Abstract
IL-24 is a newly described member of the IL-10 family. We previously demonstrated that PBMC from TB patients exhibited low levels of IL-24 and IFN-γ compared to subjects with latent tuberculosis infection (LTBI). In order to investigate the role of IL-24 in IFN-γ expression in TB patients, we stimulated PBMC from individuals with LTBI or TB patients with the Mtb-specific antigen, early secretory antigenic target-6 (ESAT-6) and measured cytokine expression using quantitative real-time PCR (qPCR). Exogenous IL-24 increased IFN-γ expression in PBMC obtained from TB patients while neutralization of IL-24 reduced IFN-γ expression in PBMC from subjects with LTBI. Exogenous IL-24 enhanced IFN-γ expression by increasing expression of IL-12 family cytokines, including IL-12α, IL-12β, IL-23α and IL-27, and by reducing FOXP3 expression in PBMC from TB patients. This is the first demonstration that IL-24 may play an important role in IFN-γ expression following infection with Mtb.
Keywords: Mycobacterium tuberculosis, Cytokine, Human
1. Introduction
The correlation between IFN-γ expression and development of tuberculosis (TB) is well established in human Mycobacterium tuberculosis (Mtb) infection [1]. T cells, NK cells and δγ T cells all secrete IFN-γ in response to Mtb antigens [2] and defects in either IFN-γ or IL-12 receptors render the host susceptible to Mtb infection [3]. Therefore, the inability to develop a robust Th1 response may contribute to ineffective anti-Mtb immunity. Different mechanisms underlying a defective Th1 response in Mtb infection have been reported, including genetic factors [3], overproduction of Th2 cytokines [4], lack of IL-12 production [5] and activation of regulatory T (Treg) cells [6].
Previously we reported that IL-24 expression is decreased in TB patients [7], prompting us to ask whether IL-24 directly modulates IFN-γ expression in individuals infected with Mtb. IL-24, also known as melanoma differentiation-associated gene-7 (mda-7), is a newly described member of the IL-10 family of cytokines and has been shown to mediate tumor suppressive and proinflammatory functions [8]. Overexpression of IL-24 via an adenovirus-mediated gene delivery system induces apoptosis selectively in cancer cells [9]. IL-24 expression has been reported to increase in virus infection [10], bacterial infection [11] and to be involved in the immunopathology of psoriasis [12]. Another potential role for IL-24 was suggested by two recent studies: we reported elevated IL-24 expression in BCG vaccinated newborns [13] while Huang et al. found that IL-19, IL-20, and IL-22, all members of the IL-24 family, are increased in macaques after BCG vaccination [14]. However, a direct connection between IL-24 and IFN-γ has not been demonstrated. In this report, we investigate the effects of IL-24 on IFN-γ expression in PBMC from Mtb-infected individuals.
2. Materials and methods
2.1. Study population
Patients with pulmonary TB were recruited from the TB Clinic at San Francisco Department of Public Health/San Francisco General Hospital. PBMC were obtained from TB patients who had received standard anti-TB therapy for <3 weeks. All patients with TB had positive cultures for Mtb. Subjects with LTBI were recruited from among health-care workers at Stanford University Medical Center. Subjects with LTBI were U.S. born and had documentation of a positive (>10 mm) tuberculin skin test (TST) using five tuberculin units of purified protein derivative, a normal chest radiograph, no history of BCG vaccination and no clinical evidence of TB. Patients known to be HIV infected or to be receiving immunosuppressive regimens were excluded. The study was approved by the institutional review boards of the University of California, San Francisco (UCSF) and Stanford University.
