Abstract
Studies using rodents have shown that behavioral responses to a stimulant are enhanced when the stimulant is given within the same context as previous stimulant administrations; this increase in effect related to context is often referred to as sensitization. We examined the role of environmental stimuli in modulating the subjective and cardiovascular effects of cocaine in humans 1) within a daily “binge” and 2) after cocaine abstinence. Ten non-treatment seeking users of smoked cocaine were admitted to the hospital for 17 consecutive days. Participants smoked cocaine (25 mg/dose) under two counterbalanced conditions: paired stimuli (same stimuli presented each session) and unpaired stimuli (varied stimuli presented each session). Under each stimulus condition, participants had cocaine test sessions for three consecutive days, no sessions for the next three days, then another cocaine test session on the following day, for a total of eight test days. Stimulus condition had no effect on cardiovascular or subjective effects so data were analyzed as a function of repeated cocaine administration over two weeks. Maximal ratings on “good drug” and “drug rating” subjective effects clusters decreased over days of repeated cocaine exposure. In contrast, baseline and peak heart rate and systolic pressure increased over days of repeated cocaine administration. Thus, repeated administration of smoked cocaine to experienced cocaine users resulted in increases in baseline blood pressure and heart rate and modest decreases in positive subjective effects. These data indicate modest tolerance rather than sensitization to the positive subjective effects of cocaine with repeated exposure.
Keywords: cocaine, stimuli, cardiovascular effects, sensitization, subjective effects, tolerance
1. Introduction
Rodents repeatedly administered a stimulant often show a greater behavioral and neural response to that stimulant than rodents who are naive to that stimulant (see Ohmori et al., 2000; Robinson et al., 1998 for review). This phenomenon, usually referred to as sensitization, has received extensive attention as a possible model for human vulnerability to drug abuse (e.g., Vezina, 2007). Further, data obtained in rodents suggest that environmental cues play a role in sensitization (see Ohmori et al., 2000; Robinson et al., 1998 for review), such that sensitization is greatest when drug is presented in a context previously associated with drug use (Carey et al., 2003; Crombag et al., 2000; see Leyton, 2007 for review). Badiani et al. (1995) demonstrated that sensitization to cocaine occurred both when the drug was given in a novel context and when given in the context that it had been given before, but the effects of cocaine were greatest when it was given in the environment previously paired with cocaine. This finding is similar to reports that amphetamine-induced sensitization was greater when administration occurred in a context previously paired with amphetamine (Chinen et al., 2006) and when an amphetamine challenge was given in a context-specific situation (Anagnostaras & Robinson, 1996). Additionally, when rats were given cocaine in an environment previously paired with cocaine, the behavioral effects were greater compared to when cocaine was administered in an environment not previously paired with cocaine (Carey & Gui, 1998).
Although an increase in some of the effects of stimulants with experience with the context it was given in has been well documented in rodents, it has been difficult to observe a similar phenomenon in humans (see Sax & Strakowski, 2001 for review). One study with cocaine abusers failed to show an enhanced cardiovascular and subjective response to cocaine when it was given in a room where they had previously received the drug (Rothman et al., 1994). However, several studies, including ours (Foltin & Haney, 2000), have reported increases in measures such as ratings of “I Want Cocaine” (Foltin & Haney, 2000), blood pressure (Cascella et al., 1989; Foltin & Haney, 2000) and heart rate (Foltin & Haney, 2000; Nagoshi et al., 1992) following placebo administration when placebo was given in a room where participants had previously received cocaine. Further, in a recent imaging study in cocaine abusers, cocaine craving was elicited following a methylphenidate “priming” dose only if it was accompanied by cocaine-related cues (Volkow et al., 2008). This evidence suggests that drug-conditioned cues play an important role in cocaine craving, and perhaps other effects of cocaine as well.
