Abstract
A dysregulation in central serotonin neurotransmission and omega-3 fatty acid deficiency have been implicated in the pathophysiology of major depression. To determine the effects of omega-3 fatty acid deficiency on indices of serotonin neurotransmission in the adult rat brain, female rats were fed diets with or without the omega-3 fatty acid precursor α-linolenic acid (ALA) during perinatal (E0–P90), post-weaning (P21–P90), and post-pubescent (P60–130) development. Ovariectomized (OVX) rats and OVX rats with cyclic estrogen treatment were also examined. Serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) content, and fatty acid composition were determined in the prefrontal cortex (PFC), and tryptophan hydroxylase-2 (TPH-2), serotonin transporter, and 5-HT1A autoreceptor mRNA expression were determined in the midbrain. ALA deficiency during perinatal (−62%, p=0.0001), post-weaning (−34%, p=0.0001), and post-pubertal (−10%, p=0.0001) development resulted in a graded reduction in adult PFC docosahexaenoic acid (DHA, 22:6n-3) composition. Relative to controls, perinatal DHA-deficient rats exhibited significantly lower PFC 5-HT content (−65%, p=0.001), significant greater 5-HIAA content (+15%, p=0.046), and a significant greater 5-HIAA/5-HT ratio (+73%, p=0.001). Conversely, post-weaning DHA-deficient rats exhibited significantly greater PFC 5-HT content (+12%, p=0.03), no change in 5-HIAA content, and a significantly smaller 5-HIAA/5-HT ratio (−9%, p=0.01). Post-pubertal DHA-deficient and OXV rats did not exhibit significant alterations in PFC 5-HT or 5-HIAA content. Only perinatal DHA-deficient rats exhibited a significant reduction in midbrain TPH-2 mRNA expression (−29%, p=0.03). These preclinical data support a causal link between perinatal omega-3 fatty acid deficiency and reduced central serotonin synthesis in adult female rats that is independent of ovarian hormones.
Keywords: Omega-3 fatty acids, docosahexaenoic acid (DHA), 5-HT, 5-HIAA, estrogen, prefrontal cortex, tryptophan hydroxylase-2, serotonin transporter, 5-HT1A, female, rat
1. Introduction
Multiple lines of clinical and postmortem evidence suggest that a dysregulation in central serotonin neurotransmission contributes to the pathophysiology of major depression and suicide (reviewed in Arango et al., 2002). For example, low cerebrospinal fluid (CSF) 5-hydroxyindoleacetic acid (5-HIAA) content is correlated with suicidal behavior (reviewed in Placidi et al., 2001), and postmortem studies have observed low midbrain serotonin (5-HT) and/or 5-HIAA content in suicides with or without depression (reviewed in Mann et al., 1989). Furthermore, medication-free depressed patients were found to exhibit reduced indices of 5-HT synthesis (Rosa-Neto et al., 2004), and significant elevations in internal jugular venoarterial 5-HIAA plasma content relative to healthy controls (Barton et al., 2008). Chronic treatment with selective serotonin reuptake inhibitors (SSRI) significantly decrease central 5-HIAA content in depressed patients (Barton et al., 2008; Lundmark et al., 1994; Potter et al., 1985; Sheline et al., 1997) and in rat prefrontal cortex (PFC)(Unceta et al., 2007). Although these findings suggest that depression and suicide are associated with perturbations in central 5-HT neurotransmission, pathogenic factors remain poorly understood and subtotal heritability estimates suggest that both genetic and environmental factors confer vulnerability (Sullivan et al., 2000).
There is an emerging body of evidence from cross-national and cross-sectional epidemiological surveys that suggest that omega-3 fatty acid deficiency may be a risk factor for major depression (reviewed in Parker et al., 2006). Meta-analyses of controlled intervention trials suggest that increasing omega-3 fatty acid intake reduces depression symptom severity in patients with affective disorders (Freeman et al., 2006; Lin & Su, 2007). Additional support comes from tissue fatty acid composition studies finding red blood cell or plasma omega-3 fatty acid deficits in suicide attempters (Garland et al., 2007; Huan et al., 2004) and patients with major depression (Assies et al., 2004; Edwards et al., 1998; Maes et al., 1999; Peet et al., 1998) relative to healthy controls. Moreover, significant and selective deficits in the principal omega-3 fatty acid in brain, docosahexaenoic acid (DHA, 22:6n-3), were found in the postmortem PFC of female, but not male, patients with major depression (McNamara et al., 2007). This gender difference is consistent with cross-sectional epidemiological surveys finding that low dietary omega-3 fatty acid intake is associated with an increased risk for depression in females but not in males (Tanskanen et al., 2001; Timomen et al., 2004). It is relevant therefore that the conversion of dietary α-linolenic acid (18:3n-3) to DHA may be positively regulated by estrogen (Giltay et al., 2004; Burdge & Wootton, 2002), and multiple findings have implicated low estrogen levels in the pathophysiology of depression and suicide in women (Grigoriadis & Kennedy, 2002; Saunders & Hawton, 2006).
