Abstract
Improved NMR detection of mass limited samples can be obtained by taking advantage of the mass sensitivity of microcoil NMR, while throughput issues can be addressed using multiple, parallel sample detection coils. We present the design and construction of a double resonance 300-MHz dual volume microcoil NMR probe with thermally-etched 440-nL detection volumes and fused silica transfer lines for high-throughput stopped-flow or flow-through sample analysis. Two orthogonal solenoidal detection coils and the novel use of shielded inductors allowed the construction of a probe with negligible radio-frequency cross talk. The probe was resonated at 1H–2D (upper coil) and 1H–13C (lower coil) frequencies such that it could perform 1D and 2D experiments with active locking frequency. The coils exhibited line widths of 0.8 to 1.1 Hz with good mass sensitivity for both 1H and 13C NMR detection. 13C directly detected 2D HETCOR spectra of 5% v/v 13C labeled acetic acid were obtained in less than 5 min. Demonstration of the probe characteristics as well as applications of the versatile two-coil double resonance probe are discussed.
Keywords: 1H NMR, 13C NMR, microcoil, dual volume probe, high throughput, double resonance, HETCOR, metabolite-profiling
INTRODUCTION
Microcoil NMR [1; 2] probes address the need to increase the intrinsic sensitivity of NMR for small samples. Smaller diameter detection coils increase the signal-to-noise ratio (SNR) for mass limited samples, thus improving the mass sensitivity[3–7]. The use of microcoils with solenoidal geometry results in an additional increase in sensitivity because they capture more magnetic flux than Helmholtz geometry coils. The construction of solenoidal microcoil NMR probes with flow-through[4] or sealed-sample[8; 9] designs provides improved sensitivity and good resolution for small and low concentrated samples as little as nanoliters to several microliters[10]. With improved sensitivity, microcoil flow-NMR can be used to advantage in conjunction with a variety of chromatographic techniques[11–16]. In particular, the use of microcoil NMR probes provides a better match to the sample volumes that elute off chromatographic columns, resulting in a significant improvement in hyphenated NMR techniques.
1H NMR has been main focus of the initial microcoil NMR work, and single resonance circuits with single or multiple sample microcoils have been employed to acquire 1D and 2D homonuclear data with improved sensitivity and good resolution[17–20]. The development of probes that incorporate multiple microcoils, such as Multiplex NMR[17; 19; 20] and two to eight-coil systems[18; 21; 22] has been driven by a desire to achieve higher sample throughput. Multiply tuned resonance circuits employing single sample coils that are based on a variety of circuit designs[23; 24] have also been used in microcoil probes to acquire 1D and 2D homonuclear experiments[16; 25]. However, little work has been done to date to employ multiple resonance circuits in conjunction with multiple sample microcoils. One example is the work by Zhang et al.[9], who designed a 15 μL observe-volume two-coil probe that operated at 15N and 1H frequencies. Both sample coils were mounted at the same height in this probe. The authors used the probe to demonstrate the acquisition of COSY and HMQC spectra at 500-MHz from two sealed samples in the same time that it takes to acquire a single spectrum with a standard probe-head. There are a variety of ways to expand on this multiple coil, multiple frequency microcoil NMR idea, such as different observe-volumes (smaller for mass limited samples, potentially larger for concentration limited samples); different frequencies between the multiple coils for variety of experiments; inclusion of a lock frequency for maintaining the line shapes for longer acquisitions; and addition of sample transfer lines for both analysis of flow-samples and easier hyphenation of NMR with other analytical tools.
With these incentives, we report the construction and characterization of a versatile double resonance 300-MHz dual volume double resonance microcoil NMR probe. The upper coil is tuned to 1H/2D frequencies and the lower coil to 1H/13C frequencies. The observe-volumes of both the coils are 440 nL. The oval sample cell has been designed to improve the fill factor using a quick-thermal-etching technique that requires only 10 min to etch a single cell. The detection coils with different multiple resonances allow for homonuclear and heteronuclear 1D or 2D NMR experiments for structural analysis. A 2D lock channel has been incorporated into the resonant circuit of the upper coil to improve the line shape for longer 2D acquisitions on either sample coil. A variety of experiments can performed by using one or both of the 1H/13C frequencies, and a combination of experiments can be run with or without the lock.
