Abstract
Background
Asthma is a complex immunologic disorder linked to altered cytokine signaling.
Objective
We tested whether asthmatic patients showed any change in cytokine-dependent signal transducer and activator of transcription (STAT) levels, focusing on the central/effector-memory CD4+CD161+ subset, which represents 15% to 25% of circulating T cells.
Methods
We quantified intracellular levels of active phosphorylated STAT (phospho-STAT) 1, 3, 5, and 6 by means of flow cytometry, without any activation or expansion.
Results
Baseline phospho-STAT1 and phospho-STAT6 levels were increased in CD4+CD161+ T cells from asthmatic patients compared with those from healthy control subjects (by 10- and 8-fold, respectively). This asthma-associated alteration was both subset specific because no change was seen in CD4+CD161−CD25+ (regulatory T cells) and CD4+CD161−CD25− subsets and isoform specific because phospho-STAT5 and phospho-STAT3 levels were unchanged. Among asthmatic patients, phospho-STAT1 and phospho-STAT6 levels correlated negatively with each other, suggesting antagonistic regulation. Oral corticosteroid (OCS) treatment significantly decreased phospho-STAT6 and IL-4 levels but not phospho-STAT1 levels. Disease parameters showing significant correlations with phospho-STAT1, phospho-STAT6, or both included age at onset, plasma IgE levels, and levels of the TH2 cytokines IL-4 and IL-10 and the TH1 cytokine IL-2. Overall, combined phospho-STAT1 and phospho-STAT6 measurements showed excellent predictive value for identifying (1) asthmatic patients versus healthy control subjects, (2) allergic versus nonallergic asthmatic patients, and (3) asthmatic patients taking versus those not taking OCSs.
Conclusion
Baseline changes in phospho-STAT1 and phospho-STAT6 levels in blood CD4+CD161+ T cells identify asthmatic patients and mirror their allergic status and response to OCSs.
Clinical implications
These results confirm the pathologic importance of activated STAT1 and STAT6 in asthma and suggest their potential use as clinical biomarkers.
Keywords: Allergy, atopy, fluorescence-activated cell sorting, immune polarization
Asthma is a chronic disease characterized by airway inflammation, obstruction, hyperresponsiveness, and remodeling.1 Asthma is generally believed to be associated with chronic abnormalities in T-cell function,2 leading notably to altered blood and lung profiles of TH1 and TH2 cytokines.3 Clinically, asthmatic patients are divided into allergic (atopic or extrinsic) and nonallergic (nonatopic or intrinsic) groups based on high blood IgE levels and positive skin test results to common aeroallergens in the former, although not in the latter.4 Although both groups feature prominent IL-5–dependent airway eosinophilia, the IL-4–dependent induction of IgE production seems mostly restricted to the allergic group.5 One also distinguishes early-onset from late-onset asthma (emerging in childhood and adulthood, respectively), with the earlier being more prone to TH2-driven, IL-4–associated allergic reactions.1
The TH2 cytokine IL-4 mediates its effects by binding to heterodimeric receptors6 formed by the IL-4 receptor (IL-4R) α chain and either the common γ chain (thus forming the type I IL-4R) or the IL-13 receptor α 1 chain (thus forming the type II IL-4R). Binding of IL-4 to the conventional type I IL-4R activates the Janus kinase family members Jak1 and Jak3, which in turn activate their target, signal transducer and activator of transcription (STAT) 6, through phosphorylation of a key tyrosine at position 641.7 Phosphorylated STAT6 (phospho-STAT6) translocates into the nucleus and turns on several key transcripts. Experiments in STAT6-knockout mice established the crucial role of STAT6 in the development of allergic asthma.8 By contrast, nonallergic asthma might be less dependent on STAT69 than on alternative STAT isoforms, such as STAT5, STAT3, or STAT1.10,11 Recent evidence suggest that IL-4 can also lead to STAT1 activation through the type II IL-4R, and so can the closely related TH2 cytokine IL-13.12,13
The pathologic events of asthma occur in the lungs. However, immune cells recruited from blood (granulocytes and lymphocytes) also play a prominent role in the disease, as reflected by the strong therapeutic effect of oral corticosteroids (OCSs).1,4 OCS are a mainstay of severe asthma treatment, providing relief of inflammatory symptoms through several mechanisms, including the anatomic redistribution of lymphocytes14 and the molecular inhibition of proinflammatory cascades.15 This supports the hypothesis that beyond well-known changes in mediator secretion (eg, abnormal blood IL-416 or IgE levels), key signaling changes involving STAT factors should also be detectable in blood cells of asthmatic patients that would distinguish allergic from nonallergic asthmatic patients and asthmatic patients taking or not taking OCSs.