One candidate mechanism by which brain DHA deficiency may increase risk for major depression and suicide is by altering central 5-HT neurotransmission. This is supported by a clinical neurochemical study finding that plasma omega-3 fatty acid concentrations were positively correlated with CSF 5-HIAA levels among healthy subjects (Hibbeln et al., 1998), and prospective studies finding that low plasma DHA composition (Sublette et al., 2006) and low CSF 5-HIAA content (Placidi et al., 2001) are both significant predictors of future suicidal behavior. Furthermore, patients with major depression exhibit blunted fenfluramine-induced cerebral glucose metabolism in the PFC (Anderson et al., 2004), and a preclinical study found that dietary omega-3 fatty acid deficiency during perinatal development blunted fenfluramine-induced elevations in extracellular 5-HT content in the rat hippocampus (Kodas et al., 2004). Moreover, young depressed suicides also exhibit elevated 5-HT2A receptor binding density in the postmortem PFC (Arango et al., 1990; Pandey et al., 2002), and perinatal omega-3 fatty acid deficiency increases 5-HT2A receptor binding in the young adult rat PFC (Delion et al., 1996). Conversely, dietary-induced elevations in DHA composition increase PFC 5-HT content (Chalon et al., 1998) and attenuate reductions in PFC 5-HT content in response to chronic stress (Vancassel et al., 2008). Together, these findings support a link between brain DHA composition and the maturation and resilience of central 5-HT synaptic transmission.
The present study had three objectives. The first objective was to determine the effects of dietary-induced brain DHA deficits initiated at different stages of brain development on 5-HT turnover in the PFC of adult female rats. Although 5-HT turnover is an indirect measure of synaptic 5-HT function, it is relevant to the pathophysiology of major depression (Barton et al., 2008; Lundmark et al., 1994; Potter et al., 1985; Sheline et al., 1997) and amenable to translational studies in rodents. We investigated DHA deficiency initiated during perinatal (E0–P90), post-weaning (P21–P90), and post-pubertal (P60–P130) development. The second objective was to determine whether brain DHA deficits alter midbrain expression of mRNA markers of presynaptic 5-HT neurotransmission, including tryptophan hydroxylase-2 (TPH-2), the rate-limiting enzyme in the biosynthesis of 5-HT in neurons (Walther et al., 2003; Zhang et al., 2004), the 5-HT transporter (SERT), and the 5-HT1A autoreceptor, which regulates 5-HT axonal firing and 5-HT release in the PFC (Sprouse & Aghajanian, 1986). Because these studies were conducted in female rats, the third objective was to determine the influence of ovarian hormones including estrogen on variables of interest by determining the effects of chronic ovariectomy with or without cyclic estrogen replacement (Becker et al., 2005).
2. Materials and methods
2.1. Animals and dietary treatments
Adult (P60) male and female Long-Evans hooded rats (Harlan Farms, Indianapolis, IN) were paired, and males removed after 2 weeks. In the perinatal condition (E0–P90), nulliparous dams received either α-linolenic acid (ALA)-fortified diet (n=4) or ALA-depleted diet (n=4) for 1 month prior to mating, and female offspring were maintained on the maternal diet following weaning into adulthood (P90). In the post-weaning condition (P21–P90), female offspring were randomly selected from 10 different litters of nulliparous dams maintained on ALA-fortified diet to receive either ALA-fortified diet or ALA-depleted diet immediately following weaning to adulthood (P90). In the post-pubertal condition (P60–P130), female rats purchased from Harlan Farms (Indianapolis, IN) were randomly assigned to either remain on ALA-fortified diet or be switched to ALA-depleted diet from P60 to P130. Both ALA-fortified and ALA-depleted diets were matched for all non-fat nutrients and fatty acids with the exception of ALA, which was not detectable in the ALA-depleted diet and represented 4.6% of total fatty acid composition in the control diet (see Table 1 in McNamara et al., 2008). Rats were housed 2 per cage with food and water available ad libitum, and were maintained under standard vivarium conditions on a 12:12 h light:dark cycle. All experimental procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee, and adhere to the guidelines set by the National Institutes of Health.
2.2. Ovariectomy and estradiol replacement
Sham and ovariectomy (OVX) surgeries were performed by Harlan Sprague-Dawley (Indianapolis, IN) in young (P56) female Long-Evans hooded rats. One half of the OVX rats were administered estradiol benzoate (Sigma-Aldrich, E8515) in sesame oil (Sigma-Aldrich, S3547) at a dose of 2 μg (s.c.) every four days from P60-P130, and one half were administered a equal volume of sesame oil. A prior study found that this cyclic estrodiol regimen was sufficient to normalize body weight gain, food intake, and sexual receptivity in OVX rats (Asarian & Geary, 2002). Rats were sacrificed 1 h after the final injection, and plasma estradiol levels determined with a DSL-4800 Ultra-sensitive estradiol radioimmunoassay kit (Diagnostic Systems Laboratories, Inc., Webster Texas).