METHODS
Detection Cell Fabrication
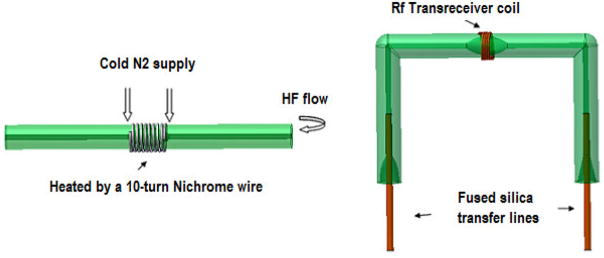
Detection cells for the probe were created using a thermal HF etching technique that is similar in principle to one described in previous work[26; 27]. However, a quick-etch technique very similar to that described in our previous publication[28] was applied and is summarized in Fig. 1. Briefly, 0.5 cm of the middle portion of a 7–8 cm section of 1.8 mm OD, 127 μm ID fused silica glass tubing (Polymicro, Phoenix, AZ) was wound with 30 AWG Nichrome wire (10 cm long, six turns). Thermal etching was performed by passing a 2 A current from voltage source (Beckman Industrial, Taiwan) and simultaneously flowing 48% HF through the glass tubing using a Harvard syringe pump (Harvard Apparatus, Holliston, MA). The pump was programmed for a 2 min infuse and 2 min refill, and then 3 alternating cycles of 1 min infuse and 1 min refill, thus resulting a total etch time of 10 min. Each end of the 0.5 cm center-etched section of the glass tubing was cooled with chilled nitrogen gas resulting in an enlarged etched volume of approximately 1 μL. The etched tube was bent into a U-shape with a torch. At this point, the tip of a 65–70 cm length of fused silica capillary tubing (360 μm OD, 70 μm ID) was inserted into each end of the sample holder and glued using polyimide sealing resin (Supelco, Bellefonte, PA). There was no need to etch the two ends of the sample tube with above protocol to fit the transfer line since the ends were being etched sufficiently from the infuse/refill flow of heated HF during the etching period. The second detection cell was etched, bent and glued with transfer lines similarly.
Figure 1.
Quick-thermal etching procedure for creating capillaries showing the main components used to create the sample detection region. A detailed description of the procedure is provided in the text. The result is an enlarged oval-shaped detection volume within the probe coil.
Probe Construction
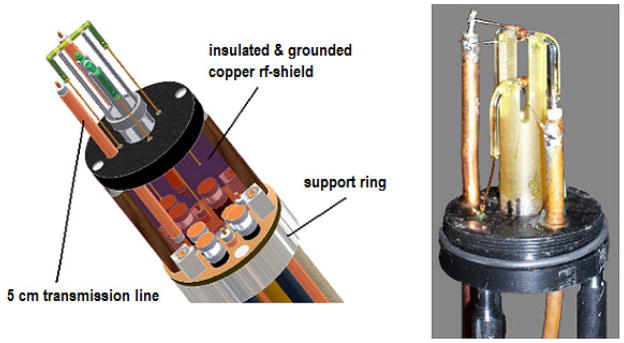
A standard bore (40 mm OD) probe body was constructed in a similar fashion to our previous work[28]. For each detection cell, two and one-half turns using two parallel wires (~40 nH with 2 cm leads), were manually wound with 150 μm OD round copper wires (California Fine Wire, Grover Beach, CA) and affixed to the sample cell using Quicktite© superglue (Henkel Loctite, Rocky Hill, CT). The coil length of 0.8 mm created an active sample volume of 440 nL. The two sample cells were then placed one cm apart and oriented orthogonally on a specially designed U-shaped Ultem plastic support (Fig. 2). AutoDesk Inventor Professional 2008 software was used to design the spatial layout of the rf coils, sample container and structural elements of the probe.
Fig. 2.
Left: Probe head image created on AutoDesk Inventor Professional 2008. Right: Real image of probe head to show cell and transmission line tuning element placement.