Previously, Miller et al17 attempted to correlate IL-4 and IgE levels to altered STAT6 expression in whole PBMC fractions from allergic asthmatic patients but failed to demonstrate any alteration. Here we hypothesized that characterizing the levels of the active (phosphorylated) form of STATs instead of total STAT levels would be more appropriate. To do so, we chose to use the phosphorylated fluorescence-activated cell sorting (phospho-FACS) methodology, which allows us to quantify expression of multiple intracellular phosphoepitopes at the single-cell level.18 Furthermore, we hypothesized that phospho-STAT levels should be measured in chosen subsets from patients instead of whole PBMC fractions. In human subjects co-expression of CD4 and CD161, without CD16 or CD56 expression (thus excluding conventional natural killer [NK] cells19), identifies a prominent central/effector-memory T-cell subset,20,21 herein referred to as CD4+CD161+, which represents approximately 15% to 25% of blood T cells.22 CD161, a C-type lectin receptor, interacts with the ceramide-generating enzyme acid sphingomyelinase,23 with long-range effects on cellular signaling and activation. 24 This CD4+CD161+ T-cell fraction also includes most invariant NK T cells, a rare subset of blood T cells (<0.1%),21 the role of which in asthma is disputed.25
Focusing on this large subset of blood CD4+CD161+ T cells, at baseline we demonstrated highly increased phospho-STAT6 and phospho-STAT1 expression in asthmatic patients compared with that seen in healthy control subjects. Moreover, within asthmatic patients, phospho-STAT1 and phospho-STAT6 levels measured in combination provide excellent discrimination between allergic and nonallergic asthmatic patients and between asthmatic patients taking or not taking OCSs, confirming the importance of functional STAT polarization in asthma patho-physiology and response to treatment.26
METHODS
Human subjects
This study was approved by the Stanford Administrative Panel on Human Subjects in Medical Research. All subjects signed informed consent forms before participating in the study. The diagnosis of allergic and nonallergic asthma was made,27 and disease severity was defined (see Table E1 in the Online Repository at www.jacionline.org for demographics and medications, including OCSs).28 Asthmatic patients not taking OCSs had not been exposed for at least 2 months to OCSs, immunotherapy, immunomodulator drugs, or high-potency steroid creams. Asthmatic patients taking OCSs had started their cure 2 or 3 days before sample collection. Asthmatic patients not taking or taking OCSs were all assessed in the presence of active disease and controlled disease. Patients with adult-onset Still disease (chronic inflammatory joint disease)29 were used as disease control subjects. None of the patients with asthma or adult-onset Still disease were undergoing exacerbations. Healthy control subjects were stable nonsmoking and nonallergic volunteers with no recent history of asthma or other lung diseases.
Quantification of phospho-STAT levels in blood CD4+CD161+ T cells
Detailed steps in this procedure are indicated in the Methods section in the Online Repository (available at www.jacionline.org). In brief, blood was collected by means of venipuncture, and CD4+CD161+ T cells were purified (purity, >92%) in 2 steps: (1) CD4+ T-cell enrichment by means of negative depletion with the CD4+ RosetteSep kit (StemCell Technology, Vancouver, British Columbia, Canada) and (2) antibody labeling with anti-human CD16119 antibody (BD Biosciences, Mississauga, Ontario, Canada), followed by positive magnetic separation. For some experiments (see Fig E1 in the Online Repository at www.jacionline.org), the CD4+CD161− fraction was further separated into CD25+ and CD25− fractions by means of further magnetic separation based on CD25 expression.30 Quantification of phospho-STAT1, phospho-STAT3, phospho-STAT5, and phospho-STAT6 levels was performed by means of FACS, as described elsewhere,18 except for 2 important modifications. First, phospho-STATs were quantified in the absence of any in vitro stimulation. Second, we included a fixable live stain to gate on viable cells only (Fig 1). Data were acquired on a digital FACS Aria (BD Biosciences) after thorough fluorescence calibration and exported to the FlowJo software (Treestar, Ashland, Ore) for compensation and analysis (see the Methods section in the Online Repository at www.jacionline.org for details).31,32
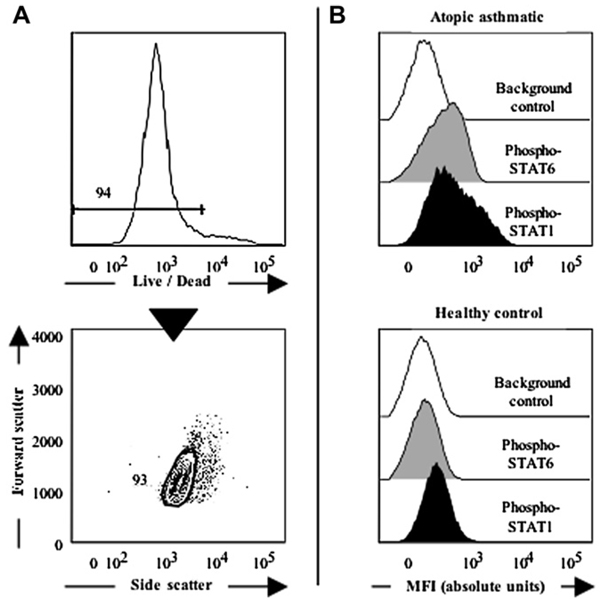
FIG 1.
Single-cell quantification of intracellular phospho-STATs in live CD4+CD161+ blood T cells by means of flow cytometry. A, The live fraction of CD4+CD161+ T cells is precisely identified based on low staining with the fixable Live/Dead probe (upper panel), followed by a scatter gate (lower panel). B, In the absence of any stimulation, live purified CD4+CD161+ T cells show distinct expression of phospho-STATs compared with background control values. Representative data from 1 allergic asthmatic patient and 1 healthy control subject are shown, as indicated.