2.2. PBMC activation and quantitative real-time polymerase chain reaction assay
PBMC were isolated by centrifugation over Ficoll and cultured in RPMI 1640 medium (GIBCo, Grand Island, NY) supplemented with L-glutamine, penicillin/streptomycin, non-essential amino acid (GIBCo), sodium pyruvate (Irvine Scientific, Santa Ana, CA) and 10% heat-inactivated pooled human sera (Gemini, Woodland, CA) in the absence or presence of 10 μg/ml ESAT-6 (Research Materials and Vaccine Testing Center, Colorado State University) for 15 h. For some experiments, recombinant human IL-24 (40 ng/ml), recombinant human IL-12 (40 ng/ml), anti-human IL-12 antibody (20 μg/ml) and anti-human IL-24 antibody (20 μg/ml) (R&D systems) were co-cultured with PBMC for 15 h. Total RNA was isolated from PBMC using RNeasy mini kits (Qiagen, Santa Clarita, CA) and was transcribed into cDNA (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. Expression of immune related genes was analyzed using qPCR with an ABI prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) (Details of PCR protocol at www.appliedbiosystems.com). All primers were purchased from Applied Biosystems, including FOXP3, IFN-γ, IL-12α, IL-12β, IL-23α, IL-24 and IL-27. The expression level of a gene in a given sample was represented as fold increase: 2−ΔΔCt where ΔΔCT = [ΔCT(sample, stimulated)] − [ΔCT(sample, unstimulated)] and ΔCT = [CT(sample)] − [CT(GUS)] where GUS is the housekeeping gene γ-glucoronidase.
2.3. ELISA
Detection of IFN-γ or IL-24 was performed using human IFN-γ or IL-24 ELISA kits (R&D). All the assay procedures were carried out according to manufacture’s guide. The limit for detection is 15.6 pg/ml for IFN-γ and 63 pg/ml for IL-24.
2.4. Statistical analysis
The Student’s t-test was used to determine significance between groups using STATA/SE.8.0 software (STATA Corporation, College Station, TX). In all instances, p < 0.05 was considered to be significant.
3. Results
In initial experiments, PBMC isolated from individuals with LTBI or TB patients were stimulated with ESAT-6 to determine the kinetics of gene expression. Cells were harvested at 2, 4, 6, 15, 24, 48, and 72 h after stimulation and mRNA for IL-24 and IFN-γ were analyzed. Transcripts for both genes were detectable between 6 and 24 h after activation and reached a maximum at 15 h (data not shown). Similar kinetics studies of cytokine expression have been previously reported [15,16]. For the remaining studies described here, cells were stimulated in vitro with ESAT-6 for 15 h and then processed for mRNA.
3.1. Low expression of IL-24 is associated with decreased IFN-γ expression in PBMC from TB patients
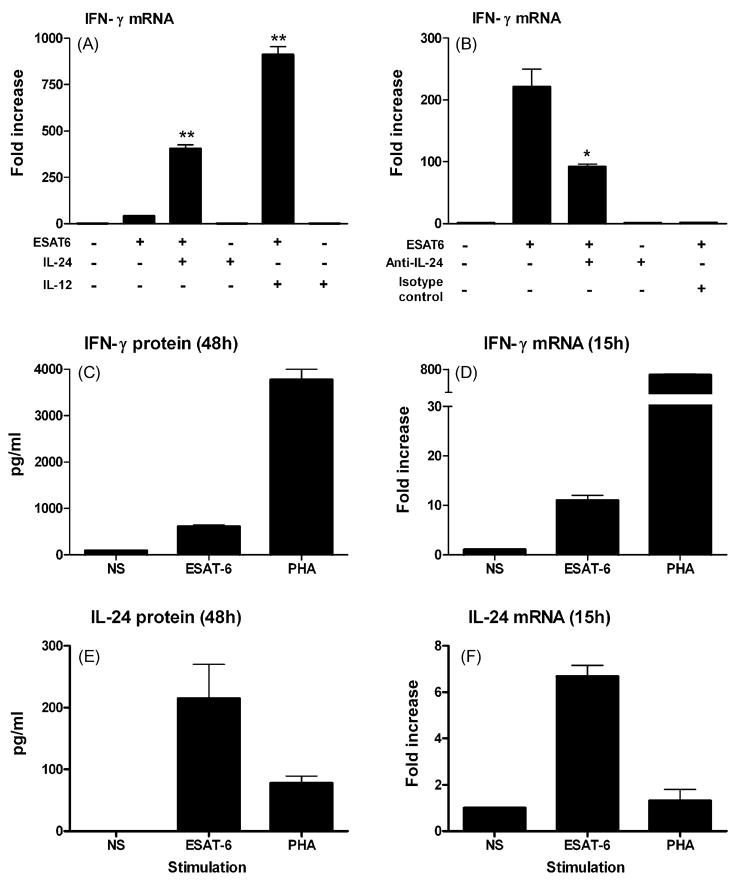
In a previous study, we demonstrated that ESAT-6 activated PBMC from TB patients expressed low levels of IL-24 and IFN-γ [7]. To establish a role of IL-24 in IFN-γ expression, PBMC from TB patients were stimulated with ESAT-6 in the absence or presence of exogenous IL-24. As shown in Fig. 1A, exogenous IL-24, similar as IL-12, dramatically increased IFN-γexpression in PBMC from TB patients. In contrast, neutralization of IL-24 decreased IFN-γ expression in PBMC of subjects with LTBI (Fig. 1B), suggesting that low levels of IL-24 may be responsible in part for defective IFN-γ expression in TB patients. We further showed that mRNA expression of IFN-γ or IL-24 is consistent with production of IFN-γ or IL-24 protein (Fig. 1C–F).