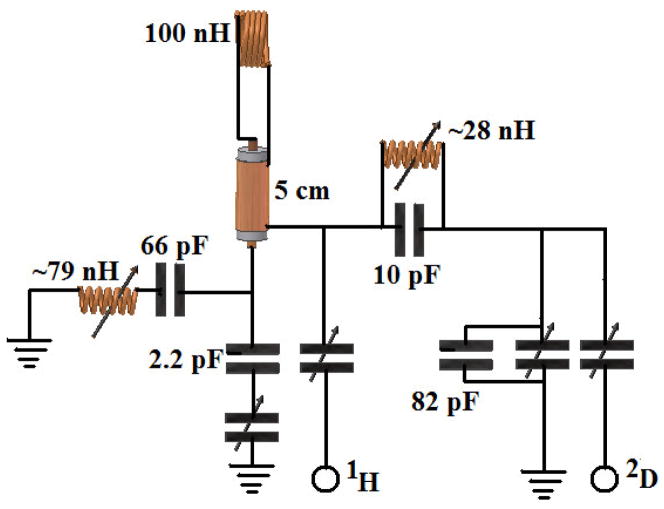
The upper and lower coils were tuned and matched using the double-resonance RF design illustrated in Fig. 3, which features LC trap and pass elements tuned for use at 7 tesla. A 5 cm, 0.181 in. OD, 50 Ω semi-rigid coaxial cable (Haverhill Manufacturing, Haverhill, MA) was used to connect the coils to the rest of the resonant circuit, and with this transmission line in place, the final coil/lead inductance was measured to be ~100 nH. Fixed value capacitors (ATC, Huntington Station, NY) and shielded 5 mm tunable inductors (Coilcraft, Cary, IL) were used in the trap and pass circuit elements, whereas fixed and tunable capacitors (0.1–9 pF, Voltronics, Denville, NJ) were used to provide tuning and matching. A single coil double resonance circuit tuned and matched to 1H/2D frequencies (300 and 46.05 MHz) is shown in Fig. 3. The circuit is based on previous NMR resonant circuit designs [16; 23; 25] and incorporates a high frequency trap (10 pF, 28 nH), low band pass filter (66 pF, 79 nH), and a variable length transmission line[28; 29] that is used both to tune the circuit and to connect the sample coil leads to the other circuit components. A similar circuit design (not shown) with different capacitor and inductor values was used for tuning and matching the 1H/13C (75.44 MHz) frequencies used for the lower detection coil. Each of the coil components was placed at the same level and connected to their respective detection coil with 5 cm transmission lines. Tunable non-magnetic shielded inductors (Coilcraft, Cary, IL) were used both to provide flexibility in tuning the trap and pass components to their respective frequencies, and to minimize the rf cross talk of the components. Shielded inductors have been widely used commercially, mainly in microwave and tele-communication circuit designs to configure traps, as tuning elements, and to prevent magnetic coupling and rf interferences that is especially important in densely packed circuit boards [30–32]. It proved advantageous to incorporate this concept while putting together all the tuning, matching, trapping and passing elements for the four resonances in a single probe circuit board of 38 mm circumference. In addition, a grounded copper sheet was placed between the reactive components of the two circuits to minimize the rf cross talk. As a result of this shield and the use of shielded inductors, it was relatively straightforward to keep the proton resonances from interacting. Finally, the detection cells and their transmission lines were surrounded by a delrin container holding FC-43 Fluorinert (3M, St. Paul, MN) to reduce the line broadening effects that can arise due to susceptibility mismatching of the probe materials[1].
Fig. 3.
Single coil/double resonance circuit diagram.
Electrical Characterization
The isolation of any two intra-ports within each coil circuit (1H and X) and two inter-ports between the upper and lower coils was measured using a 2 GS/s oscilloscope (Tektronix TDS 380, Beaverton, OR). Real-time measurements of incident and reflected power was performed by passing a radio frequency pulse from the desired transmitter channel (1H or X) to the corresponding incident probe port through a 50 dB directional coupler (BIRD Electronics, Cleveland, OH). Attenuators were used to prevent damaging the oscilloscope electronics. The incident signal consisted of a 5 μs radio frequency pulse at the optimized 90° flip angle (see ‘Experimental Section’ below) through the transmitter, and using a 1.8 sec delay between pulses.