Fluid measurements
Plasma levels of the TH1 cytokines IFN-γ, IL-2, and TNF-α and the TH2 cytokines IL-4, IL-6, IL-10, and IL-13 were measured by using the TH1/TH2 Cytometric Bead Arrays (BD Biosciences), as per the manufacturer’s guidelines. Blood IgE levels were measured with standard ELISAs.17
Data analysis
Statistical analysis was performed with the JMP 6 software (SAS Institute, Cary, NC). Between- and within-group comparisons used the nonparametric Wilcoxon rank sum and signed-rank tests, respectively. Pairwise correlations (Table I) used the Pearson test. Predictive values of tests (eg, phospho-STAT1 or plasma IL-4 levels) for subject identification (asthmatic patients vs control subjects, allergic vs nonallergic asthmatic patients, and asthmatic patients taking versus those not taking OCSs) were calculated by means of nominal logistic regression (Table II), yielding P values for negative log-likelihood χ2 tests. Predictive abilities were expressed as the area under the receiver operating characteristics (ROC) curve,33 which plots the frequency of true-positive (sensitivity) against the frequency of false-positive (1-specificity) results. Area values were considered excellent at more than 0.9 (1.0 is the maximum). For predictive abilities of combined phospho-STAT measurements (noted “phospho-STAT1 × phospho-STAT6” in Table II), the nominal logistic regression took into account the interaction between tests.33 Differences were considered significant at a P value of less than .05.
TABLE I.
Statistically significant pairwise correlations between CD4+CD161+ phospho-STAT1 and phospho-STAT6 levels and conventional disease parameters of asthma
| Parameter 1 | Parameter 2 | R (Pearson test) | P value (Pearson test) |
|---|---|---|---|
| Phospho-START1 | Age at onset | +0.81 | .0007 |
| Phospho-START1 | Plasma IgE | −0.89 | .0001 |
| Phospho-START1 | Plasma IL-2 | +0.71 | .007 |
| Phospho-START1 | Plasma IL-4 | −0.69 | .009 |
| Phospho-START1 | Plasma IL-10 | −0.66 | .0014 |
| Phospho-START1 | Phospho-STAT6 | −0.82 | .0007 |
| Phospho-START6 | Age at onset | −0.61 | .02 |
| Phospho-START6 | Plasma IgE | +0.83 | .0002 |
| Phospho-START6 | Plasma IL-2 | −0.63 | .015 |
TABLE II.
Predictive values of individual and combined phospho-STAT1 and phospho-STAT6 levels for subject group identification in comparison with plasma IL-4 and IgE levels
| Groups to identify | Proposed predictor |
P value (log likelihood) |
Area under the ROC curve |
|---|---|---|---|
| Asthmatic patients (all) vs healthy control subjects | Phospho-STAT1 | <10−4 | 0.95 |
| Phospho-STAT6 | <10−4 | 0.89 | |
| Phospho-STAT1 × Phospho-STAT6 | <10−4 | 0.96 | |
| AA (all) vs NA (all) | Phospho-STAT1 | .001 | 0.91 |
| Phospho-STAT6 | .024 | 0.69 | |
| Phospho-STAT1 × Phospho-STAT6 | .015 | 0.91 | |
| Plasma IgE | <10−4 | 1.00 | |
| Plasma IL-4 | .021 | 0.80 | |
| Asthmatic patients not taking OCSs vs asthmatic | Phospho-STAT1 | .77 | 0.61 |
| patients taking OCSs | Phospho-STAT6 | .005 | 0.82 |
| Phospho-STAT1 × Phospho-STAT6 | <10−4 | 1.00 | |
| Plasma IgE | .015 | 0.82 | |
| Plasma IL-4 | .013 | 0.65 |
See DeLong et al.33
AA, Allergic asthmatic patients; NA, nonallergic asthmatic patients.
RESULTS
Phospho-STAT6, phospho-STAT1, and phospho-STAT5 are measurable in unstimulated blood CD4+CD161+ T cells. We purified CD4+CD161+ T cells from the blood of allergic and nonallergic asthmatic patients and healthy control subjects. These cells were found in large amounts in all groups. We ascertained that the rapid purification by means of rosetting and magnetic sorting did not artifactually activate purified subsets, as shown by a lack of expression of the activation markers CD69 and CD11a (data not shown). CD4+CD161+ blood T cells contained a minor fraction of invariant NK T cells (<5%, data not shown) stained specifically by the 6B11 antibody, as described previously.21 CD4+CD161+ blood T cells were processed, without any in vitro stimulation, for intracellular phospho-STAT staining (see the Methods section). Based on Live/Dead (Invitrogen, Carlsbad, Calif) staining (Fig 1, A), purified CD4+CD161+ T cells showed excellent viability and were analytically gated, displaying significant baseline expression of phospho-STAT6, phospho-STAT1, and phospho-STAT5 in all groups (Fig 1, B). By contrast, phospho-STAT3 staining remained at levels undistinguishable from background (data not shown). All subjects were in stable condition (no ongoing exacerbation) at the time of phospho-STAT profiling. Specifically, asthmatic patients all presented with active controlled disease (taking or not taking OCSs).
