Abstract
AMPK (AMP-activated protein kinase) is highly conserved in eukaryotes, where it functions primarily as a sensor of cellular energy status. Recent studies indicate that AMPK activation strongly suppresses cell proliferation in non-malignant cells as well as in tumor cells. In this study, quercetin activated AMPK in MCF breast cancer cell lines and HT-29 colon cancer cells, and this activation of AMPK seemed to be closely related to a decrease in COX-2 expression. The application of a COX-2 inhibitor or cox-2-/- cells supported the idea that AMPK is an upstream signal of COX-2, and is required for the anti-proliferatory and pro-apoptotic effects of quercetin. The suppressive or growth inhibitory effects of quercetin on COX-2 were abolished by treating cancer cells with an AMPK inhibitor Compound C. These results suggest that AMPK is crucial to the anti-cancer effect of quercetin and that the AMPK-COX-2 signaling pathway is important in quercetin-mediated cancer control.
Keywords: AMP-activated protein kinases, apoptosis, cyclooxygenase-2, growth inhibitors, HT-29 cells, quercetin
Introduction
Quercetin is one of the most ubiquitous flavonoids found in many fruits, vegetables, nuts, and red wine, and exerts anti-inflammatory and anti-carcinogenic activities (Russo, 2007). Several pieces of evidence indicate that quercetin can modulate a number of proteins like MAPKs, Akt/protein kinase B, and phosphoinositol-3-kinase (Granado-Serrano et al., 2006; Lee et al., 2008) which are linked to cell survival, growth, apoptosis, as well as other molecular targets such as NK-κB, and COX-2, the key components of the inflammation process (O'Leary et al., 2004; AI-Fayez et al., 2006).
AMP-activated protein kinase (AMPK) is a member of a serine/threonine protein kinase that is found in all eukaryotes (Motoshima et al., 2006). The unique ability of AMPK to directly sense cellular energy status makes it an attractive target molecule for ensuring that cell division proceeds when cells have sufficient metabolic resources to support cell proliferation (Koh et al., 2007; Liang et al., 2007). A low incidence of cancer in diabetic patients on metformin has been explained in vitro by the metformin's anti-proliferatory effect through activation of AMPK (Hadad et al., 2008). AMPK activation is known to regulate apoptosis in multiple cancer cells by a signaling pathway that includes up-regulation of p53 and p21 proteins, activation of caspases, inhibition of molecules related to growth, and proliferation of cancer cells, such as COX-2, Akt, and mTOR (Jin et al., 2007; Su et al., 2007; Hwang et al., 2007).
Cyclooxygenase-2 (COX-2), the inducible form of the COX enzymes, catalyzes the conversion of arachidonic acid to prostaglandin H2, which is further converted to several other prostaglandins and plays an essential role in carcinogenesis and inflammation (Cho et al., 2005; Lee et al., 2007). COX-2 is known to be over-expressed in many cancers including breast cancer and colon cancer. In addition, inhibition of COX-2 with selective COX-2 inhibitors has been reported to effectively prevent proliferation and angiogenesis, and induce apoptosis in human breast cancer and colon cancer cell (Mazhar et al., 2006; Das et al., 2007).
In this study, we hypothesized that quercetin regulates proliferation and apoptosis through AMPK activation and that the activated AMPK inhibits COX-2 expression in both breast cancer and colon cancer cells.
Results
Quercetin induces apoptosis in MCF-7 breast cancer cells
The effect of quercetin on cell proliferation and apoptosis was evaluated. Quercetin inhibited cell growth and arrested the cell cycle at the sub-G1 phase in a dose-dependent manner (Figure 1A and B). In addition, quercetin elevated the levels of p53 and p21, the apoptosis-related genes, and reduced a survival gene, VEGF (Figure 1C). Apoptotic cell death was increased with 100 µM quercetin treatment, as shown with chromatin condensation (Figure 1D).
Figure 1.
