Abstract
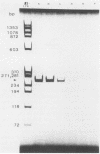
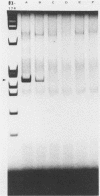
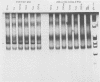
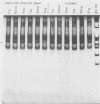
A method was developed for the purification of rotavirus RNA from fecal extracts in order to permit the sensitive identification of group A rotavirus in fecal specimens by the polymerase chain reaction. Sequential reactions with reverse transcriptase and Taq polymerase with directed primers from rotavirus gene 6 yielded characteristic 259-base-pair fragments that were then visualized by silver stain on a polyacrylamide gel. As few as 500 genomic copies of purified rotavirus RNA could be detected in this manner. However, when the method was applied to fecal samples with added rotavirus virions, inhibition was noted in many of the fecal extracts which were tested. The inhibition could be reversed by dilution of the fecal extract, but sensitivity was also reduced by a corresponding dilutional factor. The inhibition was quantitatively removed by an added step in the extraction process that utilized chromatographic cellulose fiber powder (CF11 powder) to purify the rotavirus RNA during a series of rapid washing and elution steps. After CF11 purification, rotavirus RNA could be detected in experimental fecal samples at dilutions 1,000- to 10,000-fold beyond the detection limits of standard techniques such as enzyme immunoassay and the direct visualization of RNA following polyacrylamide gel electrophoresis. Furthermore, following purification by CF11, rotavirus RNA could be detected in all of seven enzyme-linked immunosorbent assay-positive fecal samples obtained from a child with rotavirus gastroenteritis; when CF11 purification was not performed, rotavirus RNA could be detected in only four of these samples, even after the removal of inhibitors by dilution of the extracts. Large-scale identification of rotavirus in fecal specimens may be possible by use of CF11 purification of viral RNA prior to sequential reactions with reverse transcriptase and Taq polymerase in a modified polymerase chain reaction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur R. R., Dagostin S., Shah K. V. Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol. 1989 Jun;27(6):1174–1179. doi: 10.1128/jcm.27.6.1174-1179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassol S. A., Poon M. C., Pal R., Naylor M. J., Culver-James J., Bowen T. J., Russell J. A., Krawetz S. A., Pon R. T., Hoar D. I. Primer-mediated enzymatic amplification of cytomegalovirus (CMV) DNA. Application to the early diagnosis of CMV infection in marrow transplant recipients. J Clin Invest. 1989 Apr;83(4):1109–1115. doi: 10.1172/JCI113990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien A., Edgar D. B., Trela J. M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol. 1976 Sep;127(3):1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. H., Graham D. Y., Estes M. K. Detection of rotaviruses by nucleic acid hybridization with cloned DNA of simian rotavirus SA11 genes. J Infect Dis. 1985 Aug;152(2):293–300. doi: 10.1093/infdis/152.2.293. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Mason B. B., Crawford S., Cohen J. Cloning and nucleotide sequence of the simian rotavirus gene 6 that codes for the major inner capsid protein. Nucleic Acids Res. 1984 Feb 24;12(4):1875–1887. doi: 10.1093/nar/12.4.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Boeggeman E., Purcell R. H., Sereno M., Perez I., White L., Wyatt R. G., Chanock R. M., Kapikian A. Z. A dot hybridisation assay for detection of rotavirus. Lancet. 1983 Mar 12;1(8324):555–558. doi: 10.1016/s0140-6736(83)92811-8. [DOI] [PubMed] [Google Scholar]