Characteristic phospho-STAT6 and phospho-STAT1 profiles in unstimulated blood CD4+CD161+ T cells from allergic and nonallergic asthmatic patients
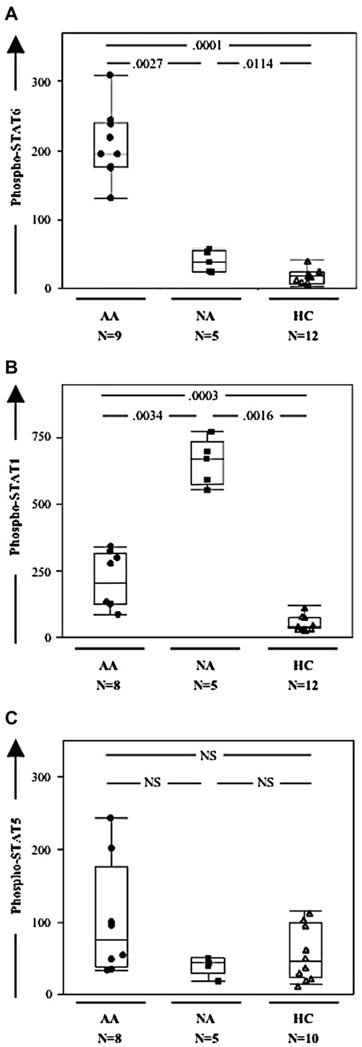
Phospho-STAT6 (Fig 2, A) and phospho-STAT1 (Fig 2, B) levels were increased in asthmatic patients compared with those seen in healthy control subjects. By contrast, phospho-STAT5 levels remained unchanged in all 3 groups (Fig 2, C). Interestingly, phospho-STAT6 levels were significantly greater in allergic compared with nonallergic asthmatic patients (Fig 2, A), whereas phospho-STAT1 levels were significantly greater in nonallergic than in allergic asthmatic patients (Fig 2, B). When considering the whole cohort of asthmatic patients, there was a strong negative correlation between phospho-STAT1 and phospho-STAT6 levels (R = −0.82, P= .0007). With regard to plasma mediators, only IL-4 and IgE levels were significantly altered between the 2 groups (both increased in allergic patients), whereas IFN-γ, IL-2, TNF-α, IL-6, IL-10, and IL-13 levels were unchanged (See Table E2 in the Online Repository at www.jacionline.org). Patients with adult-onset Still disease29 provided a further disease control group. In these patients the phospho-STAT profile in blood CD4+CD161+ T cells did not differ from that of control subjects (data not shown).
FIG 2.
Phospho-STAT6 and phospho-STAT1 levels, but not phospho- STAT5 levels, are increased in CD4+CD161+ blood T cells from asthmatic patients and distinguish allergic from nonallergic patients. A, B, and C, Box plots (with median line in box delimited by 25th and 75th quantiles ± 1.5 × interquartile range, as shown by whiskers) of phospho-STAT6, phospho-STAT1, and phospho-STAT5 values in allergic asthmatic patients (AA), nonallergic asthmatic patients (NA), and healthy control subjects (HC).
High phospho-STAT6 levels characterize allergic asthmatic patients when measured in CD4+CD161+ T cells but not in other subsets
Phospho-STAT6 expression in CD4+CD161+ T cells was unimodal (Fig 2, B); that is, there was no indication of discrete subsets20,21 with different expression levels within that population. Invariant NK T cells represented only a minor fraction of purified CD4+CD161+ T cells (<5%) and thus could not account alone for the significant difference observed between allergic asthmatic patients, nonallergic asthmatic patients, and healthy control subjects. Interestingly, phospho-STAT6 levels were significantly higher in CD4+CD161+ T cells than in CD4+CD161−CD25+ (regulatory T cells2,30) and CD4+CD161−CD25− subsets (see Fig E1 in the Online Repository at www.jacionline.org). The latter 2 subsets did not show significant differences between allergic asthmatic patients and healthy control subjects in phospho-STAT6 expression (see Fig E1), as well as in other phospho-STAT isoforms (data not shown).
Treatment with OCSs decreases phospho-STAT6 levels in CD4+CD161+ T cells from allergic asthmatic patients
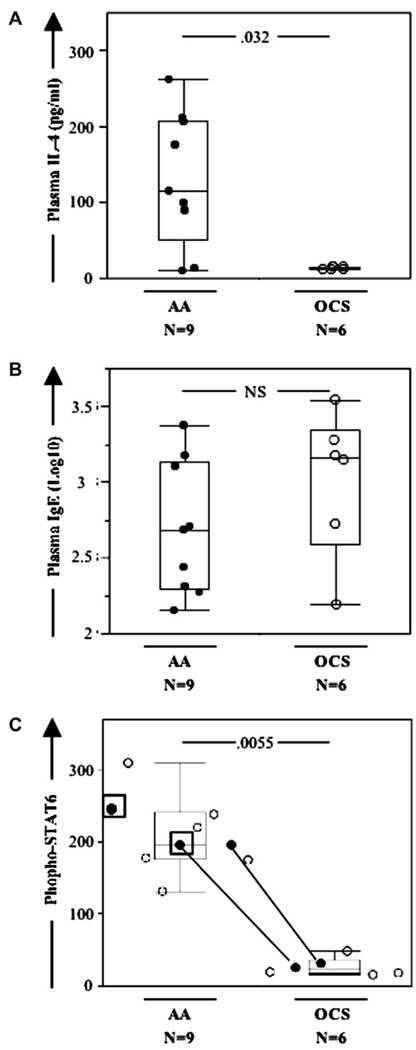
OCS treatment is a mainstay in severe asthma and asthma exacerbations, leading to decreased IL-4 levels in plasma and decreased lung symptoms.1,4 However, adherence to OCS treatment is problematic because of side effects. We assessed asthmatic patients 2 to 3 days into the course of OCS treatment and observed significantly decreased plasma IL-4 levels (Fig 3, A), as expected. By contrast, OCS treatment did not alter plasma levels of IgE (Fig 3, B) or other cytokines besides IL-4 (P > .1 for all, data not shown). Additionally, OCS treatment significantly decreased phospho-STAT6 levels within the CD4+CD161+ T-cell subset (Fig 3, B) to normal levels (P > .1 comparing asthmatic patients taking OCSs and healthy control subjects). Two patients were prescribed OCSs, but their inconsistent phospho-STAT6 values prompted us to check treatment adherence, which was indeed absent (Fig 3, C). In contrast with phospho-STAT6 levels, phospho-STAT1 and phospho-STAT5 levels were not changed by OCS treatment. The dose of OCS itself (10, 30, 40, or 60 mg) did not show any significant correlation with any of the phospho-STAT, IgE, or cytokine measurements (P > .1 for all, Pearson test for pairwise correlation).
