Abstract
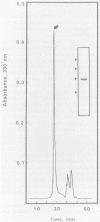
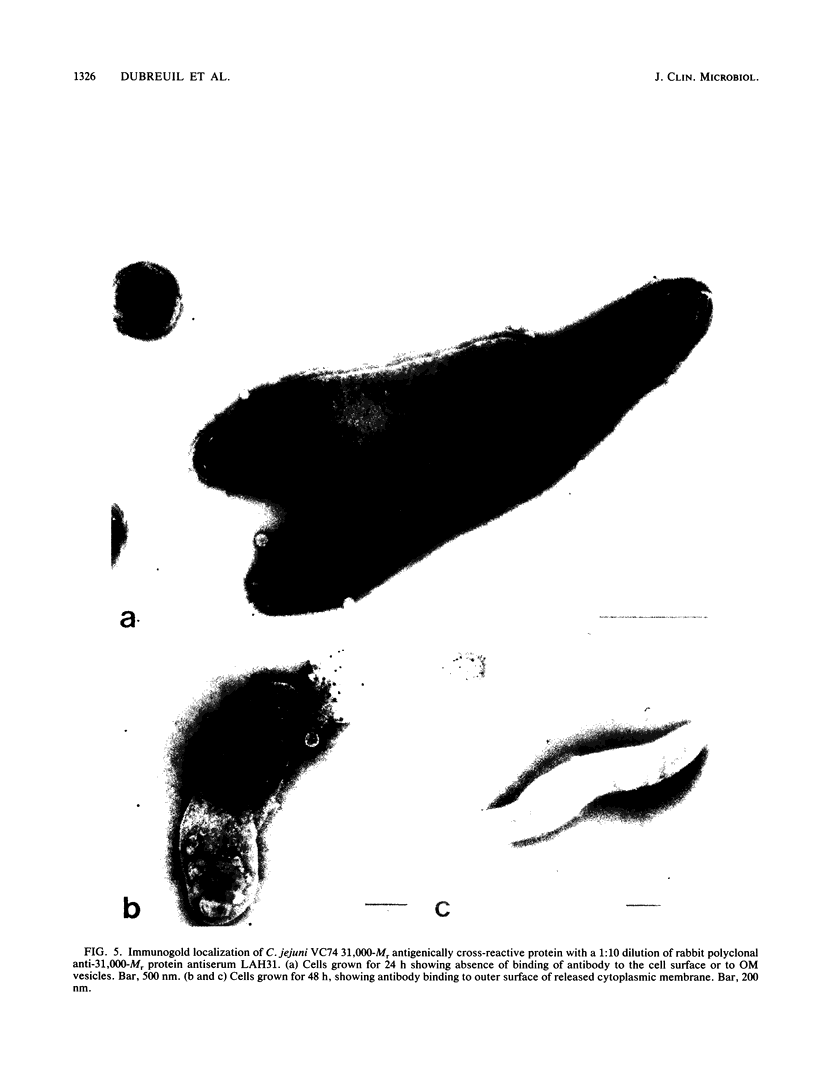
A protein antigen with an apparent molecular weight (Mr) of 31,000 was isolated from 0.2 M glycine hydrochloride (pH 2.2) extracts of a typical human fecal isolate, Campylobacter jejuni VC74. The protein was purified to homogeneity on a preparative scale by immunoaffinity chromatography followed by molecular sieving with a Superose 12 column. Isoelectric focusing under nondenaturing conditions indicated a pI of 9.3, and amino acid composition analysis showed that the protein was unusually rich in lysine, containing 14.9 mol% of this basic amino acid. Cysteine and tryptophan were absent. The protein also contained approximately 35% hydrophobic amino acid residues, and N-terminal amino acid analysis showed that 17 of the first 38 residues were hydrophobic. This amino-terminal sequence to residue 22 was virtually identical to that of an antigenically cross-reactive 31,000-Mr protein isolated from another C. jejuni strain belonging to a different heat-labile serogroup. Western blotting (immunoblotting) of glycine extracts of other C. jejuni, Campylobacter coli, and Campylobacter laridis strains belonging to different thermolabile and thermostable serotypes, as well as Campylobacter fetus, with a rabbit polyclonal antiserum raised against the purified C. jejuni VC74 protein showed that all C. jejuni, C. coli, and C. laridis strains tested contained a 31,000-Mr protein with epitopes which were antigenically cross-reactive with the C. jejuni VC74 protein. The antigenically cross-reactive epitopes of this protein were also readily detected by immunodot blot assay of glycine extracts of C. jejuni, C. coli, and C. laridis with monospecific polyclonal antisera to the 31,000-Mr protein, suggesting that this serological test could be a useful addition to those currently employed in the rapid identification of these important pathogens. Slide agglutination reactions, immunofluorescence assay, and immunogold electron microscopy with antisera to purified 31,000-Mr protein and trypsin treatment of whole cells indicated that the cross-reactive epitopes of the 31,000-Mr protein were not exposed on the cell surface. Cell fractionation analysis and immunogold electron microscopy located the protein on the outer surface of the cytoplasmic membrane. This finding suggests that the 31,000-Mr protein is not a good candidate for inclusion in a monovalent subunit Campylobacter vaccine.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrantes F. J. The nicotinic cholinergic receptor : different compositions evidenced by statistical analysis. Biochem Biophys Res Commun. 1975 Jan 20;62(2):407–414. doi: 10.1016/s0006-291x(75)80153-7. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Vasil M. L. Campylobacter jejuni outer membrane proteins are antigenic for humans. Infect Immun. 1984 Mar;43(3):986–993. doi: 10.1128/iai.43.3.986-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988 Mar;2(2):237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Caldwell M. B., Guerry P., Lee E. C., Burans J. P., Walker R. I. Reversible expression of flagella in Campylobacter jejuni. Infect Immun. 1985 Dec;50(3):941–943. doi: 10.1128/iai.50.3.941-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M. N. Campylobacter jejuni enteritis; a review. Trop Geogr Med. 1984 Sep;36(3):215–222. [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Dooley J. S., McCubbin W. D., Kay C. M., Trust T. J. Isolation and biochemical characterization of the S-layer protein from a pathogenic Aeromonas hydrophila strain. J Bacteriol. 