2.3. Tissue collection
Rats were sacrificed by decapitation during the light portion of the cycle, brains extracted and immediately immersed in ice-cold 0.9% NaCl for 2 min. The brain was then dissected on ice to isolate the midbrain (inc. dorsal raphe nuclei) and PFC, and the olfactory tubercle and residual striatal tissue were removed from the PFC. Individual tissues were placed in cryotubes, flash frozen in liquid nitrogen, and stored at −80°C. At the time of sacrifice, vaginal spears were collected onto glass slides, dried, and stained (Dipp-Kwik Stain kit, American Master/Tech Scientific Inc., Lodi, CA) to determine position in the estrous cycle (metestrus, diestrus, proestrous, estrus) based on cell morphology (Becker et al., 2005).
2.4. HPLC-ECD
PFC 5-HT content and 5-HIAA content were determined by high performance liquid chromatography with electrochemical detection (HPLC-ECD). A detailed description of the HPLC-ECD procedure has been published previously (Perez et al., 2005). Briefly, an antioxidant solution (0.4 N perchlorate, 1.34 mM ethylenediaminetetraacetic acid (EDTA) and 0.53 mM sodium metabisulfite) was added to the samples followed by homogenization using an ultrasonic tissue homogenizer (Biologics, Gainesville, VA). A fraction of the tissue homogenate was dissolved in 2% sodium dodecyl sulfate (SDS) (w/v) for protein determination (Pierce BCA Protein Reagent Kit, Rockford, IL). The remaining suspension was spun at 14,000 × g for 20 min in a refrigerated centrifuge. The supernatant was reserved for HPLC. Samples were separated on a Microsorb MV C-18 column (5 μm, 4.6×250 mm, Varian, Walnut Creek, CA) and simultaneously examined for 5-HT and 5-HIAA. Compounds were detected using a 12-channel coulometric array detector (CoulArray 5200, ESA, Chelmsford, MA) attached to a Waters 2695 Solvent Delivery System (Waters, Milford, MA). Samples were processed by a technician blinded to treatment.
2.5. Gas chromatography
Total (triglyceride, phospholipid, and cholesteryl ester) fatty acid composition was determined using the saponification and methylation methods originally described by Mecalfe et al (1966). Comparison of control female PFC DHA composition found in the present study (17.3±0.2 wt % TTL) with control female frontal cortex DHA composition found in a prior study using isolated phospholipids (17.7±0.6 wt % TTL)(Levant et al., 2007) suggest that triglyceride and cholesteryl ester DHA comprise a relatively small percentage of total DHA composition. Brain samples (~80 mg wet weight) were placed in a 20 ml glass vial into which 4 ml of 0.5N methanolic sodium hydroxide was added, and the sample heated at 80°C for 5 min. Following a 10 min cooling period, 3 ml of boron trifluoride in methanol was added to methylate the sample. After an additional five minutes of heating in the water bath (80°C), the sample vial was allowed to cool, and 2 ml of a saturated solution (6.2 M) of sodium chloride and 10 ml of hexane was added. The samples were then mixed by vortex for one minute. The hexane fraction was then transferred into a 20 ml vial containing 10 mg of sodium sulfate to dry the sample. The hexane solution was then removed for GC analysis. Samples were analyzed with a Shimadzu GC-2014 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD). The column was a DB-23 (123–2332): 30m (length), I.D. 0.32 mm wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom CA). The GC conditions were: column temperature ramping by holding at 120°C for one minute followed by an increase of 5°C/min from 120–240°C. The temperature of the injector and flame ionization detector was 250°C. A split (8:1) injection mode was used. The carrier gas was helium with a column flow rate of 2.5 ml/min. Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Analysis of fatty acid methyl esters is based on areas calculated with Shimadzu Class VP 4.3 software. Fatty acid composition data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids, wt % total). In addition to PFC DHA composition, we also determined PFC docosapenaenoic acid (DPA, 22:5n-6) and arachidonic acid (AA, 20:4n-6) compositions, and the AA:DHA. Samples were processed by a technician blinded to treatment.
2.6. Midbrain mRNA expression
The real-time reverse transcriptase polymerase chain reaction (RT-PCR) procedure has been described in detail previously (McNamara et al., 2006). Primers and fluorogenic probes (Midland Certified Reagent Company, Midland, TX) were designed using Primer Express v.2.0 software (Applied Biosystems, Foster City, CA) based on the rat mRNA sequences for GAPDH (GenBank accession number: NM_017008), TPH-2 (NM_173839), SERT (NM_013034), 5-HT1A (NM_012585). Primers used for GAPDH amplification were 5′-CTCAACTACATGGTCTACATGTTCCA-3′ and 5′-CTTCCCATTCTCAGCCTTGACT-3′, and the probe sequence was 5′-CCCACGGCAAGTT CAACGGCA-3′. The sequences of TPH-2 primers were 5′-TACCTGAGCCCA AGAGACTTCCT-3′and 5′-GCTCATGGCATGTGTCTGGTT-3′, and the probe sequence was 5′-CTCCGACCCCCTCTACACCCCG-3′. The sequences of SERT primers were 5′-AACTCCTGGAACACTGGCAACT-3′and 5′-GCAGGACATGGCGCAAGTA-3′, and the probe sequence was 5′-CAACTACTTCG CCCAGGACAACATCACC-3′. The sequences of 5-HT1A primers were 5′-TGTTGCTCATGCTGGTTCTCTAC-3′and 5′-CTGACAGTCTTGCGGATTCG-3′, and the probe sequence was 5′-CGCATCTTCAGAGCCGCACGC-3′. Each probe was conjugated to a FAM reporter at the 5′ end and a TAMRA quencher at the 3′ end. The reverse primer spanned an exon-intron junction to obviate genomic DNA contamination. Reverse transcription was performed using a 9600 GeneAmp thermocycler (Perkin-Elmer, Norwalk, CT). Relative mRNA quantities were normalized to GAPDH mRNA values obtained from the same tissue. All samples were processed by a technician blinded to treatment.