The current study examined the role of cocaine-related stimuli in modulating the subjective and cardiovascular effects of cocaine in humans 1) within a daily “binge” and 2) after a brief (3-day) cocaine abstinence. Participants smoked cocaine in the morning and afternoon for several days in a row while exposed to one of two stimulus conditions, the order of which was counterbalanced across participants. Under one condition, participants were exposed to specific, consistent stimulus conditions (paired-cue condition). Under the second condition, participants were exposed to a variety of stimulus conditions (unpaired-cue condition). We used neutral stimuli (e.g., presence of a specific colored towel) because we wanted to assess change in the response to repeatedly pairing these novel stimuli with cocaine, that we may not have been able to see if we used stimuli that would typically have been paired with cocaine in prior use (e.g., a crack vial). We hypothesized that the subjective and cardiovascular response to smoked cocaine would 1) increase across separate days of a binge only within the paired stimuli condition and 2) be greater after a period of cocaine abstinence within the paired stimuli condition.
2. Methods
2.1. Participants
Nine male and one female African-American research volunteers, 29 to 45 years of age (mean = 41.2 years) and with an average of 12.7 ± 1.3 (mean ± S.D.) years of education, participated in this study. Three additional participants were enrolled in the protocol: two participants left for personal reasons and one participant was discontinued due to the occurrence of premature ventricular contractions during baseline data collection.
To be eligible, participants had to smoke cocaine at least twice a week and spent at least $70 a week on cocaine for the past six months. All participants were cocaine-positive on urine toxicology tests during screening. Participants reported using cocaine predominately by the smoked route, on average, for the past 15.5 ± 6.4 years, using cocaine 3.3 ± 1.1 days per week, and spending US $245 ± 69 per week on crack cocaine (the cost of cocaine was about US $30/g in the New York City area when these data were collected). Eight participants met DSM-IV criteria for cocaine dependence or abuse. No participant met DSM-IV criteria for dependence on any other illicit drug or alcohol. Nine participants smoked an average of 8.8 ± 6.0 tobacco per day; one participant did not smoke tobacco. All participants passed an extensive medical evaluation prior to the study, and none were receiving psychiatric treatment. None were seeking treatment for their drug use, and none were using hormonal contraceptives, or any other prescription medication. Each signed a consent form, approved by the Institutional Review Board of The New York State Psychiatric Institute, which described the study, outlined the possible risks, and indicated that cocaine would be administered. As is customary for our studies, participants were paid for their participation at the end of the protocol in multiple weekly payments not exceeding a value of $300 each week. By receiving limited weekly payments, participants could not spend all of their study earnings on cocaine upon discharge. This payment schedule also allowed us to monitor our participants over several weeks following their inpatient stay.
2.2. Procedure
The participants were admitted to the Irving Institute for Clinical and Translational Research in the Presbyterian Hospital. Their private hospital rooms were equipped with a television, radio, and DVD player; DVD movies were provided to them. The rooms contained an air purification system, and the participants were free to smoke tobacco cigarettes in their rooms. Visitors were prohibited. Urine samples were collected each morning and rooms could be searched to enforce compliance with study procedures.
Each participant was exposed to two different stimulus conditions during laboratory sessions: (1) Paired stimuli (stimuli remained the same across laboratory sessions); and (2) Unpaired stimuli (stimuli varied each laboratory session). Stimulus conditions were counterbalanced across the first and second phases of the study. Beginning the day after inpatient admission, participants engaged in laboratory sessions twice each day for 3 days, had no sessions for 3 days then had two sessions on the following day under the Paired or Unpaired stimuli condition (Table 1). This pattern of sessions was repeated a second time under the other stimuli condition (Paired or Unpaired) immediately after the first stimuli condition sessions were completed. The same dose of cocaine (25 mg/dose) was tested during each laboratory session. Participants smoked 6 doses of cocaine per session in order to mimic the binge pattern by which cocaine is abused (Foltin et al., 2003).
Table 1.
