Abstract
The retinal activity for vision requires a precise synaptic connectivity. Shank proteins at postsynaptic sites of excitatory synapses play roles in signal transmission into the postsynaptic neuron. However, the correlation of Shank 2 expression with neuronal differentiation in the developing retina remains to be elucidated regardless of previous evidences of Shank 2 expression in retina. Herein, we demonstrated that with progression of development, Shank 2 is initially detected in the inner plexiform layer at P2, and then intensively detected in inner plexiform layer, outer plexiform layer, and ganglion cell layer at P14, which was closely colocalized to the neurofilament expression. Shank 2 was, however, not colocalized with glial fibrillary acidic protein. Shank 2 expression was increased in the differentiated retinoblastoma cells, which was mediated by ERK 1/2 activation. Moreover, Shank 2 expression was colocalized with neurofilament at the dendritic region of cells. In conclusion, our data suggests that Shank 2 is expressed in the neurons of the developing retina and could play a critical role in the neuronal differentiation of the developing retina.
Keywords: cell differentiation; extracellular signal-regulated MAP kinases; neurons; retina; SHANK 2 protein, human
Introduction
The retinal activity for vision requires a precise synaptic connectivity and an appropriate balance between excitatory and inhibitory synapses. The retina is well-organized into three distinct nuclear layers containing neuronal cell bodies and two distinct plexiform layers of synaptic contacts. The visual signals proceed through the nuclear layers, which are delivered and modulated by extensive synapses within the plexiform layers.
The proper functioning of the neuronal networks is regulated by the balance in excitatory and inhibitory synapses between two neurons with intrinsic properties (MacLean et al., 2003; Turrigiano et al., 2004). The most of these synapses occur at contacts between presynaptic axons and postsynaptic dendrites, which predominantly use glutamate as the excitatory neurotransmitter. In the excitatory synapses, cardinal components of the postsynaptic specialization including glutamate receptors are gathered in a structure known as the postsynaptic density (PSD) (Sheng, 2001).
Of PSD proteins, Shank is a scaffold protein to contain a PDZ domain that binds to the C terminus of PSD-95-associated protein GKAP, a proline-rich region that binds to cortactin, and a SAM domain that mediates multimerization (Naisbitt et al., 1999). Shank 1, Shank 2, and Shank 3 constitute a family of proteins, which can be generated by alternative splicing or different assignments of translational start site (Lim et al., 1999). The Shank proteins are differentially expressed in different regions and at different developmental stages. Shank 1 and Shank 2 are mainly expressed in brain and Shank 3 is abundant in heart and moderate in brain and spleen (Lim et al., 1999; Yao et al., 1999). Shank proteins expressed in early developmental stages are related to the development of central nervous systems (Petralia et al., 2005). In addition, Shank has been known to function as a molecular scaffold to induce the neuronal differentiation and synaptogenesis (Sala et al., 2001; Roussignol et al., 2005; Gerrow et al., 2006). Although it was reported that Shank 2 is expressed in retina synapses (Brandstätter et al., 2004), the correlation of Shank 2 expression with neuronal differentiation in the developing retina remains to be elucidated.
Herein, we demonstrated Shank 2 is expressed in the developing retina, which is related to the neuronal differentiation. Shank 2 was colocalized with neurofilament of neuronal differentiation marker, but not with glial fibrillary acidic protein (GFAP) of an astrocyte-specific marker. In addition, Shank 2 was up-regulated with neuronal differentiation mediated by ERK 1/2 activation, which was also colocalized with neurofilament at the dendritic region of the differentiated retinoblastoma cells. Therefore, our data suggests that Shank 2 may play a critical role in the neuronal differentiation of the developing retina.