2.7. Statistical analysis
Fatty acid, neurochemical, and mRNA data were analyzed with a one-way ANOVA, with diet treatment as the main factor. Pairwise comparisons used unpaired t-tests (2-tail, α=0.05). A parametric (Pearson) linear regression analysis was used to evaluate the interrelationship between PFC 5-HT content and midbrain TPH-2 mRNA expression. Group differences in estrous phase were evaluated by non-parametric Mann-Whitney U tests (2-tail, α=0.05). For these analyses, metestrus, diestrus, proestrous, and estrus phases were arbitrarily assigned numerical values (1,2,3,4, respectively). All statistical analyses were performed with GB-STAT software (Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Perinatal omega-3 fatty acid deficiency
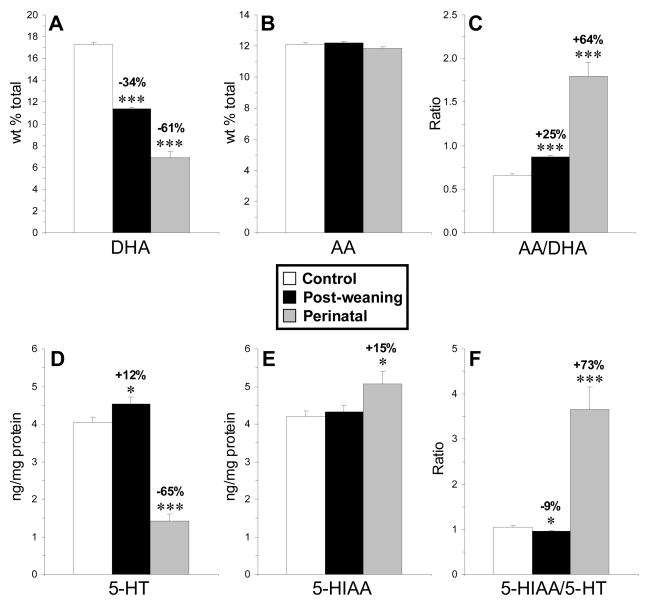
Female rats maintained on ALA-depleted diet from E0–P90 (perinatal) exhibited significant deficits in PFC DHA composition (−61%, p≤0.0001) relative to female rats maintained on ALA-fortified diet (Fig. 1A). No significant alterations in arachidonic acid (AA, 20:4n-6) composition were observed (Fig. 1B), and the AA:DHA ratio (+64%, p≤0.0001)(Fig. 1C) and DPA (22:5n-6) composition (+>99%, p≤0.0001) were significantly elevated. Perinatal DHA-deficient rats exhibited significantly lower PFC 5-HT content (−65%, p=0.001)(Fig. 1D), significantly greater 5-HIAA content (+15%, p=0.001)(Fig. 1E), and a significantly greater 5-HIAA/5-HT ratio (+73%, p=0.001) relative to controls (Fig. 1F). Control and perinatal DHA-deficient groups did not differ significantly in their position in the estrous cycle at the time of sacrifice (p=0.509).
Fig. 1.
Mean DHA composition (A), arachidonic acid (AA) composition (B ), the AA/DHA ratio (C), 5-HT content (D), 5-HIAA content (E), and the 5-HIAA/5-HT ratio (F) in the PFC of control (n=12), post-weaning (n=10), and perinatal (n=8) DHA-deficient rats. Values are means ± S.E.M. *P ≤0.05, ***P ≤ 0.001 vs. controls.
3.2. Post-weaning omega-3 fatty acid deficiency
Female rats maintained on ALA-depleted diet from P21–P90 (post-weaning) exhibited significant deficits in PFC DHA composition relative to controls (−34%, p≤0.001)(Fig. 1A). No significant alterations in AA composition were observed (Fig. 1B), and the AA:DHA ratio (+25%, p≤0.0001)(Fig. 1C) and DPA (22:5n-6) composition (+99%, p≤0.0001) were significantly elevated. Post-weaning DHA-deficient rats exhibited significantly greater PFC 5-HT content (+12%, p=0.03)(Fig. 1D), no change in 5-HIAA content (Fig. 1E), and a significantly lower 5-HIAA/5-HT ratio (−9%, p=0.001) relative to controls (Fig. 1F). Control and post-weaning DHA-deficient groups did not differ significantly in their position in the estrous cycle at the time of sacrifice (p=0.549).