- Gama R. E., Horsnell P. R., Hughes P. J., North C., Bruce C. B., al-Nakib W., Stanway G. Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J Med Virol. 1989 Jun;28(2):73–77. doi: 10.1002/jmv.1890280204. [DOI] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikala O. J., Kokkonen J. O., Leinonen M. K., Nurmi T., Mäntyjärvi R., Sarkkinen H. K. Rapid detection of rotavirus in stool by latex agglutination: comparison with radioimmunoassay and electron microscopy and clinical evaluation of the test. J Med Virol. 1983;11(2):91–97. doi: 10.1002/jmv.1890110202. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Ahluwalia G. S., Barker F. G., Horsman G., Hazelton P. R. Comparison of direct and indirect enzyme immunoassays with direct ultracentrifugation before electron microscopy for detection of rotaviruses. J Clin Microbiol. 1982 Jul;16(1):53–59. doi: 10.1128/jcm.16.1.53-59.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. H., Tuomari A. V., Mann D. R., Hamparian V. V. Latex immunoassay for rapid detection of rotavirus. J Clin Microbiol. 1984 Sep;20(3):441–447. doi: 10.1128/jcm.20.3.441-447.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Imai M., Bellamy A. R., Ikegami N., Furuichi Y., Summers D., Nuss D. L., Deibel R. Diagnosis of rotavirus infection with cloned cDNA copies of viral genome segments. J Virol. 1985 Aug;55(2):509–512. doi: 10.1128/jvi.55.2.509-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Imai M., Ikegami N., Bellamy A. R., Summers D., Nuss D. L., Deibel R., Furuichi Y. cDNA probes of individual genes of human rotavirus distinguish viral subgroups and serotypes. J Virol Methods. 1987 Mar;15(4):285–289. doi: 10.1016/0166-0934(87)90151-0. [DOI] [PubMed] [Google Scholar]
- Miotti P. G., Eiden J., Yolken R. H. Comparative efficiency of commercial immunoassays for the diagnosis of rotavirus gastroenteritis during the course of infection. J Clin Microbiol. 1985 Nov;22(5):693–698. doi: 10.1128/jcm.22.5.693-698.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D. M. Detection of enterotoxigenic Escherichia coli after polymerase chain reaction amplification with a thermostable DNA polymerase. J Clin Microbiol. 1989 Feb;27(2):261–265. doi: 10.1128/jcm.27.2.261-265.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saito I., Servenius B., Compton T., Fox R. I. Detection of Epstein-Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1989 Jun 1;169(6):2191–2198. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimans M. M., Holsappel S., van de Rijke F. M., Jiwa N. M., Raap A. K., Weiland H. T. Rapid detection of human parvovirus B19 DNA by dot-hybridization and the polymerase chain reaction. J Virol Methods. 1989 Jan;23(1):19–28. doi: 10.1016/0166-0934(89)90085-2. [DOI] [PubMed] [Google Scholar]
- Shibata D., Fu Y. S., Gupta J. W., Shah K. V., Arnheim N., Martin W. J. Detection of human papillomavirus in normal and dysplastic tissue by the polymerase chain reaction. Lab Invest. 1988 Oct;59(4):555–559. [PubMed] [Google Scholar]
- Sumazaki R., Motz M., Wolf H., Heinig J., Jilg W., Deinhardt F. Detection of hepatitis B virus in serum using amplification of viral DNA by means of the polymerase chain reaction. J Med Virol. 1989 Apr;27(4):304–308. doi: 10.1002/jmv.1890270409. [DOI] [PubMed] [Google Scholar]
- Theil K. W., McCloskey C. M., Saif L. J., Redman D. R., Bohl E. H., Hancock D. D., Kohler E. M., Moorhead P. D. Rapid, simple method of preparing rotaviral double-stranded ribonucleic acid for analysis by polyacrylamide gel electrophoresis. J Clin Microbiol. 1981 Sep;14(3):273–280. doi: 10.1128/jcm.14.3.273-280.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- Yolken R. H. Nucleic acids or immunoglobulins: which are the molecular probes of the future? Mol Cell Probes. 1988 Jun;2(2):87–96. doi: 10.1016/0890-8508(88)90030-8. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Willoughby R., Wee S. B., Miskuff R., Vonderfecht S. Sialic acid glycoproteins inhibit in vitro and in vivo replication of rotaviruses. J Clin Invest. 1987 Jan;79(1):148–154. doi: 10.1172/JCI112775. [DOI] [PMC free article] [PubMed] [Google Scholar]