Schedule of Laboratory Sessions
| Phase 1 | Phase 2 | |||||
|---|---|---|---|---|---|---|
| Day 1–3 | Day 4–6 | Day 7 | Day 8 | Day 9–11 | Day 12–14 | Day 15 |
| Cocaine Sessions | No Sessions | Cocaine Sessions | No Sessions | Cocaine Sessions | No Sessions | Cocaine Sessions |
2.3. Experimental sessions
There were two laboratory sessions on days when cocaine was available (Table 1): the first session each day began at 0900 h, and the second session each day began at 1500 h. Each session lasted approximately 2 hr. Between sessions, participants were escorted back to their rooms on the research unit for lunch.
During experimental sessions, participants were seated in a reclining chair in front of a Macintosh computer and video monitor with a mouse manipulandum. A 22-gauge catheter (Quik-Cath, Travenol Laboratories, Deerfield, IL) was inserted in a subcutaneous vein in one arm as a safety measure in case of a medical emergency during cocaine administration. Electrocardiograms (ECGs) were monitored continuously with chest electrodes (MAC PC, Marquette Electronics, Milwaukee, WI), and heart rate (HR) and blood pressure (systolic, SBP; diastolic, DBP) were recorded every 2 min (Sentry II-Model 6100 automated vital signs monitor, NBS Medical, Costa Mesa, CA) beginning 20 min prior to cocaine administration. A Macintosh computer located in an adjacent control room was used for automated data collection. The participants were monitored via a one-way mirror by a physician and research nurse (both ACLS-certified) located in the adjacent room, with whom they could communicate via an intercom system.
Each session consisted of six trials during which participants smoked crack cocaine (25 mg/dose) at 14 min intervals. Participants were told that they would smoke up to six doses of cocaine each session. Because participants were instructed to smoke all doses, this was not a self-administration procedure, and participants could choose to refuse to smoke a dose of cocaine. However, no participant refused a dose. During cocaine administration, participants were blindfolded to prevent dose-related visual cues (e.g., amount of crack being presented). Cocaine base in a glass pipe fitted with a screen (“stem”) was handed to the participant by the study nurse with instruction to take one large inhalation and to hold the inhalation as long as he or she normally would outside of the laboratory. Vaporization of the cocaine base was accomplished by holding the flame from a butane lighter above the cocaine base in the pipe. Cocaine was not given on any trial in which blood pressure and heart rate exceeded the threshold for safe drug administration [SBP > 160 mm Hg, DBP > 100 mm Hg, HR > (220 – subject age × 0.85)]. Each session ended 30 min after the last cocaine delivery.
2.4. Proximal Stimuli
Proximal stimuli consisted of visual, olfactory, and auditory stimuli. Specifically, a blue or yellow towel was draped on the computer screen in front of the participant, participants wore a hospital facemask that contained either a peppermint- or orange-scented tea bag and they listened to either New Age music or a set of tones used to test speaker function. The duration of the stimulus presentations was 2 min prior to smoking cocaine. During paired stimuli sessions, the same set of stimuli (e.g., same color towel, scented tea bag and auditory material) was presented together prior to each administration of cocaine. During unpaired stimuli sessions, varied stimuli (e.g., different colored towel, scented tea bag and auditory material) were presented together prior to each administration of cocaine. Half of the participants experienced the paired stimuli first and half experienced the unpaired stimuli first.
2.5. Subjective Effects Questionnaire
A computerized subjective effects battery was completed prior to the first cocaine dose (baseline), 4 min after each cocaine dose was delivered, and again 15 min after the last cocaine dose of the session. The battery consisted of a series of 100 mm visual analog scales (VASs) anchored by “not at all” (0 mm) at one end and “extremely” (100 mm) at the other end. To reduce the number of dependent variables, our laboratory has applied cluster analysis for those VASs, which measure the subjective effects of cocaine (e.g., Evans et al., 2002; Foltin & Haney, 2004). Five clusters of cocaine-related VASs have been noted, with the changes in one item being predictive of changes in the other items in the same cluster, but not predictive of changes in items in the other clusters (Evans et al., 2002). In all, 20 VASs were grouped into the following five independent clusters: ‘Bad drug effect’ consisted of seven items related to negative drug effects (e.g., ‘bad drug effect’, ‘anxious’), ‘self-esteem’ consisted of five items (e.g., ‘self-confident’, ‘social), ‘calm’ consisted of two items (‘calm’ and ‘able to concentrate’), ‘good drug effect’ consisted of three items (‘high’, ‘good drug effect’, and ‘stimulated’), and ‘drug rating’ consisted of three items related to the cocaine dose the participant had just received (‘drug quality’, ‘drug potency’, and ‘drug liking’). Four VAS were used to operationalize drug craving, and were labeled ‘I want…’ ‘…cocaine’, ‘…heroin’, ‘…ethanol’, and ‘…nicotine’. A final question asked the participants ‘How much would you pay for the dose you just received?’ with a response range of $0–25.