NMR Experiments
A Varian INOVA 300 MHz NMR spectrometer was used to evaluate the probe for NMR performance. Two separate samples of 40 mM sucrose (Sigma-Aldrich) and 5% v/v enriched 13CH313COOH (Cambridge Isotope, Andover, MA) were prepared in D2O. The enriched 13CH313COOH sample was prepared by diluting 5 μL 100% 13CH313COOH in 95 μL D2O. The sucrose sample was injected into the upper cell and the acetic acid sample in the lower cell using a 100 μL Hamilton syringe (Hamilton, Reno, NV) and syringe adapter (VICI Valco, Houston, TX). At 40 dB transmitter pulse power, 1H 90° flip angles for the upper and lower coils were 6.8 μs and 5.4 μs, respectively. The 13C 90° flip angle for the lower coil was 2.4 μs at 50 dB. With the lock channel engaged at all times, the upper coil was utilized for the 1D 1H experiments with solvent suppression using a presaturation pulse, whereas, the lower coil was used for 1D 1H and 13C (coupled and decoupled) experiments. Directly-detected 13C 2D HETCOR (coupled), and INADEQUATE (phase-sensitive and 1H decoupled) experiments were performed to show the utility of the probe in structural analysis. Because the NMR spectrometer consisted of one 1H channel, the spectrometer channel was manually connected to the 1H probe port of the desired coil for either the 1D or 2D proton experiment.
RESULTS AND DISCUSSION
Measurements of the electrical performance of the probe generally showed very good results, indicating a good radio frequency design and construction. The power isolation of any two intra-ports, 1H to 2D (upper coil) and 1H to 13C (lower coil), was measured to be less than 0.75% (−21 dB). The power isolation of any two inter-ports between the upper and lower coil was found to be less than 0.004% (−44 dB). Proper intra-port isolation within each coil ensures maximum power transfer to the sample coil from one port with minimal power leakage to the other port, while the proper inter-port isolation between upper and lower coils minimizes the radio frequency cross talk. Initially, both coils were shimmed with a sample consisting of a 1:9 H2O/D2O mixture with the optimized shim center placed at the lower coil. Each coil required its own unique shim values for optimal performance in terms of the full width half maximum (FWHM) line widths, which were measured to be 0.8 Hz (lower coil) and 1.1 Hz (upper coil). When the upper coil was centered in the magnet, the upper coil could be shimmed to 0.8 Hz FWHM, but the lower coil had a poorer line width (~2 Hz), but without any distortion in the line shape. The lock level stayed stable at all the times, and lock level fluctuation after changing the corresponding shim values for each coil was minimal.
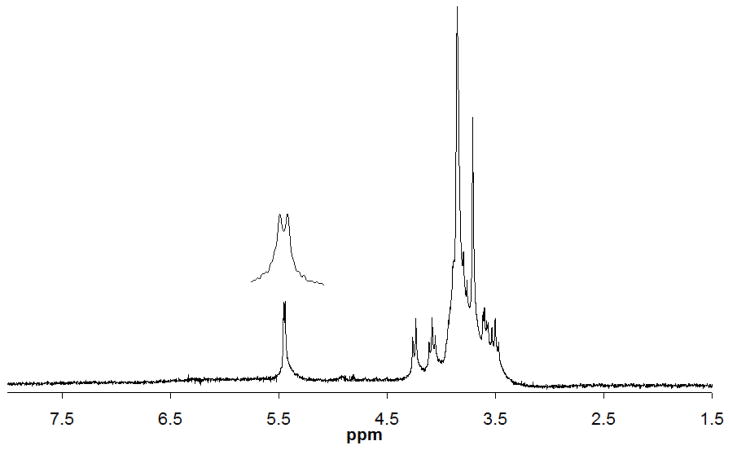
The signal-to-noise ratio of a single scan for the anomeric peak of sucrose at 5.4 ppm observed in either of the coils was close to 28.8 with the receiver gain set to 40 dB. The limit of detection (LOD) for the largest peak at 3.8 ppm using the 40 mM sucrose sample with solvent suppression and a 30 min acquisition was approximately 330 pmole for both coils, as shown in Fig. 4 (the lower coil spectrum is not shown). This picomole detection limit is similar to previous micro coil probe efforts[10].
Fig. 4.
1H NMR spectrum of 40 mM sucrose detected in the upper sample coil with water presaturation and 1024 scans (~30 min) resulting in a LOD of 330 pmol for the largest peak at 3.8 ppm.
