Abstract
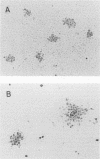
A rapid neutralization test for influenza A and B viruses was developed. In this method, a 96-well tissue culture plate was used for the preparation of cell monolayers and the peroxidase-antiperoxidase staining technique was used for the visualization of foci infected with these viruses. In the presence of trypsin and tragacanth gum, clear foci developed 1 day after infection. A linear relationship between virus dilutions and numbers of foci was observed. When neutralizing antibodies in some test sera were assayed, a good correlation was observed between the titers obtained by the focus method and those obtained by the ordinary plaque method. In addition, many serum specimens were investigated by the neutralization test, and it was demonstrated that the test is useful for serological studies of influenza.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frank A. L., Puck J., Hughes B. J., Cate T. R. Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J Clin Microbiol. 1980 Sep;12(3):426–432. doi: 10.1128/jcm.12.3.426-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman A. K., Kaverin N. V., Kharitonenkov I. G., Rudneva I. A., Sklyanskaya E. L., Zhdanov V. M. Dissociation of the haemagglutination inhibition and the infectivity neutralization in the reactions of influenza A/USSR/90/77 (H1N1) virus variants with monoclonal antibodies. J Gen Virol. 1986 Oct;67(Pt 10):2247–2251. doi: 10.1099/0022-1317-67-10-2247. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gross P. A., Davis A. E. Neutralization test in influenza: use in individuals without hemagglutination inhibition antibody. J Clin Microbiol. 1979 Sep;10(3):382–384. doi: 10.1128/jcm.10.3.382-384.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon M. W., Rota P. A., Walls H. H., Kendal A. P. Antibody response in humans to influenza virus type B host-cell-derived variants after vaccination with standard (egg-derived) vaccine or natural infection. J Clin Microbiol. 1988 Feb;26(2):333–337. doi: 10.1128/jcm.26.2.333-337.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimine T., Tadano M., Fukunaga T., Okuno Y. An improved micromethod for infectivity assays and neutralization tests of dengue viruses. Biken J. 1987 Jun;30(2):39–44. [PubMed] [Google Scholar]
- Kida H., Brown L. E., Webster R. G. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology. 1982 Oct 15;122(1):38–47. doi: 10.1016/0042-6822(82)90375-0. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D. Comparative efficacy of neuraminidase-specific and conventional influenza virus vaccines in induction of antibody to neuraminidase in humans. J Infect Dis. 1976 Oct;134(4):384–394. doi: 10.1093/infdis/134.4.384. [DOI] [PubMed] [Google Scholar]
- Okuno Y., Fukunaga T., Srisupaluck S., Kasemsarn P., Dharakul C., Sangkawibha N. Serological and virological studies on patients with dengue hemorrhagic fever (DHF) in Chanthaburi province, Thailand. I. Serological studies on paired sera from DHF patients by neutralization (N), hemagglutination inhibition (HI) and staining tests. Biken J. 1980 Sep;23(3):113–121. [PubMed] [Google Scholar]
- Okuno Y., Fukunaga T., Tadano M., Okamoto Y., Ohnishi T., Takagi M. Rapid focus reduction neutralization test of Japanese encephalitis virus in microtiter system. Brief report. Arch Virol. 1985;86(1-2):129–135. doi: 10.1007/BF01314119. [DOI] [PubMed] [Google Scholar]
- Okuno Y., Igarashi A., Fukai K. Neutralization tests for dengue and Japanese encephalitis viruses by the focus reduction method using peroxidase-anti-peroxidase staining. Biken J. 1978 Dec;21(4):137–147. [PubMed] [Google Scholar]
- Okuno Y., Sasao F., Fukunaga T., Fukai K. An application of PAP (peroxidase-anti-peroxidase) staining technique for the rapid titration of dengue virus type 4 infectivity. Biken J. 1977 Mar;20(1):29–33. [PubMed] [Google Scholar]
- Okuno Y., Yamanishi K., Lwin S., Takahashi M. Micro-neutralization test for mumps virus using the 96-well tissue culture plate and PAP (peroxidase-antiperoxidase) staining technique. Microbiol Immunol. 1985;29(4):327–335. doi: 10.1111/j.1348-0421.1985.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Quina M. A., Thein S., Auvanich W., Okuno Y., Igarashi A., Fukai K. Changes in dengue and Japanese encephalitis (JE) antibody after JE vaccination. Biken J. 1978 Dec;21(4):149–159. [PubMed] [Google Scholar]
- Raharjo E., Tadano M., Okamoto Y., Okuno Y. Development of a micro-neutralization test for chikungunya virus. Biken J. 1986 Mar;29(1):27–30. [PubMed] [Google Scholar]
- Tanishita O., Takahashi Y., Okuno Y., Yamanishi K., Takahashi M. Evaluation of focus reduction neutralization test with peroxidase-antiperoxidase staining technique for hemorrhagic fever with renal syndrome virus. J Clin Microbiol. 1984 Dec;20(6):1213–1215. doi: 10.1128/jcm.20.6.1213-1215.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita K., Sugiura A., Enomote C., Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975 Dec 30;162(1):9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- Yamada A., Imanishi J., Juang R. F., Fukunaga T., Okuno Y., Tadano M., Fukai K., Baba K., Yabuuchi H. Trial of inactivated Japanese encephalitis vaccine in children with underlying diseases. Vaccine. 1986 Mar;4(1):32–34. doi: 10.1016/0264-410x(86)90095-2. [DOI] [PubMed] [Google Scholar]
- Yoden S., Kida H., Yanagawa R. An avian influenza virus of which infectivity is neutralized by antisera lacking hemagglutination-inhibition activity. Brief report. Arch Virol. 1982;74(2-3):205–210. doi: 10.1007/BF01314713. [DOI] [PubMed] [Google Scholar]
- Zakay-Rones Z., Margalith E., Levy R., Katz E. A sensitive plaque inhibition technique for assay of antibodies to influenza virus: use to detect previous antigenic priming with influenza viruses. J Virol Methods. 1980;1(6):355–360. doi: 10.1016/0166-0934(80)90053-1. [DOI] [PubMed] [Google Scholar]