Quercetin exerts apoptotic effects on MCF-7 breast cancer cells. MCF-7 cells were treated with quercetin (25, 50, 100, 200, or 400 µM) for 6 h, and the cell viability was determined by MTT assay as described in Methods. Data are expressed as the percent relative to control (A). Cells were treated with quercetin (50, 100 or 200 µM) for 24 h, and harvested cells were fixed with 70% ethanol, and stained with 10 µg/ml propidium iodide. The cell cycle was then examined using flowcytometry (B). Cells were exposed to quercetin (25, 50, 100 or 200 µM) for 24 h, total RNA was extracted with Tri-Zol reagent, and RT-PCR was performed using specific primers for p-53, p-21, VEGF, and β-actin (C). Cells were treated with 100 µM quercetin for 12 h, and stained with 10 µM Hoechst33342 dye for 30 min, and chromatin condensation representing apoptotic cell death was examined using fluorescence microscope (D).
Quercetin activates AMPK through ROS generation in MCF-7 breast cancer cells
We investigated the effect of quercetin on AMPK activation. Quercetin strongly activated AMPK in a dose-dependent manner (Figure 2A). For closer confirmation, we treated the AMPK activator (AICAR) with quercetin in MCF-7 cells. As shown in Figure 2B, co-treatment of AICAR and quercetin increased AMPK more drastically. To examine the role of quercetin on AMPK activation, we used a synthetic AMPK inhibitor, Compound C, in cancerous cells. As expected, the elevated AMPK activity by quercetin was abrogated (Figure 2C). These results strongly indicate that quercetin induces apoptosis and activates AMPK in MCF-7 breast cancer cells. We examined the effects of quercetin on ROS generation, because ROS is proposed to be an upstream candidate of AMPK (Hwang et al., 2007). Quercetin increased AMPK activity through ROS generation and AMPK activity was reduced by the antioxidant NAC (N-acetyl-cysteine) (Figure 2D). The induction of ROS by quercetin was also determined by DCFH-DA staining (Figure 2E).
Figure 2.
Quercetin activates AMPK in MCF-7 breast cancer cells. MCF-7 cells were treated with quercetin (25, 50, 100, 200, or 400 µM) for 6 h (A), with quercetin (100 or 200 µM) or/and 1 mM AICAR for 6 h (B), and with 10 µM Compound C before being stimulated by quercetin (100 or 200 µM) for 6 h (C), and were exposed to 5 mM NAC before treatment with quercetin (100 or 200 µM) for 6 h (D). After treatment, the cells were lysed and the levels of phosphorylated AMPK, ACC, and β-actin were determined by Western blot analysis as described in Methods using specific antibodies. Intracellular ROS levels were determined as follows. Cells were treated with 100 µM quercetin for 12 h, and stained with 10 µM DCFH-DA for 30 min, and ROS generation was examined using a fluorescence microscope (E).
Quercetin inhibits COX-2 expression through AMPK activation
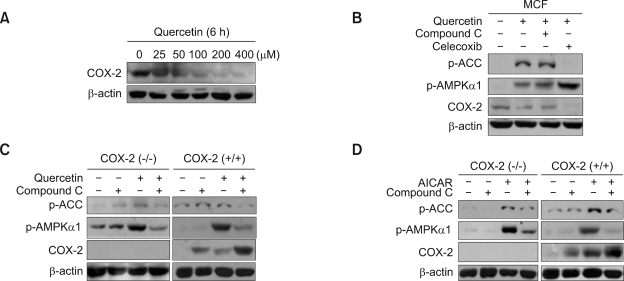
It was shown that quercetin inhibited COX-2 expression in a dose-dependent manner (Figure 3A). To explore the association of AMPK with the inhibitory effect of quercetin on COX-2 regulation, the inhibition of AMPK as well as suppression of COX-2 by celecoxib was adopted. As shown in Figure 3B, by inhibiting AMPK with Compound C, the down-regulation of COX-2 by quercetin was no longer observed. Furthermore, when the cox-2 dominant-positive or cox-2 dominant-negative fibroblast cells were treated with quercetin in the presence or absence of Compound C, it was found that quercetin activated AMPK both in the presence and absence of the cox-2 gene and inhibited COX-2 only through AMPK activation (Figure 3C). The association between AMPK and COX-2 was further confirmed by treating cox-2 dominant-positive or cox-2 dominant-negative fibroblast cells with AICAR. AMPK activation was not dependent on the presence of the cox-2 gene (Figure 3D). In addition, without activating AMPK, COX-2 expression was not inhibited. These results strongly suggest that quercetin decreased COX-2 expression by activation of AMPK in MCF-7 breast cancer cells.