2.6. Cocaine
Cocaine base, derived from cocaine hydrochloride, was prepared in pellets of 25 mg by the New York State Psychiatric Institute Pharmacy, as previously described (Foltin et al., 1990).
2.7. Data analysis
For one participant, one cocaine dose during one morning session was withheld due to elevated blood pressure. Because this was the only dose out of the 96 total possible doses that was not given to that individual, the data from this session were still included in the analyses rather than excluding all of the data from this participant. Due to technical difficulties, the last unpaired session was not included in the data analysis for one participant. To include the data from this participant, plausible estimates of the missing values were calculated using multiple imputation (Rubin & Schenker, 1991).
Eight time points were calculated for the cardiovascular measures by obtaining the mean of data collected in 10 min bins: − 14 to − 4 min before the first dose, 2 – 12 min after each dose and 14 – 24 mins after the last dose. Each VAS was collected once before the first cocaine dose, after each cocaine dose, and twice after the last cocaine dose, for a total of nine measurements within a session. Cardiovascular and VAS data were analyzed separately.
For all measures, we originally used a repeated-measures ANOVA with Stimulus Condition (paired first vs. unpaired first), Day (days 1 – 15) and Time of Day (AM vs. PM) as the within-subjects factors for baseline and peak ratings. Because we found only a few increases in effects in the afternoon session compared to the morning session, we collapsed the data across time of day. Further, because we found no difference between stimuli conditions, but found a Stimulus Condition by Day interaction, we then analyzed the data in order, using an ANOVA with Day (days 1 – 3, 7, 9 – 11 and 15) as the within-subjects factors for baseline and peak ratings, with stimulus condition as a covariate. The latter results are presented here. To further determine whether the changes in cardiovascular effects were due to changes in baseline measures, we analyzed the data in order, using an ANOVA with Day (days 1 – 3, 7, 9 – 11 and 15) as the within-subjects factors for area under the curve change score, with stimulus condition as a covariate. Planned comparisons were used to compare day 1 to all other days. The planned comparisons were single degree of freedom comparisons that used the error term for Session Day. The results were considered statistically significant at p ≤ 0.05.
Results
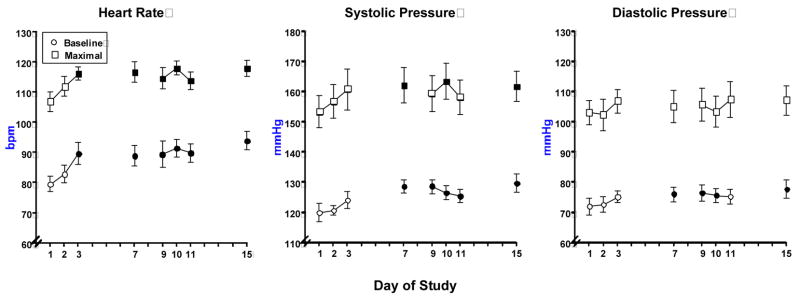
Figure 1 portrays baseline and maximal heart rate and blood pressure parameters across days, compared to day 1, independent of stimuli condition. Heart rate and blood pressure increased during the study. There was a significant increase in baseline heart rate with all days (except day 2) significantly greater than day 1 (p = 0.0001). Baseline systolic pressure was significantly greater on days 7 – 15 compared to day 1 (p ≤ 0.03). Baseline diastolic pressure was significantly greater on days 7–10 and day 15 compared to day 1 (p ≤ 0.04).