Fig. 1.
IL-24 modulates IFN-γ expression in ESAT-6 stimulated PBMC. (A) IFN-γ expression in PBMC from a TB patient is increased by exogenous IL-24 and IL-12 (40 ng/ml). Results are presented as the fold increase ± S.D. and are representative of individual experiments using PBMC from six different TB patients. **p < 0.01, Addition of IL-24 or IL-12 vs. medium. (B) IFN-γ expression in PBMC from an individual with LTBI is decreased by anti-IL-24 antibody (20 μg/ml). Results are presented as the fold increase ± S.D. and are representative of individual experiments using PBMC from three different individuals with LTBI. *p < 0.05, Addition of anti-IL-24 antibody vs. isotype control. (C and D) Messenger RNA expression of IFN-γ is consistent with production of IFN-γ protein. PBMC from TB patient was stimulated with ESAT-6 or PHA for either 15 h (mRNA) or 48 h (protein). Results are shown as the fold increase ± S.D. (mRNA) or pg/ml ± S.D. (protein) and are representative of individual experiments using PBMC from three different TB patients with similar results. (E and F) Messenger RNA expression of IL-24 is consistent with production of IL-24 protein. PBMC from TB patient was stimulated with ESAT-6 or PHA for either 15 h (mRNA) or 48 h (protein). Results are shown as the fold increase ± S.D. (mRNA) or pg/ml ± S.D. (protein) and are representative of individual experiments using PBMC from three different TB patients with similar results.
3.2. Exogenous IL-24 increases IFN-γ expression by up-regulating IL-12α, IL-12β, IL-23α and IL-27 expression in PBMC from TB patients
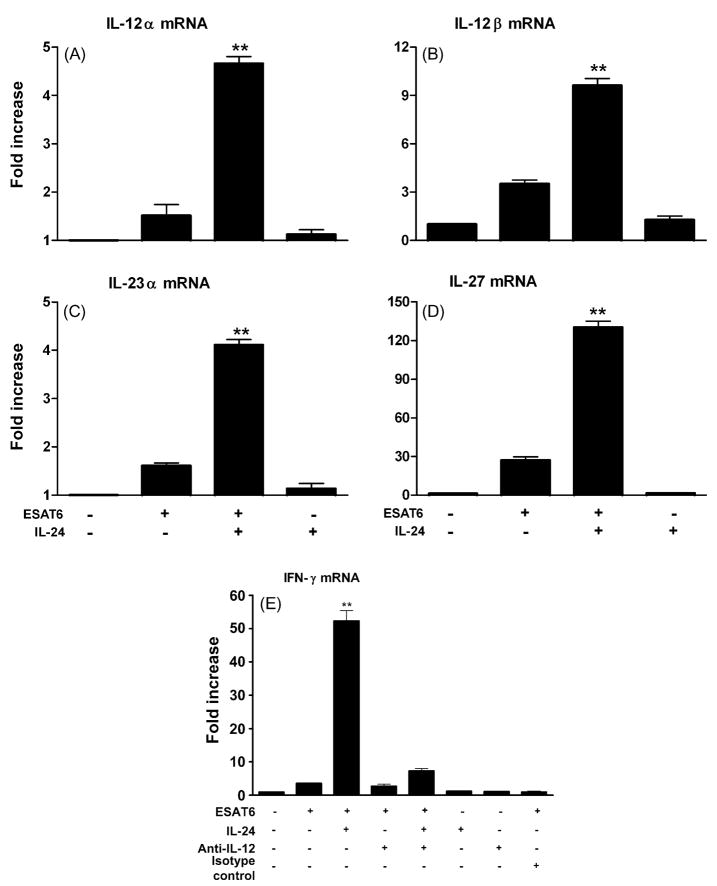
Since IL-12 and its family members are the main inducers of IFN-γ, we asked whether exogenous IL-24 could increase levels of IL-12, IL-23 and IL-27 mRNA. Exogenous IL-24 was added for 15 h to ESAT-6 stimulated PBMC from TB patients and mRNA levels were measured. There was a significant increase in expression of IL-12α, IL-12β, IL-23α and IL-27 mRNA in the presence of exogenous IL-24 in PBMC from TB patients (Fig. 2A–D). Moreover, enhancement of IFN-γ expression mediated by IL-24 was dramatically blocked in the presence of anti-IL-12 antibody (Fig. 2E). This suggests that failure to produce IL-24 may result in low expression of IL-12 family cytokines, leading to low IFN-γ expression.