FIG. 3.
OCS treatment in allergic asthmatic patients decreases plasma IL-4 and IgE levels and phospho-STAT6 levels in blood CD4+CD161+ T cells. A, B, and C, Box plots of plasma IL-4 levels, plasma IgE levels (shown as Log10 caused by lognormal distribution), and phospho-STAT6 levels in blood CD4+CD161+ T cells, respectively, for allergic asthmatic patients not taking (AA) or taking OCSs (OCS). In Fig 3, C, 2 patients (square boxes) were prescribed OCSs but did not take them. Two individuals yielded pre- and post-OCS data (solid circles with matching lines).
Correlation of phospho-STAT levels in blood CD4+CD161+ T cells with disease parameters
Next, we tested whether cell-based measurements of phospho-STATs in CD4+CD161+ T cells in asthmatic patients would correlate with conventional disease parameters (Table I). Looking at allergic and nonallergic asthmatic patients not taking OCSs, age at onset correlated positively with phospho-STAT1 and negatively with phospho-STAT6 levels. This is consistent with the notion that early- and late-onset asthma diverge in their immunologic underpinnings.1 Other demographic parameters, including functional expiratory volume in 1 second (a measure of lung function) and sex, did not correlate with phospho-STAT levels, nor did disease severity classes defined as mild, moderate, and severe. As expected, IgE levels, used clinically to distinguish allergic from nonallergic patients correlated positively with phospho-STAT6 levels (greater in allergic patients) and negatively with phospho-STAT1 levels (greater in nonallergic patients). Consistently, levels of the TH2 cytokines IL-4 and IL-10 correlated negatively with phospho-STAT1 levels, whereas levels of the TH1 cytokine IL-2 correlated positively with phospho-STAT1 and negatively with phospho-STAT6 levels.
Predictive values of individual and combined phospho-STAT1 and phospho-STAT6 measurements in blood CD4+CD161+ T cells for the clinical classification of asthmatic patients
Because of the clear discrimination between asthmatic patients and healthy control subjects and between asthmatic subgroups based on phospho-STAT1 and phospho-STAT6 measurements (see Fig E2 in the Online Repository at www.jacionline.org), we next questioned how these compared with 2 other blood-based markers of asthma, IgE and IL-4, with regard to their predictive values for patient classification. These predictive values were tested by using nominal logistic regression and expressed as areas under the ROC curves (see the Methods section). As shown in Table II, phospho-STAT1 levels yield excellent predictive value for distinguishing asthmatic patients from healthy control subjects and, among asthmatic patients, distinguishing nonallergic from allergic patients. In the latter case the phospho-STAT1 level (area under the ROC curve, 0.91) is superior to the plasma IL-4 level (area under the ROC curve, 0.80), yet remains less than the predictive value of plasma IgE (area under the ROC curve, 1.0), which is by definition the diagnostic criterion distinguishing allergic and nonallergic patients. Phospho-STAT6 levels, on the other, hand accurately distinguish patients taking or not taking OCSs. Combining these 2 measurements (phospho-STAT1 × phospho-STAT6) yielded excellent predictive value (area under the ROC curve, > 0.9) for distinguishing (1) asthmatic patients from healthy control subjects, (2) allergic from nonallergic asthmatic patients, and (3) asthmatic patients taking or not taking OCSs. Of note, when patients taking OCSs were excluded from the calculation, areas under the ROC curves for phospho-STAT1 × phospho-STAT6 were all equal to 1.0 for distinguishing asthmatic patients from healthy control subjects and allergic from nonallergic asthmatic patients.
DISCUSSION
Asthma is a complex airway disease with a strong immunologic component. Here we show that baseline expression of phosphorylated forms of STAT6 and STAT1 within a prominent central/effector-memory subset of blood lymphocytes, namely CD4+CD161+ T cells, distinguishes asthmatic patients from healthy control subjects, as well as allergic from nonallergic asthmatic patients, and tracks their adherence and response to OCS treatment. These results provide new insights into the clinical course of human asthma.