Figure 1.
Mean baseline and maximal responses of heart rate, systolic pressure and diastolic pressure in response to cocaine (25 mg) as a function of day. Bars represent the mean + 1 S.E.M. Closed symbols represent a significant difference from Day 1 for baseline and peak ratings.
Figure 1 also shows that there was a significant increase in peak heart rate on all days (except day 2) compared to day 1 (p ≤ 0.02). Further, peak systolic pressure was significantly greater during days 7, 10 and 15 than on day 1 (p ≤ 0.05). There were no significant differences in peak diastolic pressure across days.
There were no significant effects of day in the area under the curve change score for heart rate or blood pressure, indicating that the increases observed in heart rate were due to baseline increases.
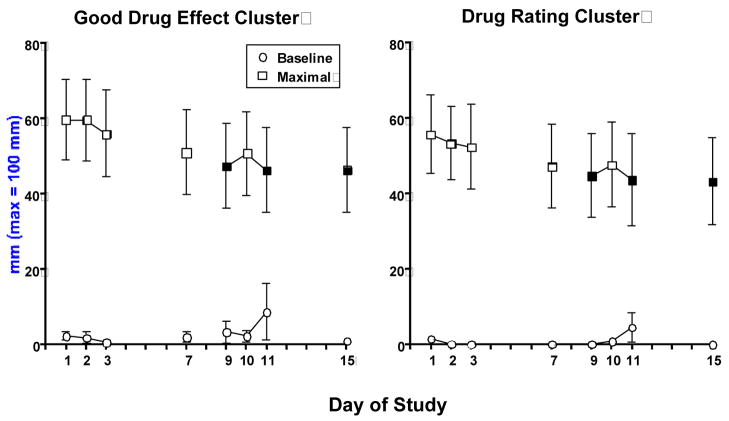
Figure 2 portrays selected baseline and maximal subjective effects across days, compared to day 1, independent of stimulus condition. Baseline ratings of the Bad Drug Effect cluster were significantly lower on days 2, 3, 9 and 15 than on day 1 (p ≤ 0.03; data not shown). There were no effects of day on any other baseline subjective effects ratings. In contrast to cardiovascular measures, peak subjective ratings decreased during the study. Peak ratings on the Good Drug Effect cluster were significantly less on days 9, 11 and 15 than on day 1 (p ≤ 0.03). Further, peak scores on the Drug Rating cluster were significantly less on days 9, 11 and 15 than on day 1 (p ≤ 0.04). Peak ratings on the Bad Drug Effect cluster were small but significantly less on all days (range: 14.0 – 16.0 mm) except day 2 compared to day 1 (p ≤ 0.04; mean ± SEM: 18.0 ± 5.0 mm). There were no significant effects of day on ratings of “I Want Cocaine,” “How much would you pay for the dose you just received?” or the self-esteem or calm cluster.
Figure 2.
Mean baseline and maximal scores on ratings of the Good Drug Cluster and Drug Rating cluster in response to cocaine (25 mg) as a function of day. Bars represent the mean + 1 S.E.M. Closed symbols represent a significant difference from Day 1 for baseline and peak ratings.
Discussion
In our human laboratory model of an intermittent cocaine binge, blood pressure and heart rate during baseline and after cocaine administration increased across study days. In contrast, the positive subjective effects of cocaine decreased across days. No effect of stimulus condition was observed. This shows that there were changes in the effects of cocaine after repeated cocaine administration in the same laboratory context, regardless of whether it was in the presence of proximal stimuli that were consistently paired with cocaine or proximal stimuli that were not consistently paired with cocaine. Change in self-report across study days was largely specific to a decrease in positive subjective effects since there were little or no changes across days on other subjective-effects measures. Although we observed a significant decrease in the peak ratings on the Bad Drug Effect cluster, the changes were slight.