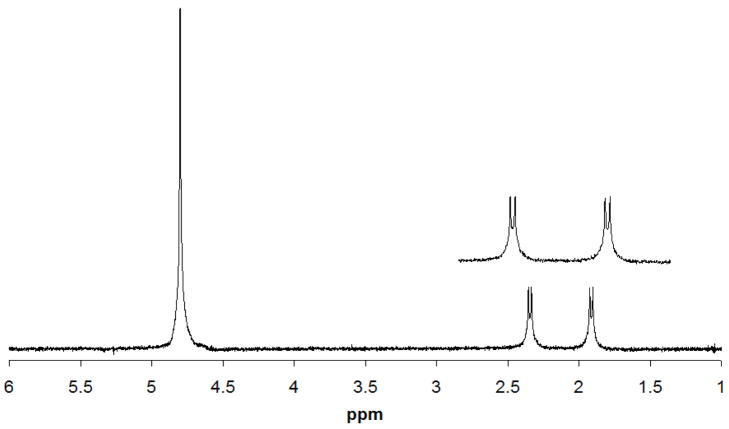
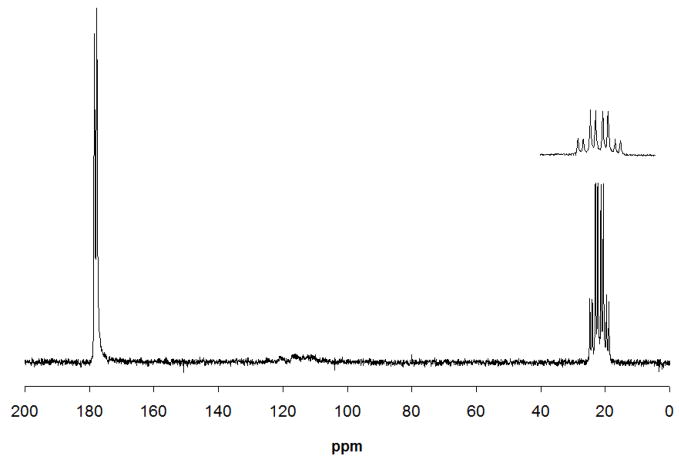
Figures 5A and 5B show 1D 1H (coupled) and 13C (coupled) spectra, respectively, collected from the lower coil for a sample of 5% v/v 13CH313COOH in D2O. The LOD for the 13C=O peak at 176 ppm for this sample located in the lower coil was 3.5 nmol for a 40 min acquisition using a 1D 13C experiment (Fig. 5B). The 1H decoupling efficiency of the lower coil was tested by acquiring a standard WALTZ decoupled 13C experiment (Fig. 6). A decoupling power of only 17 dB (12.6 mW) was required to achieve effective decoupling.
Fig. 5.
Fig. 5A: Single scan 1H (coupled) spectrum of 5% v/v 13CH313COOH detected in the lower sample coil.
Fig. 5B: 13C (coupled) spectrum of 5% v/v 13CH313COOH (512 scans; ~40 min) in the lower coil, resulting in a LOD of 3.5 nmol for the carbonyl peak located at 176 ppm.
Fig. 6.
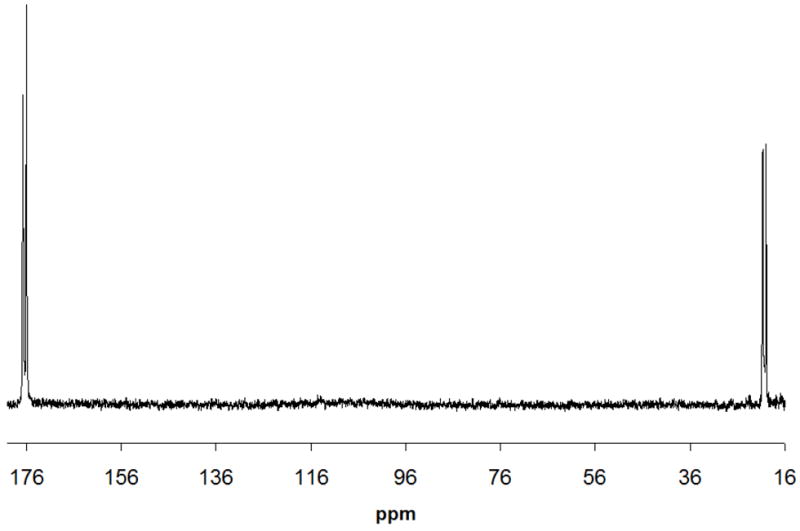
13C (decoupled) spectrum of 5% v/v 13CH313COOH (512 scans; ~40 min) detected in the lower coil. A decoupling power of 17 dB was used. 13C-13C coupling is still evident in the spectrum.