Fig. 2.
IL-24 enhances IFN-γ expression by up-regulating IL-12α, IL-12β, IL23α and IL-27 expression in PBMC from TB patients. (A–D) PBMC from TB patients were stimulated with ESAT-6 (10 μg/ml) in the presence or absence of exogenous IL-24 (40 ng/ml). Results are presented as the fold increase ± S.D. and are representative of individual experiments using PBMC from six different TB patients. **p < 0.01, Addition of IL-24 vs. medium. (E) PBMC from TB patients were stimulated with ESAT-6 (10 μg/ml) in the presence of either IL-24 or anti-IL-12 antibody or both for 15 h. Results are presented as the fold increase ± S.D. and representative of individual experiments using PBMC from three different TB patients. **p < 0.01, Addition of IL-24 vs. medium.
3.3. Exogenous IL-24 reduces FOXP3 expression in PBMC from TB patients
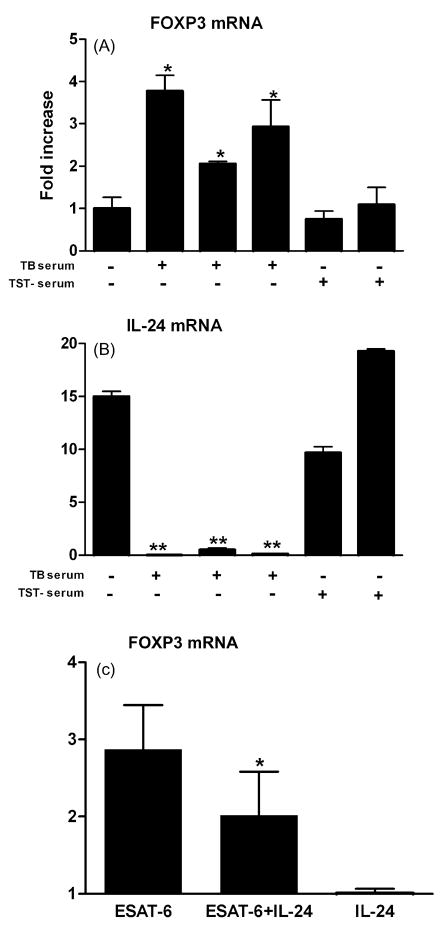
Recent studies have shown that CD4+CD25+FOXP3+ T regulatory (Treg) cells are associated with low levels of IFN-γ in patients infected with Mtb [12,17]. This prompted us to ask whether IL-24 modulates IFN-γ expression by affecting FOXP3 expression, a marker associated with Treg cells. To explore this, PBMC from individuals with LTBI were stimulated with ESAT-6 in the presence of sera from TB patients or TST donors. Sera from TB patients (n = 3) consistently induced higher levels of FOXP3 mRNA and abrogated expression of IL-24 while sera from TST donors (n = 2) did not affect expression of either FOXP3 or IL-24 (Fig. 3A and B). Moreover, addition of exogenous IL-24 decreased FOXP3 expression in PBMC from TB patients (Fig. 3C, p < 0.05), suggesting that IL-24 may enhance IFN-γ expression by down-regulating FOXP3 expression in PBMC from TB patients.