Figure 3.
Quercetin down-regulates COX-2 through AMPK activation. MCF-7 cells were treated with quercetin (25, 50, 100, 200, or 400 µM) for 6 h (A) and pre-treated with Compound C (10 µM) or Celecoxib (50 µM) for 30 min, and treated with quercetin (100 µM) for 6 h (B). Further, cox-2 negative and cox-2 positive cells were exposed with 10 µM Compound C for 30 min before treatment with 100 µM quercetin for 6 h (C) or treatment with 1 mM AICAR (D). The cells were lysed and the levels of phosphorylated AMPK, ACC, COX-2, and β-actin were determined by Western blot analysis as described in Methods using specific antibodies.
Quercetin exerts apoptotic effects by controlling the AMPK-COX-2 signaling pathway in HT-29 colon cancer cells
The apoptotic effect of quercetin was also observed in HT-29 colon cancer cells. Quercetin arrested the cell cycle at the sub-G1 phase, and induced chromatin condensation, which was assessed by Hoechst 33342 staining (Figure 4A). AMPK was activated and COX-2 was inhibited by quercetin in a dose-dependent manner in HT-29 cells (Figure 4B), and the expressions of both AMPK and COX-2 were abolished by Compound C (Figure 4C). Further, quercetin blocked cell proliferation at 50 µM but AMPK inhibition with Compound C led to increased cell population (Figure 4D). These results indicate that quercetin also regulates the AMPK-COX-2 signaling pathway in HT-29 cells and AMPK may be a key regulator of cell growth or apoptosis in cancerous cells when they are treated with a flavonoid such as quercetin.
Figure 4.
Quercetin exhibits apoptotic effects through regulation of AMPK-COX-2 signaling in HT-29 colon cancer cells. HT-29 cells were treated with quercetin (25, 50 or 100 µM) for 24 h. The cells were harvested and stained with 10 µg/ml propidium iodide and then the cell cycle was examined using flowcytometry (A, top). Treated cells were stained with 10 µM Hoechst33342 dye for 30 min, and chromatin condensation representing apoptotic cell death was examined using a fluorescence microscope (A, bottom). HT-29 cells were treated with quercetin (10, 25, 50, 100, or 200 µM) for 6 h (B), and cells were pre-exposed to Compound C (5, 10 or 20 µM) for 30 min before treatment with 50 µM quercetin for 6 h (C). The cells were lysed and the levels of phosphorylated AMPK, ACC, COX-2, and β-actin were determined by Western blot analysis as described in Methods using specific antibodies. Cells were pre-treated with Compound C (5 or 10 µM) for 30 min, and treated with 50 µM quercetin for 6 h and cell viability was examined by MTT assay. Data are expressed as the percent relative to control (D).