Predictive value in asthma of baseline phospho-STAT1 and phospho-STAT6 levels within blood CD4+CD161+ T-cells, as measured by a rapid FACS-based method
Our study contrasts with a previous study9 that failed to show changes in STAT6 expression in blood cells from allergic asthmatic patients. However, another previous study17 assayed total STAT6 levels instead of phospho-STAT6 levels and used mixed PBMCs instead of purified subsets. Our results clearly show that phospho-STAT expression is not equivalent in all T-cell subsets, with the range of phospho-STAT6 alterations in CD4+CD161+ T cells significantly exceeding that measured in CD4+CD161−CD25+ regulatory T cells.23
Altered phospho-STAT regulation was not only subset specific but also specific for the STAT1 and STAT6 isoforms because baseline phospho-STAT5 expression was unchanged across groups, and phospho-STAT3 levels were always less than background. Phospho-STAT profiling in CD4+CD161+ T cells yields a relevant set of blood cell markers reflecting ongoing immune polarization in patients, which correlate with fluid mediators (eg, IgE and specific TH1/TH2 cytokines). Further supporting this notion is the excellent predictive value of CD4+CD161+ phospho-STAT1 and phospho-STAT6 levels in discriminating between groups of subjects, exceeding, for example, the predictive value of plasma IL-4 levels in identifying allergic versus nonallergic asthmatic patients. Importantly, CD4+CD161+ cells make up 15%to 25% of blood T cells21 and therefore represent an easily accessible population in which to follow the course of asthma in vivo.
Our finding of phospho-STAT alterations in unstimulated blood CD4+CD161+ T cells contrasts with previous phosphoepitope studies of human blood lymphocytes. Indeed, such studies invariably relied on strong in vitro biochemical activation of cells to maximize phosphoepitope detection.18 Here we designed our purification procedure for quick isolation of the cells of interest, without any detectable activation (see the Methods section). Our results demonstrate that with careful choice of the subset of interest, phosphoepitope profiling can carry significant value without any recourse to exogenous stimulation, focusing on freshly isolated cells. This approach is of significance for further research in asthma and in other diseases in which phosphorylation pathways play an important pathologic role and for which it could yield both novel mechanistic leads and clinically relevant biomarkers.
Alterations in phospho-STAT1 and phospho-STAT6 signaling mirror the allergic status and OCS treatment in asthmatic patients but not disease severity or pulmonary function
Our results identify a clear association of asthma with baseline increases in phospho-STAT6 and phospho-STAT1 levels and support the hypothesis that coordinated regulation34,35 of phospho-STAT6 and phospho-STAT1 represents a key determinant in allergic versus nonallergic asthma.10,11,26 In addition, the strong negative correlation (R=−0.82, P=.0007) between the 2 activated transcription factors among the entire asthmatic cohort further supports the notion that these 2 STAT isoforms function, at least in part, in an antagonistic fashion. Furthermore, our results identified age at onset as correlating positively with phospho-STAT1 levels and negatively with phospho-STAT6 levels. This suggests that STAT-dependent immune polarization in asthmatic patients might proceed differently according to age at onset, with early-onset disease more susceptible to fall into the high phospho-STAT6/IL-4/IgE, low phospho-STAT1 category, which is consistent with previous data.1 Interestingly, severity and pulmonary function indices did not correlate with phospho-STAT levels in CD4+CD161+ blood T cells (or other systemic markers of immune polarization, such as plasma IL-4 and IgE levels). Thus historical, organ-specific, and systemic biomarkers in asthma are not equivalent and are best combined in clinical practice.4
Our study further documents decreased phospho-STAT6 expression in CD4+CD161+ T cells and decreased plasma IL-4 levels on OCS treatment, which is consistent with the known inhibitory effect of OCSs on the STAT6/IL-4 pathway.36 The absence of decrease in phospho-STAT1 levels, as well as in plasma levels of IgE or cytokines other than IL-4, suggests a relative specificity of OCSs toward the STAT6/IL-4 pathway. OCSs altered phospho-STAT6 expression in blood CD4+CD161+ cells independently of dose (10–60 mg/d) and as early as 2 days on treatment, which suggests that this central/effector-memory subset can be quickly reprogrammed in vivo, even at low OCS doses. Alternatively, blood CD4+CD161+ T cells, although harboring a central/effector-memory phenotype, might not be long lived37 but rather undergo rapid in vivo turnover, possibly accelerated by OCSs. A third explanation for this phenomenon could be the OCS-induced sequestration of polarized CD4+CD161+ cells to lymphoid tissues other than blood.14 In the absence of OCSs, inhaled corticosteroids in relatively high doses (twice a day, 100–250 µg, based on disease severity28) did not normalize phospho-STAT expression, which confirms the nonequivalence of inhaled and OCSs.4
Clinical implications and perspectives
Further assessment of CD4+CD161+ T cells in asthmatic patients after OCS treatment has been tapered off will help in the investigation of how quickly they return to pathologic phospho-STAT levels in the subset. Indeed, the effect of OCSs on phospho-STAT6 levels has to be reversible because most allergic asthmatic patients assessed while in stable condition had undergone previous OCS treatment (>2 months before the study). Poor adherence to OCSs because of side effects can lead to poor control of asthma symptoms and increased disease severity and frequency of exacerbations.38 Poor adherence to OCSs can also lead to erroneous classification of patients as OCS insensitive39 and use of costlier anti-IgE antibody (omalizumab) treatment.5 It will also be interesting to assess asthmatic patients who are truly OCS insensitive and test whether the clinical efficiency of omalizumab itself (a B cell–directed treatment) is reflected by changes in the phospho-STAT profile of CD4+CD161+ blood T cells or other blood lymphocyte subsets (eg, circulating B cells).