Fig. 3.
Sera from TB patients enhance expression of FOXP3 and inhibit expression of IL-24 in PBMC from individuals with LTBI. (A and B) PBMC from a donor with LTBI were stimulated with ESAT-6 (10 μg/ml) for 15 h in the absence or presence of sera from three different TB patients or from two different TST donors. Results are presented as the fold increase ± S.D. for each gene in ESAT-6 stimulated cells compared to resting cells. Similar results were obtained using PBMC from four different individuals with LTBI. *p < 0.05, **p < 0.01, TB sera vs. AB sera. (C) IL-24 decreases expression of FOXP3. PBMC from a TB patient were stimulated for 15 h with ESAT-6 (10 μg/ml) in the presence of exogenous IL-24 (40 ng/ml). FOXP3 mRNA levels are presented as the fold increase ± S.D. in ESAT-6 stimulated cells compared to resting cells. Results are representative of individual experiments using PBMC from three different TB patients. *p < 0.05, Addition of IL-24 vs. medium.
4. Discussion
In this study, we demonstrate a previously unrecognized role for IL-24 in host defense against Mtb infection. Individuals with latent Mtb infection produce high levels of IL-24 and IFN-γ while patients with TB produce low levels of IL-24 and IFN-γ [7]. Exogenous IL-24 enhances expression of IFN-γ while neutralization of IL-24 results in low expression of IFN-γ. IL-24 also enhances expression of the IFN-γ inducing cytokines IL-12, IL-23, and IL-27. Finally, IL-24 reduces FOXP3 expression, suggesting that it may be involved in modulation of FOXP3 expression.
IL-24 has been reported to activate human monocytes to produce TNF-α and IL-6 as well as to kill human melanoma cell lines [8]. IL-24 expression is induced in human PBMC by LPS and is regulated at the post-transcriptional level through stabilization of IL-24 mRNA [15]. In humans, IL-24 expression is increased at 2 days in cells committed to Th1 differentiation [18]. Treatment of PBMC with IL-24 resulted in the induction of IL-6, IFN-γ, TNF-α, IL-1β, IL-12, and GM-CSF [19], consistent with our finding that addition of exogenous IL-24 to ESAT-6 stimulated PBMC from TB patients induced high levels of IFN-γ, IL-12, and IL-6.
IL-24 signals either through its cell surface receptors as a classical cytokine [20] or intracellularly in a non-receptor-mediated manner. The mechanism by which IL-24 increases IFN-γ expression is still unknown.
It is notable that although IL-10 and IL-24 belong to the same family, they elicit opposite functional effects, as IL-10 is a major suppressor of the immune response and inflammation [21], while IL-24 is immunomodulatory. In some conditions, IL-19, IL-20 and IL-24 are distinct from classical interleukins and constitute a separate subfamily within the IL-10 family [22].
Another interesting finding is that sera from TB patients contain unknown factors that significantly increase FOXP3 expression and at the same time decrease IL-24 expression in PBMC from individuals with LTBI. Of note, we recently reported that IL-2 and IL-9 expression is elevated in ESAT-6 stimulated PBMC from TB patients [7,23]. The IL-2 and IL-9 receptors share the common gamma chain that is also involved in IL-4, IL-7, IL-15 and IL-21 signaling [24]. Activated Treg may further inhibit IL-24 expression.
In conclusion, we demonstrate a new role for IL-24 in modulating INF-γ expression in the recall immune response to ESAT-6 in Mtb-infected individuals. Understanding of detailed pathways affected by IL-24 and other cytokines in the immune response in Mtb infection may provide information relevant for vaccine development.
Supplementary Material
Acknowledgments
We are grateful to Colorado State University and the NIH, NIAID Contract NO 1 AI-75320, entitled “Tuberculosis Research Materials and Vaccine Testing” for providing ESAT-6 used in this study and to the TB Clinic at the San Francisco Department of Public Health. We thank Irina Rudoy and Cynthia Merrifield for recruitment of TB patients, blood drawing and data collection, Shu-Chen Lyu for technical assistance, and Gary Schoolnik and Peter Small for helpful discussions.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.imlet.2007.11.018.