3.3. Post-pubertal DHA deficiency
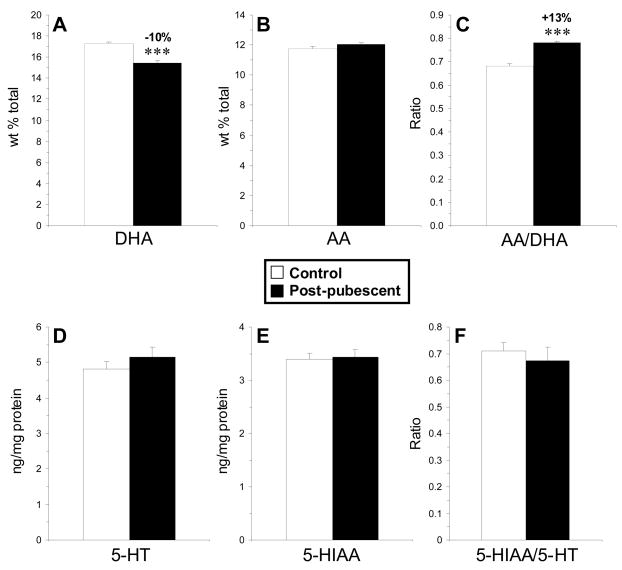
Female rats maintained on ALA-depleted diet from P60–P130 (post-pubertal) exhibited a significant deficit in PFC DHA composition (−10%, p≤0.0001) relative to controls (Fig. 2A). No significant alterations in AA composition were observed (Fig. 2B), and the AA:DHA ratio (+13%, p≤0.0001)(Fig. 2C) and DPA (22:5n-6) composition (+99%, p≤0.0001) were significantly elevated. PFC 5-HT content (Fig. 2D), 5-HIAA content (Fig. 2E), and the 5-HIAA/5-HT ratio (Fig. 2F) did not differ between post-pubertal DHA-deficient rats and controls (p>0.05). Controls and perinatal DHA-deficient groups did not differ significantly in their position in the estrous cycle at time of sacrifice (p=0.842).
Fig. 2.
Mean DHA composition (A), arachidonic acid (AA) composition (B), the AA/DHA ratio (C), 5-HT content (D), 5-HIAA content (E), and the 5-HIAA/5-HT ratio (F) in the PFC of control (n=8) and post-pubescent DHA-deficient (n=8) rats. Values are means ± S.E.M. ***P ≤ 0.001 vs. controls.
3.4. Ovariectomy
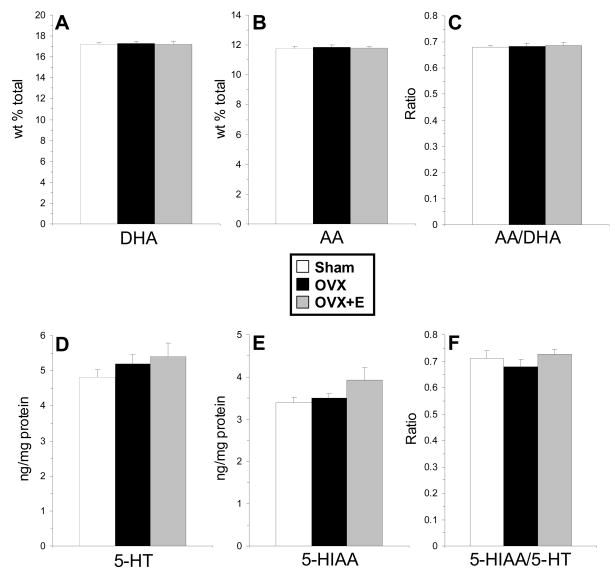
Consistent with a previous report (Asarian & Geary, 2002), vehicle-treated OVX rats (353±5.8 g) exhibited significantly greater body weight gain over 70 d (P60–P130) relative to controls (283±2.3 g, p≤0.0001), and this weight gain was significantly attenuated in OVX rats receiving cyclic estradiol treatment (317±5.9 g, p=0.007). Plasma estradiol concentrations in OVX+E rats 1 h after the final estradiol injection were 13.9±1.2 pg/ml, and plasma estradiol concentrations in OVX were below the limit of detection (<5 pg/ml). There were no significant group differences in PFC DHA composition, F(2,21)=0.06, p=0.94 (Fig. 3A), AA composition, F(2,21)=0.11, p=0.89 (Fig. 3B), the AA:DHA ratio, F(2,21)=0.05, p=0.95 (Fig. 3C), or DPA composition, F(2,21)=0.19, p=0.83. Similarly, there were no significant group differences in PFC 5-HT content, F(2,21)=1.04, p=0.38 (Fig. 3D), 5-HIAA content, F(2,21)=2.07, p=0.17 (Fig. 3E), or the 5-HIAA/5-HT ratio, F(2,21)=0.84, p=0.45 (Fig. 3F).
Fig. 3.
Mean DHA composition (A), arachidonic acid (AA) composition (AA)(B), the AA/DHA ratio (C), 5-HT (D), 5-HIAA (E), and the 5-HIAA/5-HT ratio (F) in the PFC of sham controls (n=8), OVX rats (n=8), and OVX rats with cyclic estrogen treatment (OVX+E)(n=8). Values are means ± S.E.M.