Results
Shank 2 expression in the developing retina
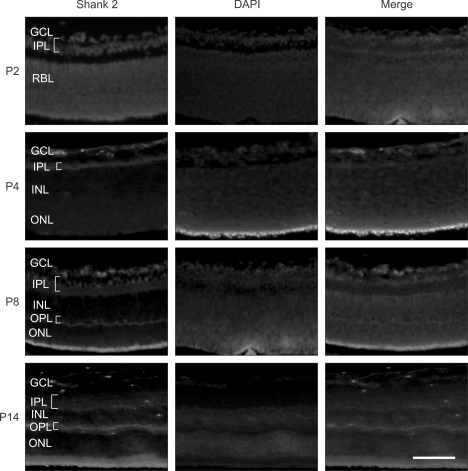
To investigate Shank 2 expression in the developing retina, fluorescence immunohistochemistry was performed (Figure 1). In normal retina of mouse, just after birth, the retina is composed of two layers of neuronal cells (ganglion cell layer, GCL and retinoblast layer, RBL) and one plexiform layer between nuclear layers. At P2, Shank 2 was detected in GCL including nerve fiber layer and IPL, where the synapses of ganglion cells, bipolar cells and amacrine cells are mainly formed. With differentiation of retina, RBL differentiates into two layers of inner nuclear layer, INL, and outer nuclear layer, ONL. From P3-4, the retina has three nuclear layer of GCL, INL, and ONL, and two plexiform layers of inner plexiform layer, IPL, and outer plexiform layer, OPL (Vecino et al., 2004). At P4, Shank2 was also detected in IPL and the newly dividing region which would later become OPL, where the most synapses of photoreceptor, horizontal cells, and bipolar cells are formed. At P8, when three distinct nuclear layers and two distinct plexiform layers were almost divided, Shank 2 was intensively expressed in IPL and OPL. At P14, Shank 2 was also strongly expressed in GCL, as well as IPL and OPL. Also, shank2 was expressed in inner or outer segments of photoreceptor. These results suggest that Shank 2 expression might be related to neuronal differentiation in the developing retina.
Figure 1.
Shank 2 expression in the developing retina. In the developing retina at P2, P4, P8, and P14, retinal sections of 2 disc diameter supero-temporal from the optic disc in the developing retina were evaluated. The immunohistochemistry for Shank 2 (red) was performed, and nuclei were labeled (blue) with DAPI. Each figure is representative ones from six independent experiments. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RBL, retinoblast layer. Scale bar, 200 µm.
Colocalization of Shank 2 with neurofilament in the developing retina
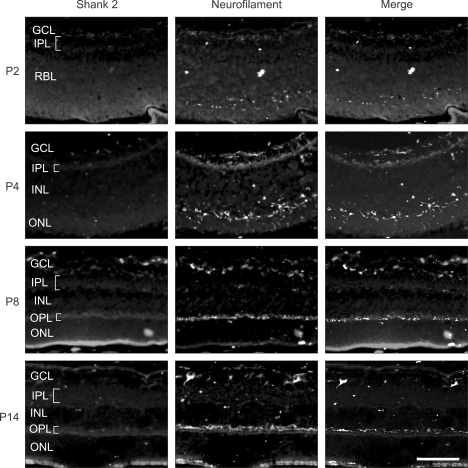
To address the correlation of Shank 2 expression with neuronal differentiation in developing retina, colocalization of Shank 2 with neurofilament, a neuronal differentiation marker, was evaluated by double immunofluorescence staining (Figure 2). At P2, neurofilament was detected mainly in GCL and IPL, and freckly within RBL, where would be OPL. With differentiation of retina, from P4, neurofilament was also detected in OPL as well as IPL and GCL. Interestingly, Shank 2 was colocalized with neurofilament through all the development stages. Although shank2 staining is very diffuse in all retinal layers, shank2 is colocalized with neurofilament. It seems that shank2 expresses basically in the retina and it is strongly localized in the dendrites regions. Considered that neurofilament is to be critical in neuronal differentiation, these results suggest that Shank 2 might be involved in neuronal differentiation in the developing retina.
Figure 2.
Colocalization of Shank 2 with neurofilament in the developing retina. In the developing retina at P2, P4, P8, and P14, retinal sections of 2 disc diameter supero-temporal from the optic disc in the developing retina were evaluated. The immunohistochemistry for Shank 2 (red) and neurofilament (green) was performed. Each figure is representative ones from six independent experiments. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RBL, retinoblast layer. Scale bar, 200 µm.