Discussion
Quercetin, an anti-oxidative polyphenolic flavonoid, has shown chemotherapeutic potential in various systems (Kandaswami et al, 2005; Ramos et al., 2007; Thomasset et al., 2007). In the present experiments, we hypothesized that quercetin mediates the induction of cell apoptosis through the activation of AMPK signaling. We also hypothesized that AMPK activity is necessary for the down-regulation of COX-2 expression in MCF-7 breast cancer cells. We observed that quercetin (25-200 µM) inhibited cell growth, arrested the cell cycle at the sub-G1 phase, and induced apoptotic death in both breast and colon cancer cells. In other reports, quercetin inhibited cell growth at 30 µM in HCT-116 colon cancer cells and also quercetin indicated apoptotic effect up to 100 µM in MCF-7 cells (Van der woude et al., 2003). When quercetin was supplemented with different concentrations (20, 100, 150 mg/day), plasma concentrations of quercetin were indicated at 255 nM, 497 nM, 1,292 nM and these concentrations were too low for improvement of plasma antioxidant status (Egert et al., 2008).
We showed that quercetin activated AMPK effectively, similar to a synthetic AMPK activator. Also, recently, it is reported that quercetin activated AMPK and induced apoptosis in adipocytes (Ahn et al., 2008). Our data are in agreement with this observation that AMPK is responsible for major effects exerted by quercetin. We also showed that, AMPK was necessary in quercetin-induced apoptosis in cancer cells, since the abolishment of AMPK activity by treatment with an AMPK inhibitor was no longer growth inhibitory. The present study indicates that a mechanism through which quercetin activates AMPK might involve an up-stream target, ROS. The distinctive generation of ROS by the quercetin treatment was abolished completely by NAC, a ROS scavenger.
We then investigated the possibility of COX-2 as a downstream target of AMPK. The importance of COX-2 in the development of breast and colon cancers has been emphasized in many cancer reports. According to the US Women's Health Initiative Observational Study of 80,741 postmenopausal women, NSAID usage for 5-9 years resulted in a 21% reduction of breast cancer incidence (Harris et al., 2003). In addition, treatment of familial ademomatous polyposis (FAP) patients with celecoxib for 6 months reduced the number of colorectal polyps closely associated with developing colon cancers (Steinbach et al., 2000). We have found that quercetin exerts anti-proliferatory effects on MCF-7 breast cancer cells mediated by the activation of AMPK in parallel with suppression of COX-2 expression. It was shown that the activation process of AMPK by quercetin seemed to be independent of COX-2 involvement, since quercetin stimulated AMPK expression in celecoxibtreated and cox-2 dominant-negative cells. However, the inhibition of COX-2 by quercetin could be reversed in cells treated with an AMPK inhibitor, Compound C. Thus we conclude that quercetin regulates AMPK regardless of the presence of COX-2, and AMPK exerts an important role in COX-2 regulation by quercetin. It has been reported that quercetin inhibited adipogenesis through regulating MAPK as well as AMPK signaling (Ahn et al., 2008). Furthermore, even though the relationship between COX-2 and MAPK pathway is well established, MAPK pathway is not the only way to regulate COX-2 expression, and in the cancer cell systems we applied, MAPK might be also connected to the AMPK or COX-2 regulatory process of quercetin. Thus the relationships among AMPK, MAPK and COX-2 need to be scrutinized.
We determined the role of quercetin in regulating cancer cell proliferation and apoptosis, and the possible involvement of an AMPK-COX-2 signaling pathway exerting these regulatory processes. The activation of AMPK by quercetin appeared to be necessary in the regulation of COX-2 in MCF-7 breast and HT-29 colon cancer cells. Based on the findings of this study, we propose that the anti-cancer activity of quercetin carry out through AMPK expression that targets COX-2 regulation in breast cancer cells as well as colon cancer cells.
Methods
Cell culture and reagents
MCF-7 cells and HT-29 cells were obtained from the American Type Culture Collection (Manassas, VA) and grown in RPMI 1640 medium containing 10% FBS at 37℃ in a 5% CO2 atmosphere. Quercetin, and AICAR were purchased from Sigma-Aldrich (St. Louis, MO). Compound C was obtained from Calbiochem (San Diego, CA). Specific antibodies that recognize the phosphorylated forms of AMPK Thr172, ACC Ser79 and COX-2, and β-actin were obtained from Cell Signaling Technology (Danvers, MA). Celecoxib (Celebrex) was supplied by Pharmacia (Seoul, South Korea).