In the current study, the increase in maximal cardiovascular effects of cocaine, mostly during the first week, at first glance appears to parallel the reports of sensitization to drug effects in rodents. For example, in rats, the cardiovascular effects of cocaine after repeated cocaine administration increased across days and after days of abstinence (Tella et al., 1991). Further, Walsh et al. (2000) found an increase in cardiovascular effects in response to i.v. doses of cocaine in humans. However, in the current study, there was also a significant increase in baseline heart rate and blood pressure, and no difference in the area under the curve change score for cardiovascular effects. This suggests that participants did not have a sensitized heart rate and blood pressure response to cocaine but rather a conditioned response (increased baseline) to the stimuli previously associated with cocaine administration (e.g., laboratory room, staff, etc.). Other studies in humans have shown that baseline cardiovascular effects were increased on a test day following repeated exposure to i.v. cocaine in the same room (Cascella et al., 1989; Nagoshi et al., 1992). The fact that participants’ cardiovascular effects were already increased prior to cocaine administration may have been a result of anticipation and the knowledge that they were about to receive cocaine (expectancy effects). This increase in cardiovascular effects peaked within a few days, and did not increase further during the second week of cocaine exposure. It is possible that this peak effect occurred quickly as cocaine users continued to use cocaine within the same laboratory context. Given that there was an increase in resting heart rate and blood pressure baseline without an increase in the size of the effect of cocaine, we conclude that this is not evidence of sensitization.
In contrast to the increase observed in cardiovascular effects, the positive subjective effects of cocaine showed modest decreases, mainly across the first study week, suggestive of tolerance. This is supported by prior work from our laboratory that showed tolerance to cocaine’s positive subjective effects across days of “binge” cocaine use (Ward et al., 1997). Further, acute tolerance to the positive subjective effects in response to cocaine has been observed within a single session (Foltin & Fischman, 1991; Foltin & Haney, 2004). However, other studies in humans have found small or no changes in the subjective effects of stimulants across repeated administration (e.g., Wachtel & de Wit, 1999; Rothman et al., 1994). In a clinical retrospective study, Bartlett et al. (1997) concluded that sensitization occurred to the psychosis-inducing effects of cocaine, but not to the positive subjective effects of cocaine. However, this was based on participants describing how they felt using cocaine during the laboratory session compared to their first use of cocaine outside of the laboratory and retrospective self-report may not be reliable. With respect to our study, it is possible that “sensitization” may not be relevant to long-term heavy users in that if sensitization does occur, it may occur early in an individual’s cocaine use history.
The dissociation between cardiovascular and subjective effects in humans has been observed in a previous study, where there was an increase in the cardiovascular effects of oral cocaine, but no corresponding increase in the subjective effects (Kollins & Rush, 2002). Thus, the dissociation between the effects of cocaine on subjective ratings and cardiovascular measures was not entirely unexpected. The mechanism by which cocaine produces positive subjective effects has yet to be fully determined, but is likely related to the mesolimbic dopaminergic system (Breiter et al., 1997). However, the mechanism by which cocaine increases cardiovascular effects is most likely related to the adrenergic system (Tella et al., 1991) and thus involves a different neural system than that associated with the subjective effects.
Interestingly, we did not see an effect of proximal stimuli condition (i.e., colored towel, music and odor) in the current study; we observed changes in cocaine’s effects across days regardless of whether participants were exposed to paired or unpaired stimuli first. Thus, the laboratory, or something other than the specific proximal stimuli set used in this study, was predictive of changes in heart rate and blood pressure baseline. In rats, cues previously paired with i.v. cocaine maintained cocaine responding during placebo administration (Panlilio et al., 2005). There are few data examining the effects of conditioning on the response to cocaine in humans and most studies have examined the response to cocaine-paired stimuli in the presence of placebo or no drug. For example, in our previous study, cues previously paired with smoked cocaine produced increases in the subjective effects “Anxious,” “Tired” and “I Want Cocaine” and cardiovascular effects during placebo administration (Foltin & Haney, 2000). Thus, it appears that cocaine-associated stimuli can increase the subjective effects of cocaine compared to stimuli that are not associated with cocaine, whether in the presence of cocaine, placebo or no drug. A key difference between the previous studies and the present study is that the previous studies specifically paired one set of stimuli with cocaine and another set of stimuli with placebo. Proximal stimuli are effective when different stimuli are paired with cocaine and placebo. In the current study two sets of proximal stimuli were associated with cocaine, and there was no placebo pairing. Under these circumstances, being in the laboratory, or something other than the proximal stimuli set used in this study, was predictive of the increase in baseline blood pressure and heart rate.