In our study a disease control group of patients with Still disease did not demonstrate alterations in phospho-STAT levels similar to those seen in asthmatic patients. Further studies are required to assess whether such alterations in phospho-STAT pathways are limited to asthma or shared with other allergic, autoimmune, and chronic inflammatory syndromes.2,40,41 It will be important for future studies to investigate the exact role and in vivo turnover of CD4+CD161+ blood T cells. The biology of these cells, starting with the functional importance of the surface C-type lectin receptor CD161 and its membrane associates, such as the enzyme acid sphingomyelinase,23 are only starting to be unraveled. Additional studies should also include patients with poorly controlled asthma or those undergoing inflammatory exacerbations.38 Thus with further validation, single-cell assays of phospho-STAT levels might evolve into clinically relevant biomarkers42 to monitor not only ongoing immune polarization in asthma but also the effects of therapies.
Acknowledgments
We thank the asthmatic patients and healthy control subjects who provided samples for this study. We also thank R. Moss, K. Atkuri, D. Parks, J. Tung, E. Ghosn, and L. Herzenberg for help and advice.
Supported by the Mary Hewitt Loveless Foundation, the Walter and Idun Berry Fellowship, the Parker B. Francis Foundation, and the American Association of Allergy, Asthma & Immunology Special Interest Award.
Abbreviations used
- FACS
Fluorescence-activated cell sorting
- IL-4R
IL-4 receptor
- NK
Natural killer
- OCS
Oral corticosteroid
- ROC
Receiver operating characteristic
- STAT
Signal transducer and activator of transcription
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
REFERENCES
- 1.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 2.Umetsu DT, Dekruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238–255. doi: 10.1111/j.0105-2896.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 3.O’Byrne PM. Cytokines or their antagonists for the treatment of asthma. Chest. 2006;130:244–250. doi: 10.1378/chest.130.1.244. [DOI] [PubMed] [Google Scholar]
- 4.Bel EH. Clinical phenotypes of asthma. Curr Opin Pulm Med. 2004;10:44–50. doi: 10.1097/00063198-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Buhl R. Anti-IgE antibodies for the treatment of asthma. Curr Opin Pulm Med. 2005;11:27–34. doi: 10.1097/01.mcp.0000147860.83639.30. [DOI] [PubMed] [Google Scholar]
- 6.Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10:493–499. doi: 10.1016/j.molmed.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 8.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 9.Christodoulopoulos P, Cameron L, Nakamura Y, Lemiere C, Muro S, Dugas M, et al. TH2 cytokine-associated transcription factors in atopic and nonatopic asthma: evidence for differential signal transducer and activator of transcription 6 expression. J Allergy Clin Immunol. 2001;107:586–591. doi: 10.1067/mai.2001.114883. [DOI] [PubMed] [Google Scholar]
- 10.Pernis AB, Rothman PB. JAK-STAT signaling in asthma. J Clin Invest. 2002;109:1279–1283. doi: 10.1172/JCI15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann N, Mishra A, King NE, Fulkerson PC, Doepker MP, Nikolaidis NM, et al. Transcript signatures in experimental asthma: identification of STAT6-dependent and -independent pathways. J Immunol. 2004;172:1815–1824. doi: 10.4049/jimmunol.172.3.1815. [DOI] [PubMed] [Google Scholar]
- 12.Ratthe C, Pelletier M, Chiasson S, Girard D. Molecular mechanisms involved in interleukin-4-induced human neutrophils: expression and regulation of suppressor of cytokine signaling. J Leukoc Biol. 2007;81:1287–1296. doi: 10.1189/jlb.0306209. [DOI] [PubMed] [Google Scholar]
- 13.Wang IM, Lin H, Goldman SJ, Kobayashi M. STAT-1 is activated by IL-4 and IL-13 in multiple cell types. Mol Immunol. 2004;41:873–884. doi: 10.1016/j.molimm.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Parrillo JE, Fauci AS. Mechanisms of glucocorticoid action on immune processes. Annu Rev Pharmacol Toxicol. 1979;19:179–201. doi: 10.1146/annurev.pa.19.040179.001143. [DOI] [PubMed] [Google Scholar]
- 15.Barnes PJ, Adcock IM. How do corticosteroids work in asthma? Ann Intern Med. 2003;139:359–370. doi: 10.7326/0003-4819-139-5_part_1-200309020-00012. [DOI] [PubMed] [Google Scholar]
- 16.Shirai T, Inui N, Suda T, Chida K. Correlation between peripheral blood T-cell profiles and airway inflammation in atopic asthma. J Allergy Clin Immunol. 2006;118:622–626. doi: 10.1016/j.jaci.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Miller RL, Eppinger TM, McConnell D, Cunningham-Rundles C, Rothman P. Analysis of cytokine signaling in patients with extrinsic asthma and hyperimmunoglobulin E. J Allergy Clin Immunol. 1998;102:503–511. doi: 10.1016/s0091-6749(98)70141-1. [DOI] [PubMed] [Google Scholar]