Shank 2 expression in the retinal astrocytes
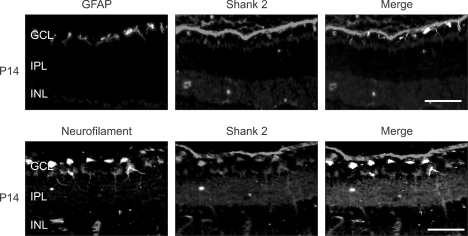
In the central nervous system including brain and retina, the astrocyte regulates synaptic transmission and neurovascular coupling, as same as the neuronal cell (Haydon et al., 2006). Therefore, we checked whether Shank 2 is also expressed in the retinal astrocyte (Figure 3). Shank 2 was detected in IPL, OPL, and GCL, whereas GFAP, an astrocyte marker, was detected only in the outermost region of GCL. However, Shank 2 was not colocalized with GFAP in GCL. Theses data indicate that Shank 2 is primarily expressed in neuronal cells rather than astrocytes of the retina.
Figure 3.
Shank 2 expression in the retinal astrocytes. In the developing retina on P14, retinal sections of 2 disc diameter supero-temporal from the optic disc in the developing retina were evaluated. The immunohistochemistry for Shank 2 (red), neurofilament (green), and GFAP (green) was performed. Each figure is representative ones from six independent experiments. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer. Scale bar, 500 µm.
Shank 2 expression in neuronal differentiation via ERK 1/2 activation
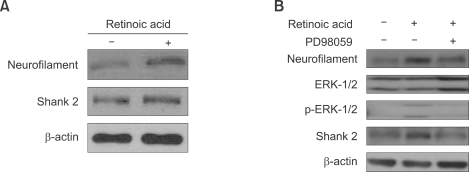
To determine whether Shank 2 expression is related to the neuronal differentiation via ERK 1/2 activation, we assessed Shank 2 expression in differentiated retinoblastoma cells treated with all-trans retinoic acid (Kim et al., 2007a) and addressed the relationship with ERK 1/2 activation (Jung et al., 2006). As shown in Figure 4A, with treatment of all-trans retinoic acid (10 µM) in SNUOT-Rb1 cells, the expression of neurofilament and Shank 2 were increased together. To confirm the relationship of Shank 2 expression with neuronal differentiation via ERK 1/2 activation, we investigate whether the inhibition of ERK 1/2 blocks the differentiation of retinoblastoma cells (Figure 4B). With treatment of all-trans retinoic acid, Shank 2 expression was increased, which was accompanied by the phosphorylations of ERK1/2. SNUOT-Rb1 cells treated with 50 µM PD98059, an inhibitor of MEK-1, and 10 µM all-trans retinoic acid inhibited the phosphorylation of ERK 1/2 and the expression of Shank 2. These data suggest that Shank 2 expression is related to the neuronal differentiation via ERK 1/2 activation.
Figure 4.
Shank 2 expression in neuronal differentiation via ERK 1/2 activation. SNUOT-Rb1 cells were treated with 10 µM retinoic acid or 50 µM PD98059, a specific inhibitor of MEK-1. Shank 2, neurofilament, ERK 1/2 and phosphor-ERK 1/2 were detected by Western blotting analysis. Data are representative of at least three independent experiments. β-actin was served as a loading control.
Colocalization of Shank 2 with neurofilament at the contacts of outgrowing neurites
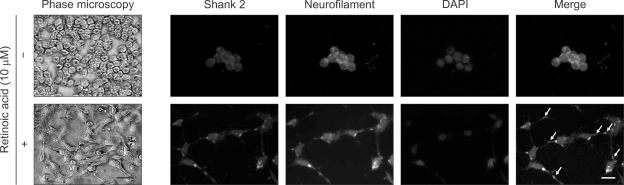
To investigate the localization of Shank 2 in the differentiated retinoblastoma cells, we performed immunocytochemistry for Shank2 and neurofilament (Figure 5). While Shank 2 was diffusely expressed around the nucleus in the undifferentiated cells, it was strongly expressed at the contacts of outgrowing neuritis in the differentiated cells. Interestingly, Shank 2 was colocalized with neurofilament in the differentiated retinoblastoma cells, as similar as in the developing retina (Figure 2). These data provide that Shank 2 increased in neuronal differentiation is colocalized with neurofilament at the contacts of outgrowing neuritis.
Figure 5.