The proximal stimuli we used in the study (i.e., colored towels, music and odors) were specifically designed to be distinct from stimuli associated with typical “street” drug use. We may have seen an increased subjective response to proximal stimuli if we had used stimuli from the participants’ natural drug-using environment. However, the fact that the stimulus categories were similar for each phase (e.g., always a towel, etc.), regardless of their detailed properties (e.g., color, scent, etc.), most likely made coming to the laboratory more salient. The expectation of receiving cocaine, coming to the laboratory rooms for sessions and being in the study itself were likely the most salient stimuli in this protocol.
There were several limitations to this study. First, if we had presented proximal stimuli for longer during a session or in more sessions, had a longer abstinence phase, or used more salient stimuli, we may have observed conditioning to the proximal stimuli. Second, we did not readily have access to cardiovascular effects on days when there were no sessions. Thus, we could not determine if the increases observed during baseline were due to being in the laboratory or if they remained elevated even outside of the laboratory. Third, we did not repeat the stimulus presentations with different doses of cocaine, thus we do not know if we would have observed greater effects of cocaine in response to stimuli paired with other doses of cocaine. Fourth, we did not use a placebo control, so it is not known whether different changes would have been observed in subjective and cardiovascular effects if the laboratory was consistently paired with placebo. Fifth, it is also possible that since our participants were drug users with long histories of cocaine use, changes in the subjective effects of cocaine across the duration of this study may have been difficult to observe.
In conclusion, experienced non-treatment seeking cocaine users had increased maximal cardiovascular response to cocaine upon repeated dosing across days. However, this increase was due to an increase in baseline blood pressure and heart rate in a manner suggesting that these increases in blood pressure and heart rate were a result of participants coming to the laboratory for cocaine sessions (i.e., a consistent context). In contrast, there were minimal changes in baseline subjective effects but evidence for modest tolerance development across sessions to the maximal positive subjective effects of cocaine. This divergence suggests that individuals may become somewhat tolerant to the subjective effects of cocaine, even while developing a greater cardiovascular baseline, when cocaine is taken in a consistent context. Although we did not observe any adverse physical consequences under the controlled conditions of the current study, such dissociation between subjective effects and heart rate and blood pressure could increase the risk of adverse cardiovascular effects during a cocaine binge outside of the laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: Modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Badiani A, Browman KE, Robinson TE. Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res. 1995;674:291–298. doi: 10.1016/0006-8993(95)00028-o. [DOI] [PubMed] [Google Scholar]
- Bartlett E, Hallin A, Chapman B, Angrist B. Selective sensitization to the psychosis-inducing effects of cocaine: a possible marker for addiction relapse vulnerability? Neuropsychopharmacology. 1997;16:77–82. doi: 10.1016/S0893-133X(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Gui J. Cocaine conditioning and cocaine sensitization: what is the relationship? Behav Brain Res. 1998;92:67–76. doi: 10.1016/s0166-4328(97)00126-5. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E. Cocaine-conditioned behavioral effects: a role for habituation processes. Pharmacol Biochem Behav. 2003;74:701–712. doi: 10.1016/s0091-3057(02)01072-9. [DOI] [PubMed] [Google Scholar]
- Cascella N, Muntaner C, Kumor KM, Nagoshi CT, Jaffe JH, Sherer MA. Cardiovascular responses to cocaine placebo in humans: A preliminary report. Biol Psychiatry. 1989;25:285–295. doi: 10.1016/0006-3223(89)90176-5. [DOI] [PubMed] [Google Scholar]