- 18.Perez OD, Nolan GP. Phospho-proteomic immune analysis by flow cytometry: from mechanism to translational medicine at the single-cell level. Immunol Rev. 2006;210:208–228. doi: 10.1111/j.0105-2896.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- 19.Loza MJ, Metelitsa LS, Perussia B. NKT and T cells: coordinate regulation of NK-like phenotype and cytokine production. Eur J Immunol. 2002;32:3453–3462. doi: 10.1002/1521-4141(200212)32:12<3453::AID-IMMU3453>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 20.Iliopoulou EG, Karamouzis MV, Missitzis I, Ardavanis A, Sotiriadou NN, Baxevanis CN, et al. Increased frequency of CD4+ cells expressing CD161 in cancer patients. Clin Cancer Res. 2006;12:6901–6909. doi: 10.1158/1078-0432.CCR-06-0977. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol. 2006;176:211–216. doi: 10.4049/jimmunol.176.1.211. [DOI] [PubMed] [Google Scholar]
- 22.Lanier LL, Chang C, Phillips JH. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol. 1994;153:2417–2428. [PubMed] [Google Scholar]
- 23.Pozo D, Vales-Gomez M, Mavaddat N, Williamson SC, Chisholm SE, Reyburn H. CD161 (human NKR-P1A) signaling in NK cells involves the activation of acid sphingomyelinase. J Immunol. 2006;176:2397–2406. doi: 10.4049/jimmunol.176.4.2397. [DOI] [PubMed] [Google Scholar]
- 24.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 25.Ho LP. Natural killer T cells in asthma—toward increased understanding. N Engl J Med. 2007;356:1466–1468. doi: 10.1056/NEJMe078014. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Khurana Hershey GK. Signal transducer and activator of transcription signals in allergic disease. J Allergy Clin Immunol. 2007;119:529–541. doi: 10.1016/j.jaci.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 27.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 28.Bousquet J. Global initiative for asthma (GINA) and its objectives. Clin Exp Allergy. 2000;30 suppl 1:2–5. doi: 10.1046/j.1365-2222.2000.00088.x. [DOI] [PubMed] [Google Scholar]
- 29.Efthimiou P, Paik PK, Bielory L. Diagnosis and management of adult onset Still’s disease. Ann Rheum Dis. 2006;65:564–572. doi: 10.1136/ard.2005.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi HZ, Li S, Xie ZF, Qin XJ, Qin X, Zhong XN. Regulatory CD4+CD25+ T lymphocytes in peripheral blood from patients with atopic asthma. Clin Immunol. 2004;113:172–178. doi: 10.1016/j.clim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 32.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: a guide for the perplexed. Nat Immunol. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 33.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 34.Wurtz O, Bajenoff M, Guerder S. IL-4-mediated inhibition of IFN-gamma production by CD4+ T cells proceeds by several developmentally regulated mechanisms. Int Immunol. 2004;16:501–508. doi: 10.1093/intimm/dxh050. [DOI] [PubMed] [Google Scholar]
- 35.Yu CR, Mahdi RM, Ebong S, Vistica BP, Chen J, Guo Y, et al. Cell proliferation and STAT6 pathways are negatively regulated in T cells by STAT1 and suppressors of cytokine signaling. J Immunol. 2004;173:737–746. doi: 10.4049/jimmunol.173.2.737. [DOI] [PubMed] [Google Scholar]
- 36.So EY, Kim SH, Park HH, Cho BS, Lee CE. Corticosteroid inhibits IL-4 signaling through down-regulation of IL-4 receptor and STAT6 activity. FEBS Lett. 2002;518:53–59. doi: 10.1016/s0014-5793(02)02635-2. [DOI] [PubMed] [Google Scholar]
- 37.Robertson JM, MacLeod M, Marsden VS, Kappler JW, Marrack P. Not all CD4+ memory T cells are long lived. Immunol Rev. 2006;211:49–57. doi: 10.1111/j.0105-2896.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 38.Chanez P, Wenzel SE, Anderson GP, Anto JM, Bel EH, Boulet LP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007;119:1337–1338. doi: 10.1016/j.jaci.2006.11.702. [DOI] [PubMed] [Google Scholar]
- 39.Horne R. Compliance, adherence, and concordance: implications for asthma treatment. Chest. 2006;130 suppl:65S–72S. doi: 10.1378/chest.130.1_suppl.65S. [DOI] [PubMed] [Google Scholar]
- 40.Adcock IM, Chung KF, Caramori G, Ito K. Kinase inhibitors and airway inflammation. Eur J Pharmacol. 2006;533:118–132. doi: 10.1016/j.ejphar.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 41.Tirouvanziam R. Neutrophilic inflammation as a major determinant in the progression of cystic fibrosis. Drug News Perspect. 2006;19:610–614. doi: 10.1358/dnp.2006.19.10.1068008. [DOI] [PubMed] [Google Scholar]
- 42.Deykin A. Biomarker-driven care in asthma: are we there? J Allergy Clin Immunol. 2006;118:565–568. doi: 10.1016/j.jaci.2006.06.007. [DOI] [PubMed] [Google Scholar]