Colocalization of Shank 2 with neurofilament at the contacts of outgrowing neuritis. SNUOT-Rb1 cells were treated with 10µM retinoic acid. Neuronal differentiation with retinoic acid was addressed by the morphological changes of neurite extensions. The immunocytochemistry for Shank 2 (red) and neurofilament (green) was performed, and nuclei were labeled (blue) with DAPI. Arrows indicate the colocalization of Shank 2 and neurofilament at the dendritic region of cells. Each figure is representative ones from six independent experiments. Scale bar, 10 µm.
Discussion
The synaptogenesis is a critical event in the development of the neuronal retina. Although spontaneous activity through gap junctions occurs in the retina before first chemical synapses appear (Cook et al., 1995; Wong et al., 1998), the vertical pathway to carry the main visual information from photoreceptors via bipolar cells to ganglion cells is composed of chemical synapses (Maslim et al., 1986). Therefore, chemical transmission via neurotransmitters is the major form of signal flow in the retina. The main neurotransmitters in the vertebrate retina are glutamate, GABA and glycine (Brandstätter et al., 1998; Wässle et al., 1998). The differential expression of neurotransmitter receptors among retinal neurons significantly adds to functional diversity for visual information (Yang, 2004).
Shank proteins are major components of the postsynaptic density and display several proteinprotein interaction domains (Sheng et al., 2000), which are the master scaffolding proteins in excitatory postsynaptic region (Sheng, 2001). Glutamate is the principal excitatory neurotransmitter in retinal synaptic circuitry (Brandstätter et al., 1998). By linking various glutamate receptors and intracellular signaling proteins, Shank enhances the signal transmission and regulates the differentiation or synaptogenesis of neuron (Ozma et al., 1998; Sala et al., 2001; Roussignol et al., 2005; Gerrow et al., 2006). Among three members of the Shank family, we focused our studies on Shank 2 because the role of Shank 2 in neuronal differentiation of the developing retina remains to be elucidated though the expression of Shank 2 in retina synapses has been known (Brandstätter et al., 2004).
In the present study, we demonstrated Shank 2 is expressed in the developing retina, which is related to the neuronal differentiation. Shank 2 was initially detected by P2 in the inner plexiform layer. As development progressed, Shank 2 was intensely expressed in inner plexiform layer, outer plexiform layer, and ganglion cell layer with emergence of the outer plexiform layer and neuronal maturation. Interestingly, Shank 2 is expressed in the plexiform layers even before the completion of plexiform layer formation, and also closely related with neurofilament expression during all the developmental stages of retina. Based on the facts that synapses of retinal neurons are formed in the plexiform layers and the expression of neurofilament is critical in neuronal differentiation, our results suggest that Shank 2 might be involved in the neuronal development and differentiation in retina. Shank 2 was colocalized exclusively with neurofilament of neuronal differentiation marker, but not with GFAP of an astrocyte-specific marker. With differentiation of retinoblastoma cells, Shank 2 was up-regulated with neuronal differentiation mediated by ERK 1/2 activation. Moreover, Shank 2 expression was colocalized with neurofilament at the contacts of outgrowing neurites from differentiated retinoblastoma cells.
In conclusion, our data suggests that Shank 2 is expressed in the neurons of developing retina and could play a critical role in the neuronal differentiation of the developing retina. In the future, further studies about the coordinated regulation of Shank 2 and other neurotrophic factors such as neurotrophins and insulin growth factor-1 (Bovolenta et al., 1996; Frade et al., 1996) in the retina could help not only to understand the mechanism of retinal neuronal differentiation and synapsis to join individual neurons into a functional network of the retina, but also to develop therapeutic approaches to restore disrupted neuronal differentiation in various retinal degenerations.
Methods
Animals
C57BL/6 mice were purchased from Samtako (Korea). Care, use, and treatment of all animals in this study were in strict agreement with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6 mice were kept in the condition of a standard 12-h dark light cycles and in around 23℃ of room temperature. Eyes for normal retinal development experiments were enucleated from C57BL/6 mice sacrificed on P2, P4, P8, and P14. Each group contained 10 animals.
Cell culture
The human retinoblastoma cells, SNUOT-Rb1, which was newly established by our group (Kim et al., 2007a, b), were cultured in RPMI-1640 containing penicillin-streptomycin (10 ml/l, Gibco BRL, Invitrogen, CA) and 10% FBS (Gibco BRL, Invitrogen, CA) in 5% CO2 incubator at 37℃. The culture medium was changed every third day. To induce differentiation of cells, all-trans retinoic acid (10 µM) was supplied into the culture media up to 10 days. The cultured cells were observed daily under a phase-contrast microscope.