References
- 1.Kawakami K. Promising immunotherapies with Th1-related cytokines against infectious diseases. J Infect Chemother. 2003;9:201–9. doi: 10.1007/s10156-003-0263-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann SH. Is the development of a new tuberculosis vaccine possible? Nat Med. 2000;6:955–60. doi: 10.1038/79631. [DOI] [PubMed] [Google Scholar]
- 3.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 4.van Crevel R, Ottenhoff TH, van der Meer JW. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev. 2002;15:294–309. doi: 10.1128/CMR.15.2.294-309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–15. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 7.Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, et al. Messenger RNA expression of IL-8, FOXP3, and IL-12beta differentiates latent tuberculosis infection from disease. J Immunol. 2007;178:3688–94. doi: 10.4049/jimmunol.178.6.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mumm JB, Ekmekcioglu S, Poindexter NJ, Chada S, Grimm EA. Soluble human MDA-7/IL-24: characterization of the molecular form(s) inhibiting tumor growth and stimulating monocytes. J Interferon Cytokine Res. 2006;26:877–86. doi: 10.1089/jir.2006.26.877. [DOI] [PubMed] [Google Scholar]
- 9.Madireddi MT, Su ZZ, Young CS, Goldstein NI, Fisher PB. Mda-7, a novel melanoma differentiation associated gene with promise for cancer gene therapy. Adv Exp Med Biol. 2000;465:239–61. doi: 10.1007/0-306-46817-4_22. [DOI] [PubMed] [Google Scholar]
- 10.Garn H, Schmidt A, Grau V, Stumpf S, Kaufmann A, Becker M, et al. IL-24 is expressed by rat and human macrophages. Immunobiology. 2002;205:321–34. doi: 10.1078/0171-2985-00135. [DOI] [PubMed] [Google Scholar]
- 11.Buzas K, Megyeri K. Staphylococci induce the production of melanoma differentiation-associated protein-7/IL-24. Acta Microbiol Immunol Hung. 2006;53:431–40. doi: 10.1556/AMicr.53.2006.4.2. [DOI] [PubMed] [Google Scholar]
- 12.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–40. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 13.Wu B, Huang C, Garcia L, Ponce de Leon A, Osornio JS, Bobadilla-del-Valle M, et al. Unique gene expression profiles in infants vaccinated with different strains of Mycobacterium bovis bacille Calmette-Guerin. Infect Immun. 2007;75:3658–64. doi: 10.1128/IAI.00244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang D, Qiu L, Wang R, Lai X, Du G, Seghal P, et al. Immune gene networks of mycobacterial vaccine-elicited cellular responses and immunity. J Infect Dis. 2007;195:55–69. doi: 10.1086/509895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poindexter NJ, Walch ET, Chada S, Grimm EA. Cytokine induction of interleukin-24 in human peripheral blood mononuclear cells. J Leukoc Biol. 2005;78:745–52. doi: 10.1189/jlb.0205116. [DOI] [PubMed] [Google Scholar]
- 16.Doherty TM, Demissie A, Menzies D, Andersen P, Rook G, Zumla A. Effect of sample handling on analysis of cytokine responses to Mycobacterium tuberculosis in clinical samples using ELISA, ELISPOT and quantitative PCR. J Immunol Methods. 2005;298:129–41. doi: 10.1016/j.jim.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Roberts T, Beyers N, Aguirre A, Walzl G. Immunosuppression during active tuberculosis is characterized by decreased interferon- gamma production and CD25 expression with elevated forkhead Box P3, transforming growth factor- beta, and interleukin-4 mRNA levels. J Infect Dis. 2007;195:870–8. doi: 10.1086/511277. [DOI] [PubMed] [Google Scholar]
- 18.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 19.Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168:6041–6. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Tan Z, Zhang R, Kotenko SV, Liang P. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J Biol Chem. 2002;277:7341–7. doi: 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]
- 21.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 22.Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, et al. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol. 2006;15:991–1004. doi: 10.1111/j.1600-0625.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, et al. IL-9 is associated with an impaired Th1 immune response in patients with tuberculosis. Clin Immunol. 2008;126:202–10. doi: 10.1016/j.clim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Knoops L, Renauld JC. IL-9 and its receptor: from signal transduction to tumorigenesis. Growth Factors. 2004;22:207–15. doi: 10.1080/08977190410001720879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.