Measurement of cell viability
Cells were seeded on 96-well micro plates at 4,000 cells/well and were incubated with curcumin at the indicated concentration and for the indicated time period. The medium was removed and the cells were then incubated with 100 µl of MTT (Sigma-Aldrich, St. Louis, MO) solution (2 mg/ml MTT in PBS) for 4 h. Optical densities of the solutions were determined by an ELISA reader.
Cell cycle analysis by FACS
Total cells were harvested by trypsinization, collected by centrifugation, washed with PBS, fixed with 70% ethanol, and stained in PBS containing 10 µg/ml propidium iodide (Sigma-Aldrich) and 300 µg/ml RNase. After sorting out viable cells, fluorescence intensity was measured by flow cytometry (Becton-Dickinson Biosciences, San Diego, CA) using excitation and emission wavelengths of 488 and 525 nm, respectively.
Detection of apoptosis by Hoechst 33342 chromatin staining
Cells were stained with 10 µM Hoechst 33342 dye (Sigma-Aldrich) for 30 min and then, fixed with 3.7% para-formaldehyde for 15 min. After washing with PBS, the fluorescence intensity was assessed using a fluorescence microscope.
RNA isolation and RT-PCR
Total RNA was extracted with Tri-Zol reagent (Life Technologies, Glasgow, UK) according to the manufacturer's instructions, and cDNA synthesis was performed with 1 µg total RNA. Synthesized cDNA was used for amplification of a specific target. The amplified products were visualized on 1% agarose gels.
Protein extract and western blot analysis
The cells were washed with PBS, scraped into lysis buffer (50 mM Tris-HCl [pH 7.4], 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, 1 mM NaF, 1 µg/ml aprotinin, 1 µg/ml leupeptin, and 1 µg/ml pepstatin), and subjected to western blot analysis with specific antibodies.
Detection of reactive oxygen species
MCF-7 cells were treated with 10 µM 2', 7' dichlorofluorescein diacetate (DCFH-DA) (Sigma-Aldrich) for 30 min, and then cells were fixed with 3.7% para-formaldehyde for 15 min. After washing with PBS, fluorescence intensity was assessed using fluorescence microscope.
COX-2 positive and negative cells
COX-2 positive and negative mouse fibroblast cells were gifts from Dr. Zigang Dong (University of Minnesota). Genotypes of these cells were confirmed using PCR primers of 5'-ATCCTAGCACTGCATCCTGC-3', 5'-CACCATAGAATCCAGTCCGC-3' and 5'-CTTGGGTGGAGAGGCTATTC-3'. These cell lines were cultured in low glucose DMEM containing 10% FBS.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD). (KRF 2007B443-HJ-R-0101-S000100)
Abbreviations
- AICAR
5-aminoimidazole-4-carboxamide-ribose
- AMPK
AMP-activated protein kinase
- COX-2
cyclooxygenase-2
References
- 1.Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373:545–549. doi: 10.1016/j.bbrc.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 2.AI-Fayez M, Cai H, Tunstall R, Steward WP, Gescher AJ. Differential modulation of cyclooxygenase-mediated prostaglandin production by the putative cancer chemopreventive flavonoids tricin, apigenin and quercetin. Cancer Chemother Pharmacol. 2006;58:816–825. doi: 10.1007/s00280-006-0228-3. [DOI] [PubMed] [Google Scholar]
- 3.Cho JW, Park K, Kweon R, Jang BC, Baek WK, Suh MH, Kim CW, Lee KS, Suh SI. Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT) by inhibiting activation of AP-1: p38 MAP kinase and JNK as potential upstream targets. Exp Mol Med. 2005;37:186–192. doi: 10.1038/emm.2005.25. [DOI] [PubMed] [Google Scholar]
- 4.Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68:1089–1100. doi: 10.1016/j.bcp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Das D, Arber N, Jankowski JA. Chemoprevention of colorectal cancer. Digestion. 2007;76:51–67. doi: 10.1159/000108394. [DOI] [PubMed] [Google Scholar]