3.5. Midbrain mRNA expression
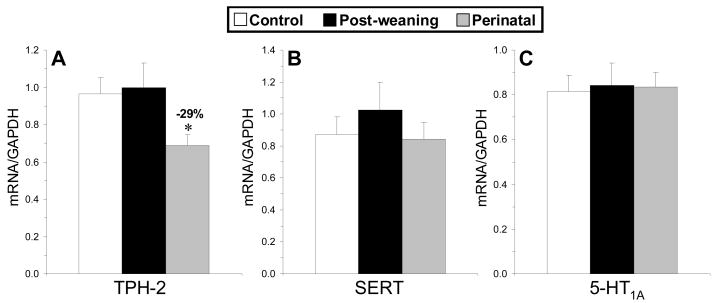
Among control, perinatal, and post-weaning DHA-deficient rats, midbrain GAPDH mRNA expression did not differ between groups, F(2,28)=0.272, p=0.764. A significant main effect was observed for TPH-2, F(2,28)=4.95, p=0.01, but not for SERT, F(2,28)=0.49, p=0.612, or 5-HT1A, F(2,27)=0.28, p=0.971, mRNA expression (Fig. 4A–C). Post-hoc tests found that the perinatal DHA-deficient group exhibited significantly lower TPH-2 mRNA expression relative to controls (−29%, p=0.03), and the difference between perinatal and post-weaning DHA-deficient groups approached significance (−31%, p=0.055)(Fig. 4A). PFC 5-HT content was positively correlated with midbrain TPH-2 mRNA expression (r = +0.57, p=0.001) and TPH-2/GAPDH mRNA expression (r = +0.49, p=0.007).
Fig. 4.
Mean tryptophan hydroxlase-2 (TPH-2)(A), serotonin transporter (SERT)(B), and 5-HT1A (C) mRNA expression (mRNA/GAPDH mRNA) in the midbrain of control (n=12), post-weaning (n=10), and perinatal (n=8) DHA-deficient rats. Values are means ± S.E.M. *P ≤0.05 vs. controls.
4. Discussion
The main finding of this study is that dietary-induced DHA deficits are associated with significant alterations in 5-HT and 5-HIAA content and 5-HT turnover (5-HT/5-HIAA) in the PFC of adult female rats, and that this effect is dependent on when omega-3 fatty acid depleted diets were introduced during perinatal development. Specifically, perinatal (E0–P90) omega-3 fatty acid deficiency increased PFC 5-HT turnover, post-weaning (P21–P90) omega-3 fatty acid deficiency decreased PFC 5-HT turnover, and post-pubescent omega-3 fatty acid deficiency did not alter PFC 5-HT turnover. Alterations in female PFC 5-HT turnover could not be attributed to position in the estrous cycle at time of sacrifice, and chronic ovariectomy resulting in plasma estradiol depletion did not alter PFC 5-HT turnover. These findings indicate that ovarian hormones including estrogen do not directly regulate PFC 5-HT turnover. Furthermore, only perinatal DHA-deficient rats exhibited significantly lower midbrain TPH-2 mRNA expression. Collectively, these findings demonstrate that deficits in omega-fatty acids during perinatal development reduce 5-HT synthesis and increase 5-HT turnover independently of ovarian hormones.
The greater 5-HT turnover observed in the PFC of perinatal DHA-deficient rats is attributable to both lower 5-HT content and elevated 5-HIAA content. Perinatal DHA-deficient rats also exhibited lower midbrain expression of TPH-2, the rate-limiting enzyme in neuronal 5-HT biosynthesis (Walther et al., 2003; Zhang et al., 2004), and among control, perinatal, and post-weaning DHA-deficient rats, PFC 5-HT content was positively correlated with midbrain TPH-2 mRNA expression. These data suggest that the lower 5-HT content observed in the PFC of perinatal DHA-deficient rats are due to reductions in TPH-mediated 5-HT synthesis. The prior finding that perinatal DHA-deficient rats exhibit greater basal extracellular 5-HT content in the hippocampus (Kodas et al., 2004) suggest that the reduction in PFC 5-HT content observed in perinatal DHA-deficient rats may reflect adaptive reductions in 5-HT synthesis in response to elevated basal 5-HT release and/or impaired reuptake. Additional studies will be required to clarify the synaptic mechanisms mediating elevated 5-HT turnover in the PFC of perinatal DHA-deficient rats.
The magnitude of the DHA deficit observed in the PFC of adult rats was dependent on when dietary omega-3 fatty acid deficiency was initiated. The graded PFC DHA deficits observed in perinatal (−61%), post-weaning (−34%), and post-pubescent (−10%) rats is consistent with age-related reductions in the loss half-life of DHA in response to dietary ALA insufficiency (Bourre et al., 1992; DeMar et al., 2004). Furthermore, the effects of DHA deficiency on PFC 5-HT turnover were also dependent on when dietary omega-3 fatty acid deficiency was initiated, and suggest that there is a neurodevelopmental window (E0–P21) in which 5-HT systems are vulnerable to dysregulation in response to DHA deficiency. This is also supported by the prior finding that the blunted fenfluramine-stimulated 5-HT release observed in perinatal DHA-deficient rats could be normalized if dietary omega-3 fatty acid fortification was initiated on P0, P7, or P14, but not when initiated on P21 despite the normalization of DHA composition (Kodas et al., 2004). Importantly, this neurodevelopmental window (E0-P21) coincides with the perinatal maturation of central 5-HT synapses (~E13-P14)(Hansson et al., 1998; Zhou et al., 2000). It will be of interest to determine whether the greater PFC 5-HT turnover observed in perinatal DHA-deficient rats can be normalized with early (<P21) postnatal omega-3 fatty acid intervention.