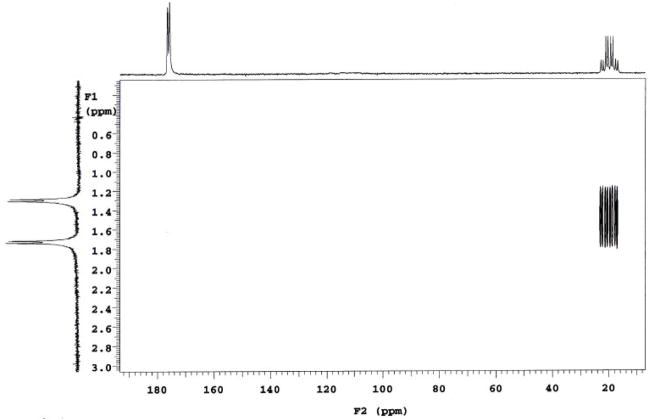
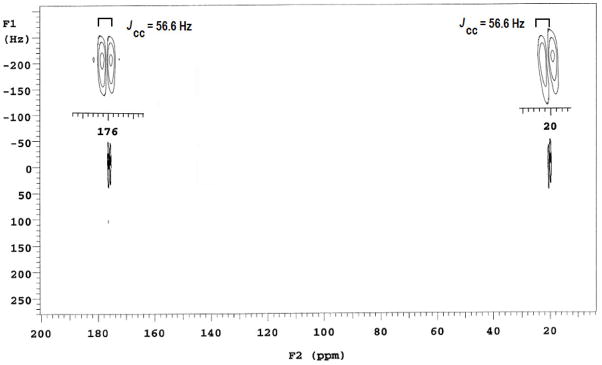
13C-directly-detected 2D HETCOR spectra of the same acetic acid sample were obtained from the lower coil. Both coupled (Fig. 7) and decoupled HETCOR spectra (not shown) were easily obtained with good sensitivity and resolution in less than 5 min using 32 increments and 8 scans per increment. In Fig. 7, HETCOR plot correlates the carbon multiplet at 20 ppm to the proton multiplet at 1.5 ppm. In addition, a 2D INADEQUATE (phase sensitive and 1H decoupled) spectrum was obtained at 64 increments, 36 scans per increment. The spectral data was processed with sinebell appodization and is shown in Fig. 8. A long pre-delay of 5 sec was used to prevent overheating of both the probe and the sample during long experimental period. The Jcc coupling between methyl and carbonyl carbons was measured to be the same value of 56.6 Hz at both peak positions in the F2 dimension, as shown in the figure.
Fig. 7.
HETCOR (coupled) spectrum of 5% doubly 13C labeled acetic acid (CH3COOH) acquired in 4 min 43 sec (32 increments and 8 scans per increment). Only the methyl-carbon directly attached to its protons are visible (as a quartet of doublets) in the HETCOR plot.
Fig. 8.
INADEQUATE (phase-sensitive and 1H decoupled) spectrum of 5% v/v 13CH313COOH in D2O acquired in the lower coil of duo-volume probe. Parameters include nt= 36, ni= 64, d1= 5 secs, at= 200 ms and sinebell appodization. The insets show the 13C-13C coupling measured from center to center of the contour plot to be 56.6 Hz for the two regions. (Sum of the offsets in the F1 dimension is scaled to 0 Hz).
The sensitivity, resolution, flow design, and variety of 1D/2D homonuclear/heteronuclear NMR experiments offered by the probe provide important capabilities for future applications in separation-based metabolomics studies. Multidimensional chromatographic-NMR studies using different hyphenation techniques can be employed with this two coil probe either in stopped-flow or flow-through mode. One such approach could be to send metabolic eluates from a chromatographic column to the top coil of the probe, and any interesting/unknown peak could be sent to the lower coil for 2D NMR structural analysis. Another technique could be to collect chromatographic bands in different storage loops and use an automatic sample handler to send the bands selectively to desired coil of the dual volume probe. In addition, to expedite the whole analytical process, instead of using a single channel receiver, spectrometers with two 1H channel receivers, if available, can be configured to connect to each of the 1H channels of the probe, to allow faster analysis of flow-samples. Alternatively, hardware modifications[5] can be made for single receiver systems. The future work of our dual volume probe will be focused on such applications.
CONCLUSIONS
The design and evaluation of a dual coil, double resonance microcoil NMR probe is described which has the flexibility to carry out a number of 1D and 2D NMR experiments using sample flow injection or hyphenation with HPLC or other separation methods. This versatile microcoil NMR probe can be utilized to acquire spectra of metabolites or other small molecules of interest using as little as 400 nL sample volumes and sub-nmol analyte quantities. We have shown that high resolution and good line shape can be achieved using this 1H/13C and 1H/2D circuit design. The probe has good sensitivity as we were able to acquire spectra in a short time from either sample coils with negligible cross talk. The probe should be useful in a variety of applications including structural identification where small volume samples are encountered.