- 6.Egert S, Wolffram S, Bosy-Westphal A, Boesch-Saadatmandi C, Wagner AE, Frank J, Rimbach G, Muller MJ. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008;138:1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 7.Granado-Serrano AB, Martin MA, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2) J Nutr. 2006;136:2715–2721. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- 8.Hadad SM, Fleming S, Thompson AM. Targeting AMPK: A new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol. 2008;67:1–7. doi: 10.1016/j.critrevonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, Loar A, Rodabough RJ, White E, McTiernan A. Women's Health Initiative, Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Woman's Health Initiative. Cancer Res. 2003;63:6096–6101. [PubMed] [Google Scholar]
- 10.Hwang JT, Ha J, Park OJ. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemoresistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem Biophys Res Commun. 2005;332:433–440. doi: 10.1016/j.bbrc.2005.04.143. [DOI] [PubMed] [Google Scholar]
- 11.Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, Park OJ. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007;247:115–121. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Jin Q, Feng L, Behrens C, Bekele BN, Wistuba II, Hong WK, Lee HY. Implication of AMP-activated protein kinase and Akt-regulated survivin in lung cancer chemopreventive activities of deguelin. Cancer Res. 2007;67:11630–11639. doi: 10.1158/0008-5472.CAN-07-2401. [DOI] [PubMed] [Google Scholar]
- 13.Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 14.Koh H, Chung J. AMPK links energy status to cell structure and mitosis. Biochem Biophys Res Commun. 2007;362:789–792. doi: 10.1016/j.bbrc.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Lee EJ, Choi EM, Kim SR, Park JH, Kim H, Ha KS, Kim YM, Kim SS, Choe M, Kim JI, Han JA. Cyclooxygenase-2 promotes cell proliferation, migration and invasion in U2OS human osteosarcoma cells. Exp Mol Med. 2007;39:469–476. doi: 10.1038/emm.2007.51. [DOI] [PubMed] [Google Scholar]
- 16.Lee KW, Kang NJ, Heo YS, Rogozin EA, Pugliese A, Hwang MK, Bowden GT, Bode AM, Lee JH, Dong Z. Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res. 2008;68:946–955. doi: 10.1158/0008-5472.CAN-07-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 18.Mazhar D, Ang R, Wazman J. COX inhibitors and breast cancer. Br J Cancer. 2006;94:346–350. doi: 10.1038/sj.bjc.6602942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motochima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation-AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Leary KA, de Pascual-Tereasa S, Needs PW, Bao YP, O'Brien NM, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat Res. 2004;551:245–254. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signaling pathways. Mol Nutr Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 22.Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol. 2007;74:533–544. doi: 10.1016/j.bcp.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Steinbach G, Lynch P, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 24.Su RY, Chao Y, Chen TY, Huang DY, Lin WW. 5-Aminoimidazole-4-carboxamide riboside sensitizes TRAIL- and TNFα-induced cytotoxicity in colon cancer cells through AMP-activated protein kinase signaling. Mol Cancer Ther. 2007;6:1562–1571. doi: 10.1158/1535-7163.MCT-06-0800. [DOI] [PubMed] [Google Scholar]
- 25.Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals-promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120:451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 26.Van der Woude H, Gliszczynska-Swinlo A, Struijs K, Smmets A, Alink GM, Rietjens IM. Biphasic modulation of cell proliferation by quercetin at concentrations physiologically relevant in humans. Cancer Let. 2003;200:41–47. doi: 10.1016/s0304-3835(03)00412-9. [DOI] [PubMed] [Google Scholar]