor in the human placenta. Am J Obstet Gynecol. 1978;130:216–219. doi: 10.1016/0002-9378(78)90369-1. [DOI] [PubMed] [Google Scholar]
- Somoza GM, Miranda LA, Strobl-Mazzulla P, Guilgur LG. Gonadotropin-releasing hormone (GnRH): from fish to mammalian brains. Cell Mol Neurobiol. 2002;22:589–609. doi: 10.1023/A:1021888420271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Mesiano S, Lee JY, Jaffe RB. Proliferation and apoptosis in the human adrenal cortex during the fetal and perinatal periods: implications for growth and remodeling. J Clin Endocrinol Metab. 1999;84:1110–1115. doi: 10.1210/jcem.84.3.5513. [DOI] [PubMed] [Google Scholar]
- Taga M, Tanaka K, Liu T, Minaguchi H, Sakamoto S. Effect of prolactin on the secretion of dehydroepiandrosterone (DHEA), its sulfate (DHEA-S), and cortisol by the human fetal adrenal in vitro. Endocrinol Jpn. 1981;28:321–327. doi: 10.1507/endocrj1954.28.321. [DOI] [PubMed] [Google Scholar]
- Timmermans PB, Chiu AT, Herblin WF, Wong PC, Smith RD. Angiotensin II receptor subtypes. Am J Hypertens. 1992;5:406–410. doi: 10.1093/ajh/5.6.406. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Rojas K, Overhauser J, Durkin AS, Nierman WC, Chrousos GP. Mapping the human melanocortin 2 receptor (adrenocorticotropic hormone receptor; ACTHR) gene (MC2R) to the small arm of chromosome 18 (18p11.21-pter) Genomics. 1993;18:454–455. doi: 10.1006/geno.1993.1499. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. Plasma levels and secretion rate of steroids with anabolic activity in man. Environ Qual Saf. 1976;(Suppl):171–180. [PubMed] [Google Scholar]
- Vuorenoja S, Rivero-Muller A, Kiiveri S, Bielinska M, Heikinheimo M, Wilson DB, Huhtaniemi IT, Rahman NA. Adrenocortical tumorigenesis, luteinizing hormone receptor and transcription factors GATA-4 and GATA-6. Mol Cell Endocrinol. 2007;269:38–45. doi: 10.1016/j.mce.2006.11.013. [DOI] [PubMed] [Google Scholar]
- White RB, Eisen JA, Kasten TL, Fernald RD. Second gene for gonadotropin-releasing hormone in humans. Proc Natl Acad Sci U S A. 1998;95:305–309. doi: 10.1073/pnas.95.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitebread S, Mele M, Kamber B, de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;163:284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- Ye P, Mariniello B, Mantero F, Shibata H, Rainey WE. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol. 2007;195:39–48. doi: 10.1677/JOE-07-0037. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Oktem M, Yilmaz AO. Fetal and maternal adrenal steroid levels and labor. Int J Gynaecol Obstet. 2004;85:274–275. doi: 10.1016/j.ijgo.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Yin H, Cheng KW, Hwa HL, Peng C, Auersperg N, Leung PC. Expression of the messenger RNA for gonadotropin-releasing hormone and its receptor in human cancer cell lines. Life Sci. 1998;62:2015–2023. doi: 10.1016/s0024-3205(98)00173-8. [DOI] [PubMed] [Google Scholar]