- Chinen CC, Faria RR, Frussa-Filho R. Characterization of the rapid-onset type of behavioral sensitization to amphetamine in mice: role of drug-environment conditioning. Neuropsychopharmacology. 2006;31:151–159. doi: 10.1038/sj.npp.1300789. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 1989;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Smoked and intravenous cocaine in humans: acute tolerance, cardiovascular and subjective effects. J Pharmacol Exp Ther. 1991;257:247–261. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Nestadt G, Stromberger H, Cornell EE, Pearlson GD. Demonstration of naturalistic methods for cocaine smoking by human volunteers. Drug Alcohol Depend. 1990;26:145–154. doi: 10.1016/0376-8716(90)90121-t. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology. 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Intranasal cocaine in humans: acute tolerance, cardiovascular and subjective effects. Pharmacol Biochem Behav. 2004;78:93–101. doi: 10.1016/j.pbb.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Ward AS, Haney M, Hart CL, Collins ED. The effects of escalating doses of smoked cocaine in humans. Drug Alcohol Depend. 2003;70:149–157. doi: 10.1016/s0376-8716(02)00343-5. [DOI] [PubMed] [Google Scholar]
- Kollins SH, Rush CR. Sensitization to the cardiovascular but not subject-rated effects of oral cocaine in humans. Biol Psychiatry. 2002;51:143–150. doi: 10.1016/s0006-3223(01)01288-4. [DOI] [PubMed] [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Nagoshi C, Kumor KM, Muntaner C. Test–retest stability of cardiovascular and subjective responses to intravenous cocaine in humans. Br J Addict. 1992;87:591–599. doi: 10.1111/j.1360-0443.1992.tb01961.x. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Abekawa T, Ito K, Koyama T. Context determines the type of sensitized behaviour: a brief review and a hypothesis on the role of environment in behavioural sensitization. Behav Pharmacol. 2000;11:211–221. doi: 10.1097/00008877-200006000-00005. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Yasar S, Nemeth-Coslett R, Katz JL, Henningfield JE, Solinas M, Heishman SJ, Schindler CW, Goldberg SR. Human Cocaine-Seeking Behavior and its Control by Drug-Associated Stimuli in the Laboratory. Neuropsychopharmacology. 2005;30:433–443. doi: 10.1038/sj.npp.1300599. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Brownman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Gorelick DA, Baumann MH, Guo XY, Herning RI, Pickworth WB, Gendron TM, Koeppl B, Thomson LE, III, Henningfield JE. Lack of evidence for context-dependent cocaine-induced sensitization in humans: Preliminary studies. Pharmacol Biochem Behav. 1994;49:583–588. doi: 10.1016/0091-3057(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Rubin DB, Schenker N. Multiple imputation in health-care data bases: an overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM. Behavioral sensitization in humans. J Addict Dis. 2001;20:55–65. doi: 10.1300/J069v20n03_06. [DOI] [PubMed] [Google Scholar]
- Tella SR, Schindler CW, Goldberg SR. Rapid sensitization to the cardiovascular effects of cocaine in rats. Eur J Pharmacol. 1991;194:119–122. doi: 10.1016/0014-2999(91)90133-b. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization, drug addiction and psychopathology in animals and humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1553–1555. doi: 10.1016/j.pnpbp.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel SR, de Wit H. Subjective and behavioral effects of repeated d-amphetamine in humans. Behav Pharmacol. 1999;10:271–281. doi: 10.1097/00008877-199905000-00004. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Haberny KA, Bigelow GE. Modulation of intravenous cocaine effects by chronic oral cocaine in humans. Psychopharmacology. 2000;150:361–373. doi: 10.1007/s002130000439. [DOI] [PubMed] [Google Scholar]
- Ward AS, Haney M, Fischman MW, Foltin RW. Binge cocaine self-administration by humans: smoked cocaine. Behav Pharmacol. 1997;8:736–744. doi: 10.1097/00008877-199712000-00009. [DOI] [PubMed] [Google Scholar]