Immunohistochemistry
The enucleated mouse eyes used for immunohistochemistry were immersion fixed in 4% formaldehyde for 15 min at room temperature and subsequently submerged into O.C.T. compound (Sakura Finetechnical Co. Ltd. Tokyo, Japan) followed by freezing at -80℃. 8 µm-thick serial sections were prepared from frozen blocks. The sections were incubated with the primary antibodies in a humidified chamber overnight. The following primary antibodies were used; goat anti-Shank 2 (1:100, Santa-Cruz Biotechnology, Santa Cruz, CA), rabbit anti-neurofilament (1:100, Chemicon, Temecula, CA), rabbit anti-GFAP (1:100, Dako, Sanfrancisco, CA). Alexa Fluor 546 donkey anti-goat IgG (1:400, Molecular probes, Eugene, OR), Alexa Fluor 488 donkey anti-rabbit IgG (1:400, Molecular probes, Eugene, OR) were used as secondary antibodies. The nuclei were stained with 4', 6-diamidino-2-phenolindole (DAPI, Sigma-Aldrich Co., St. Louis, MO). The slides were mounted with Faramount Aqueous mounting medium (DAKO, Glostrup, Denmark) and observed under fluorescence microscope (BX16, Olymphus, Tokyo, Japan).
Western blotting analysis
Western blotting was performed using standard western blotting methods. The protein concentration in the cytosolic fraction was measured using a BCA protein assay kit (Pierce, Rockford, IL). For western blot analysis, anti-Shank 2 (Santa-Cruz Biotechnology, Santa Cruz, CA), anti-neurofilament (Chemicon, Temecula, CA), phospho-ERK1/2, or ERK1/2 (Cell Signaling Technology, Beverly, MA) antibodies were used at the concentration of 1:1000, and HRP-conjugated anti-rabbit IgG or anti-mouse IgG were used at 1:5000 dilution. To ensure the equal loading of protein in each lane, the blots were stripped and reprobed with an antibody against β-actin. The blots were scanned using a flatbed scanner.
Immunocytochemistry
SNUOT-Rb1 Cells were grown and seeded on Deckglaser coverslips (Carolina Biological, Burlington, NC). Retinoblastoma cells were fixed in 4% paraformaldehyde for over night at 4℃. The primary antibodies were diluted in PBS and added to the specimen followed by incubation for over night at room temperature. The following primary antibodies were used; goat anti-Shank 2 (1:100, Santa-Cruz Biotechnology, Santa Cruz, CA), and rabbit anti-neurofilament (1:100, Chemicon, Temecula, CA). Alexa Fluor 546 donkey anti-goat IgG (1:400, Molecular probes, Eugene, OR), Alexa Fluor 488 donkey anti-rabbit IgG (1:400, Molecular probes, Eugene, OR) were used as secondary antibodies. The nuclei were stained with 4', 6-diamidino-2-phenolindole (DAPI, Sigma-Aldrich Co., St. Louis, MO). The slides were mounted with Faramount Aqueous mounting medium (DAKO, Glostrup, Denmark) and observed under fluorescence microscope (Axio observer, Carl Zeiss, Chester, VA).
Acknowledgments
This work was supported by KT& G research grant (06-2006-113-9) for neonatal diseases from the Seoul National University Children's Hospital and by R01-2004-000-10212-0 from the Basic Research Program of the Korea Science and Engineering Foundation.