Acknowledgments
Partial financial support from the NIH (1R01GM085291–01) for this project is gratefully acknowledged. We thank Dr. Robert Santini and Tim Selby of the Jonathan Amy Facility for Chemical Instrumentation, Randy Replogle and Pat Mullen of the Precision Machining Facility, and John Pirolo of the Purdue Glass Lab for their assistance in this work. We also thank Dr. Naganagowda Gowda and Dr. Murthy Shanaiah for useful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olson DL, Peck TL, Webb AG, Magin RL, Sweedler JV. High-resolution microcoil H-1-NMR for mass-limited, nanoliter-volume samples. Science. 1995;270:1967–1970. [Google Scholar]
- 2.Lacey ME, Subramanian R, Olson DL, Webb AG, Sweedler JV. High-resolution NMR spectroscopy of sample volumes from 1 nL to 10 μL. Chemical Reviews. 1999;99:3133–3152. doi: 10.1021/cr980140f. [DOI] [PubMed] [Google Scholar]
- 3.Peck TL, Magin RL, Lauterbur PC. Design and analysis of microcoils for NMR microscopy. Journal of Magnetic Resonance Series B. 1995;108:114–124. doi: 10.1006/jmrb.1995.1112. [DOI] [PubMed] [Google Scholar]
- 4.Wu N, Webb L, Peck TL, Sweedler JV. Online NMR detection of amino-acids and peptides in microbore-LC. Analytical Chemistry. 1995;67:3101–3107. doi: 10.1021/ac00114a002. [DOI] [PubMed] [Google Scholar]
- 5.Olson DL, Norcross JA, O’Neil-Johnson M, Molitor PF, Detlefsen DJ, Wilson AG, Peck TL. Microflow NMR: Concepts and capabilities. Analytical Chemistry. 2004;76:2966–2974. doi: 10.1021/ac035426l. [DOI] [PubMed] [Google Scholar]
- 6.Webb AG. Microcoil nuclear magnetic resonance spectroscopy. Journal of Pharmaceutical and Biomedical Analysis. 2005;38:892–903. doi: 10.1016/j.jpba.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 7.Brey WW, Edison AS, Nast RE, Rocca JR, Saha S, Withers RS. Design, construction, and validation of a 1-mm triple-resonance high-temperature-superconducting probe for NMR. Journal of Magnetic Resonance. 2006;179:290–293. doi: 10.1016/j.jmr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Fisher G, Petucci C, MacNamara E, Raftery D. NMR probe for the simultaneous acquisition of multiple samples. Journal of Magnetic Resonance. 1999;138:160–163. doi: 10.1006/jmre.1999.1725. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Sweedler JV, Webb AG. A probe design for the acquisition of homonuclear, heteronuclear, and inverse detected NMR spectra from multiple samples. Journal of Magnetic Resonance. 2001;153:254–258. doi: 10.1006/jmre.2001.2441. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian R, Lam MM, Webb AG. RF microcoil design for practical NMR of mass-limited samples. Journal of Magnetic Resonance. 1998;133:227–231. doi: 10.1006/jmre.1998.1450. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran O, Spraul M. LC-NMR-MS in drug discovery. Drug Discovery Today. 2003;8:624–631. doi: 10.1016/s1359-6446(03)02749-1. [DOI] [PubMed] [Google Scholar]
- 12.Jayawickrama DA, Sweedler JV. Dual microcoil NMR probe coupled to cyclic CE for continuous separation and analyte isolation. Analytical Chemistry. 2004;76:4894–4900. doi: 10.1021/ac049390o. [DOI] [PubMed] [Google Scholar]
- 13.Almeida VK, Larive CK. Insights into cyclodextrin interactions during sample stacking using capillary isotachophoresis with on-line microcoil NMR detection. Magnetic Resonance in Chemistry. 2005;43:755–761. doi: 10.1002/mrc.1626. [DOI] [PubMed] [Google Scholar]
- 14.Albert K. HPLC-NMR-coupling. Nachrichten Aus Der Chemie. 2006;54:428–432. [Google Scholar]