The present findings may take on additional significance in view of prior preclinical evidence for altered 5-HT neurotransmission in the frontal cortex of putative animal models of depression. For example, 5-HT turnover is significantly elevated in the frontal cortex of olfactory bulbectomized rats (Zhou et al., 1998), and both 5-HT and 5-HIAA content are elevated in the PFC of Flinders sensitive line (FSL) rats, a genetic rat model of depression (Zangen et al., 1997). Additionally, the present findings suggest that the perinatal DHA-deficient rat has face and construct validity as a model of depression because patients with major depression also exhibit PFC DHA deficits (McNamara et al., 2007), elevated central 5-HIAA content (Barton et al., 2008), and reduced indices of 5-HT synthesis (Rosa-Neto et al., 2004). Furthermore, chronic SSRI treatment decreases central 5-HIAA content in patients with major depression (Barton et al., 2008; Lundmark et al., 1994; Potter et al., 1985; Sheline et al., 1997), and decrease both 5-HT and 5-HIAA content in the rat PFC (Unceta et al., 2007). Interestingly, chronic SSRI treatment also increase rat midbrain TPH-2 mRNA expression (Shishkina et al., 2007). It will be of interest, therefore, to determine in future studies the effects of chronic SSRI treatment on PFC 5-HT turnover and midbrain TPH-2 mRNA expression in perinatal DHA-deficient rats to evaluate this models predictive validity.
In summary, the present data provide preclinical support for a causal link between perinatal omega-3 fatty acid deficiency and abnormalities in central 5-HT function in the adult female rat brain that are dissociable from estrogenic effects. Consistent with deficits in central 5-HT synthesis, the lower PFC 5-HT content found in perinatal DHA-deficient rats were associated with lower midbrain TPH-2 mRNA expression. The present and previous data also support a neurodevelopmental window (E0–P21) in which DHA is required for the normal development and functional maturation of rat 5-HT pathways. Collectively, these findings contribute to a growing body of evidence suggesting that perinatal deficits in brain DHA accrual is a neurodevelopmental risk factor for dysegulation in central neurotransmitter systems implicated in the pathophysiology of recurrent psychiatric disorders (reviewed inMcNamara & Carlson, 2006).
Acknowledgments
This work was supported in part by National Institute of Mental Health grants MH073704 and MH074858 to R.K.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AD, Oquendo MA, Parsey RV, Milak MS, Campbell C, Mann JJ. Regional brain responses to serotonin in major depressive disorder. J Affect Disord. 2004;82:411–417. doi: 10.1016/j.jad.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, Reis DJ, Mann JJ. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. 1990;47:1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Assies J, Lieverse R, Vreken P, Wanders RJ, Dingemans PM, Linszen DH. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol Psychiatry. 2001;49:510–522. doi: 10.1016/s0006-3223(00)00986-0. [DOI] [PubMed] [Google Scholar]
- Barton DA, Esler MD, Dawood T, Lambert EA, Haikerwal D, Brenchley C, Socratous F, Hastings J, Guo L, Wiesner G, Kaye DM, Bayles R, Schlaich MP, Lambert GW. Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Arch Gen Psychiatry. 2008;65:38–46. doi: 10.1001/archgenpsychiatry.2007.11. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Dumont OS, Piciotti MJ, Pascal GA, Durand GA. Dietary alpha-linolenic acid deficiency in adult rats for 7 months does not alter brain docosahexaenoic acid content, in contrast to liver, heart and testes. Biochim Biophys Acta. 1992;1124:119–122. doi: 10.1016/0005-2760(92)90087-c. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–420. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- Chalon S, Delion-Vancassel S, Belzung C, Guilloteau D, Leguisquet AM, Besnard JC, Durand G. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J Nutr. 1998;128:2512–2519. doi: 10.1093/jn/128.12.2512. [DOI] [PubMed] [Google Scholar]
- Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J Neurochem. 1996;66:1582–1591. doi: 10.1046/j.1471-4159.1996.66041582.x. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Jr, Marangell LB, Richardson AJ, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- Garland MR, Hallahan B, McNamara M, Carney PA, Grimes H, Hibbeln JR, Harkin A, Conroy RM. Lipids and essential fatty acids in patients presenting with self-harm. Br J Psychiatry. 2007;190:112–117. doi: 10.1192/bjp.bp.105.019562. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, Kennedy SH. Role of estrogen in the treatment of depression. Am J Ther. 2002;9:503–509. doi: 10.1097/00045391-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience. 1998;83:1185–1201. doi: 10.1016/s0306-4522(97)00444-2. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Linnoila M, Umhau JC, Rawlings R, George DT, Salem N., Jr Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biol Psychiatry. 1998;44:235–242. doi: 10.1016/s0006-3223(98)00141-3. [DOI] [PubMed] [Google Scholar]
- Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC, Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89:695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- Levant B, Ozias MK, Carlson SE. Specific brain regions of female rats are differentially depleted of docosahexaenoic acid by reproductive activity and an (n-3) fatty acid-deficient diet. J Nutr. 2007;137:130–134. doi: 10.1093/jn/137.1.130. [DOI] [PubMed] [Google Scholar]
- Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- Lundmark J, Wålinder J, Alling C, Manniche PM, Dalgaard L. The effect of paroxetine on cerebrospinal fluid concentrations of neurotransmitter metabolites in depressed patients. Eur Neuropsychopharmacol. 1994;4:1–6. doi: 10.1016/0924-977x(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Arango V, Marzuk PM, Theccanat S, Reis DJ. Evidence for the 5-HT hypothesis of suicide. A review of post-mortem studies. Br J Psychiatry. 1989;155:7–14. [PubMed] [Google Scholar]
- McNamara RK, Levant B, Taylor B, Ahlbrand R, Liu Y, Sullivan JR, Stanford K, Richtand NM. C57BL/6J mice exhibit reduced dopamine D3 receptor-mediated locomotor-inhibitory function relative to DBA/2J mice. Neuroscience. 2006;143:141–153. doi: 10.1016/j.neuroscience.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;74:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Sullivan J, Richtand NM, Jandacek R, Rider T, Tso P, Campbell N, Lipton J. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult DBA/2J mice: relationship with ventral striatum dopamine concentrations. Synapse. 2008;62:725–735. doi: 10.1002/syn.20542. [DOI] [PubMed] [Google Scholar]
- Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Pandey SC, Pesold C, Roberts RC, Conley RR, Tamminga CA. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- Perez SE, Lazarov O, Koprich JB, Chen EY, Rodriguez-Menendez V, Lipton JW, Sisodia SS, Mufson EJ. Nigrostriatal dysfunction in familial Alzheimer’s disease-linked APPswe/PS1DeltaE9 transgenic mice. J Neurosci. 2005;25:10220–10229. doi: 10.1523/JNEUROSCI.2773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placidi GP, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50:783–791. doi: 10.1016/s0006-3223(01)01170-2. [DOI] [PubMed] [Google Scholar]
- Potter WZ, Scheinin M, Golden RN, Rudorfer MV, Cowdry RW, Calil HM, Ross RJ, Linnoila M. Selective antidepressants and cerebrospinal fluid. Lack of specificity on norepinephrine and serotonin metabolites. Arch Gen Psychiatry. 1985;42:1171–1177. doi: 10.1001/archpsyc.1985.01790350045009. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Diksic M, Okazawa H, Leyton M, Ghadirian N, Mzengeza S, Nakai A, Debonnel G, Blier P, Benkelfat C. Measurement of brain regional alpha-[11C]methyl-L-tryptophan trapping as a measure of serotonin synthesis in medication-free patients with major depression. Arch Gen Psychiatry. 2004;61:556–563. doi: 10.1001/archpsyc.61.6.556. [DOI] [PubMed] [Google Scholar]
- Saunders KE, Hawton K. Suicidal behaviour and the menstrual cycle. Psychol Med. 2006;36:901–912. doi: 10.1017/S0033291706007392. [DOI] [PubMed] [Google Scholar]
- Sheline Y, Bardgett ME, Csernansky JG. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1997;17:11–14. doi: 10.1097/00004714-199702000-00003. [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. (-)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. Eur J Pharmacol. 1986;128:295–298. doi: 10.1016/0014-2999(86)90782-x. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamäki H, Lehtonen J, Vartiainen E. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52:529–531. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Räsänen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82:447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Unceta N, Barrondo S, de Azúa IR, Gómez-Caballero A, Goicolea MA, Sallés J, Barrio RJ. Determination of fluoxetine, norfluoxetine and their enantiomers in rat plasma and brain samples by liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:519–528. doi: 10.1016/j.jchromb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Vancassel S, Leman S, Hanonick L, Denis S, Roger J, Nollet M, Bodard S, Kousignian I, Belzung C, Chalon S. n-3 polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. J Lipid Res. 2008;49:340–348. doi: 10.1194/jlr.M700328-JLR200. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Zangen A, Overstreet DH, Yadid G. High serotonin and 5-hydroxyindoleacetic acid levels in limbic brain regions in a rat model of depression: normalization by chronic antidepressant treatment. J Neurochem. 1997;69:2477–2483. doi: 10.1046/j.1471-4159.1997.69062477.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- Zhou D, Grecksch G, Becker A, Frank C, Pilz J, Huether G. Serotonergic hyperinnervation of the frontal cortex in an animal model of depression, the bulbectomized rat. J Neurosci Res. 1998;54:109–116. doi: 10.1002/(SICI)1097-4547(19981001)54:1<109::AID-JNR11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK. Expression of serotonin transporter protein in developing rat brain. Dev Brain Res. 2000;119:33–45. doi: 10.1016/s0165-3806(99)00152-2. [DOI] [PubMed] [Google Scholar]