Abbreviations
- GCL
ganglion cell layer
- GFAP
glial fibrillary acidic protein
- INL
inner nuclear layer
- IPL
inner plexiform layer
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- PSD
postsynaptic density
- RBL
retinoblast layer
References
- 1.Brandstätter JH, Koulen P, Wässle H. Diversity of glutamate receptors in the mammalian retina. Vision Res. 1998;38:1385–1397. doi: 10.1016/s0042-6989(97)00176-4. [DOI] [PubMed] [Google Scholar]
- 2.Brandstätter JH, Dick O, Boeckers TM. The postsynaptic scaffold proteins ProSAP1/Shank2 and Homer1 are associated with glutamate receptor complexes at rat retinal synapses. J Comp Neurol. 2004;475:551–563. doi: 10.1002/cne.20194. [DOI] [PubMed] [Google Scholar]
- 3.Bovolenta P, Frade JM, Martí E, Rodríguez-Peña MA, Barde YA, Rodríguez-Tébar A. Neurotrophin-3 antibodies disrupt the normal development of the chick retina. J Neurosci. 1996;16:4402–4410. doi: 10.1523/JNEUROSCI.16-14-04402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook JE, Becker DL. Gap junctions in the vertebrate retina. Microsc Res Tech. 1995;31:408–419. doi: 10.1002/jemt.1070310510. [DOI] [PubMed] [Google Scholar]
- 5.Frade JM, Martí E, Bovolenta P, Rodríguez-Peña MA, Pérez-García D, Rohrer H, Edgar D, Rodríguez-Tébar A. Insulin-like growth factor-I stimulates neurogenesis in chick retina by regulating expression of the alpha 6 integrin subunit. Development. 1996;122:2497–2506. doi: 10.1242/dev.122.8.2497. [DOI] [PubMed] [Google Scholar]
- 6.Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 8.Jung HS, Kim HS, Lee MJ, Shin HY, Ahn HS, Ryu KH, Seoh JY, Kim CJ, Jang JJ. Arsenic trioxide concentration determines the fate of Ewing's sarcoma family tumors and neuroblastoma cells in vitro. FEBS Lett. 2006;580:4969–4975. doi: 10.1016/j.febslet.2006.07.077. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kim JH, Yu YS, Kim DH, Kim CJ, Kim KW. Establishment and characterization of a novel, spontaneously immortalized retinoblastoma cell line with adherent growth. Int J Oncol. 2007;31:585–592. [PubMed] [Google Scholar]
- 10.Kim JH, Kim JH, Yu YS, Kim DH, Min BH, Kim KW. Anti-tumor activity of arginine deiminase via arginine deprivation in retinoblastoma. Oncol Rep. 2007;18:1373–1377. [PubMed] [Google Scholar]
- 11.Lim S, Naisbitt S, Yoon J, Hwang JI, Suh PG, Sheng M, Kim E. Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development. J Biol Chem. 1999;274:29510–29518. doi: 10.1074/jbc.274.41.29510. [DOI] [PubMed] [Google Scholar]
- 12.MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM. Activity-independent homeostasis in rhythmically active neurons. Neuron. 2003;37:109–120. doi: 10.1016/s0896-6273(02)01104-2. [DOI] [PubMed] [Google Scholar]
- 13.Maslim J, Stone J. Synaptogenesis in the retina of the cat. Brain Res. 1986;373:35–48. doi: 10.1016/0006-8993(86)90313-6. [DOI] [PubMed] [Google Scholar]
- 14.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 15.Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- 16.Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–452. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roussignol G, Ango F, Romorini S, Tu JC, Sala C, Worley PF, Bockaert J, Fagni L. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci. 2005;25:3560–3570. doi: 10.1523/JNEUROSCI.4354-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sala C, Piëch V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- 19.Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113:1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- 20.Sheng M. Molecular organization of the postsynaptic specialization. Proc Natl Acad Sci USA. 2001;98:7058–7061. doi: 10.1073/pnas.111146298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turrigiano GG, Nelson SB. Homestatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 22.Vecino E, Hernández M, García M. Cell death in the developing vertebrate retina. Int J Dev Biol. 2004;48:965–974. doi: 10.1387/ijdb.041891ev. [DOI] [PubMed] [Google Scholar]
- 23.Wässle H, Koulen P, Brandstätter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- 24.Wong WT, Sanes JR, Wong RO. Developmentally regulated spontaneous activity in the embryonic chick retina. J Neurosci. 1998;18:8839–8852. doi: 10.1523/JNEUROSCI.18-21-08839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang XL. Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol. 2004;73:127–150. doi: 10.1016/j.pneurobio.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Yao I, Hata Y, Hirao K, Deguchi M, Ide N, Takeuchi M, Takai Y. Synamon, a novel neuronal protein interacting withsynapse-associated protein 90/postsynaptic density-95- associated protein. J Biol Chem. 1999;274:27463–27466. doi: 10.1074/jbc.274.39.27463. [DOI] [PubMed] [Google Scholar]