- 15.Djukovic D, Liu S, Henry I, Tobias B, Raftery D. Signal enhancement in HPLC/microcoil NMR using automated column trapping. Analytical Chemistry. 2006;78:7154–7160. doi: 10.1021/ac0605748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grynbaum MD, Kreidler D, Rehbein J, Purea A, Schuler P, Schaal W, Czesla H, Webb A, Schurig V, Albert K. Hyphenation of gas chromatography to microcoil H-1 nuclear magnetic resonance spectroscopy. Analytical Chemistry. 2007;79:2708–2713. doi: 10.1021/ac0617767. [DOI] [PubMed] [Google Scholar]
- 17.Hou T, MacNamara E, Raftery D. NMR analysis of multiple samples using parallel coils: improved performance using reference deconvolution and multidimensional methods. Analytica Chimica Acta. 1999;400:297–305. [Google Scholar]
- 18.Li Y, Wolters AM, Malawey PV, Sweedler JV, Webb AG. Multiple solenoidal microcoil probes for high-sensitivity, high-throughput nuclear magnetic resonance spectroscopy. Analytical Chemistry. 1999;71:4815–4820. doi: 10.1021/ac990855y. [DOI] [PubMed] [Google Scholar]
- 19.MacNamara E, Hou T, Fisher G, Williams S, Raftery D. Multiplex sample NMR: an approach to high-throughput NMR using a parallel coil probe. Analytica Chimica Acta. 1999;397:9–16. [Google Scholar]
- 20.Macnaughtan MA, Hou T, Xu J, Raftery D. High-throughput nuclear magnetic resonance analysis using a multiple coil flow probe. Analytical Chemistry. 2003;75:5116–5123. doi: 10.1021/ac034400r. [DOI] [PubMed] [Google Scholar]
- 21.Wolters AM, Jayawickrama DA, Webb AG, Sweedler JW. NMR detection with multiple solenoidal microcoils for continuous-flow capillary electrophoresis. Analytical Chemistry. 2002;74:5550–5555. doi: 10.1021/ac025903k. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Ciobanu L, Edison AS, Webb AG. An eight-coil high-frequency probehead design for high-throughput nuclear magnetic resonance spectroscopy. Journal of Magnetic Resonance. 2004;170:206–212. doi: 10.1016/j.jmr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Kan S, Fan M, Courtieu J. A single-coil triple resonance probe for NMR experiments. Review of Scientific Instruments. 1980;51:887–890. [Google Scholar]
- 24.Subramanian R, Webb AG. Design of solenoidal microcoils for high-resolution 13C NMR spectroscopy. Anal Chem. 1998;70:2454–2458. doi: 10.1021/ac980299s. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Logan TM, Edison AS, Webb A. Design of small volume HX and triple-resonance probes for improved limits of detection in protein NMR experiments. Journal of Magnetic Resonance. 2003;164:128–135. doi: 10.1016/s1090-7807(03)00184-8. [DOI] [PubMed] [Google Scholar]
- 26.Pusecker K, Schewitz J, Gfrorer P, Tseng LH, Albert K, Bayer E. On line coupling of capillary electrochromatography, capillary electrophoresis, and capillary HPLC with nuclear magnetic resonance spectroscopy. Analytical Chemistry. 1998;70:3280–3285. doi: 10.1021/ac980063o. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XF, Webb AG. Magnetic resonance microimaging and numerical simulations of velocity fields inside enlarged flow cells used for coupled NMR microseparations. Analytical Chemistry. 2005;77:1338–1344. doi: 10.1021/ac048532b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry ID, Park GHJ, Kc R, Tobias B, Raftery D. Design and construction of a microcoil NMR probe for the routine analysis of 20-μL samples. Concepts in Magnetic Resonance Part B-Magnetic Resonance Engineering. 2008;33B:1–8. [Google Scholar]
- 29.Cross VR, Hester RK, Waugh JS. Single coil probe with transmission-line tuning for Nuclear Magnetic Double-Resonance. Review of Scientific Instruments. 1976;47:1486–1488. [Google Scholar]
- 30.Young John C, Butler Chalmers M. Inductance of a Shielded Coil. IEEE Transactions on Antennas and Propagation. 2001;49:944–953. [Google Scholar]
- 31.Scherz Paul. Practical Electronics for Inventors. McGraw-Hill, NY: 2006. [Google Scholar]
- 32.Bowick Chris. Rf Circuit Design. Howard W. Sams; IN: 1982. [Google Scholar]