1988 Jun;170(6):2631–2638. doi: 10.1128/jb.170.6.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil J. D., Logan S. M., Cubbage S., Eidhin D. N., McCubbin W. D., Kay C. M., Beveridge T. J., Ferris F. G., Trust T. J. Structural and biochemical analyses of a surface array protein of Campylobacter fetus. J Bacteriol. 1988 Sep;170(9):4165–4173. doi: 10.1128/jb.170.9.4165-4173.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell C. L., Totten P. A., Quinn T. C., Patton D. L., Holmes K. K., Stamm W. E. Characterization of Campylobacter-like organisms isolated from homosexual men. J Infect Dis. 1984 Jan;149(1):58–66. doi: 10.1093/infdis/149.1.58. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Harris L. A., Logan S. M., Guerry P., Trust T. J. Antigenic variation of Campylobacter flagella. J Bacteriol. 1987 Nov;169(11):5066–5071. doi: 10.1128/jb.169.11.5066-5071.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S. M. Hippurate hydrolysis by Campylobacter fetus. J Clin Microbiol. 1980 Apr;11(4):435–437. doi: 10.1128/jcm.11.4.435-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmeland L. M. A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6368–6370. doi: 10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Antigenic cross-reactivity of major outer membrane proteins in enterobacteriaceae species. J Gen Microbiol. 1979 Apr;111(2):293–302. doi: 10.1099/00221287-111-2-293. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Hébert G. A., Hollis D. G., Weaver R. E., Lambert M. A., Blaser M. J., Moss C. W. 30 years of campylobacters: biochemical characteristics and a biotyping proposal for Campylobacter jejuni. J Clin Microbiol. 1982 Jun;15(6):1065–1073. doi: 10.1128/jcm.15.6.1065-1073.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert G. A., Hollis D. G., Weaver R. E., Steigerwalt A. G., McKinney R. M., Brenner D. J. Serogroups of Campylobacter jejuni, Campylobacter coli, and Campylobacter fetus defined by direct immunofluorescence. J Clin Microbiol. 1983 Mar;17(3):529–538. doi: 10.1128/jcm.17.3.529-538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper M. H. The independent distribution of amino acid near neighbor pairs into polypeptides. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1018–1024. doi: 10.1016/0006-291x(77)90523-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeGendre N., Matsudaira P. Direct protein microsequencing from Immobilon-P Transfer Membrane. Biotechniques. 1988 Feb;6(2):154–159. [PubMed] [Google Scholar]
- Lior H. New, extended biotyping scheme for Campylobacter jejuni, Campylobacter coli, and "Campylobacter laridis". J Clin Microbiol. 1984 Oct;20(4):636–640. doi: 10.1128/jcm.20.4.636-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lior H., Woodward D. L., Edgar J. A., LaRoche L. J. Serotyping by slide agglutination of Campylobacter jejuni and epidemiology. Lancet. 1981 Nov 14;2(8255):1103–1104. doi: 10.1016/s0140-6736(81)91293-9. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Location of epitopes on Campylobacter jejuni flagella. J Bacteriol. 1986 Nov;168(2):739–745. doi: 10.1128/jb.168.2.739-745.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983 Nov;42(2):675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy E. C., Doyle D., Burda K., Corbeil L. B., Winter A. J. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect Immun. 1975 Mar;11(3):517–525. doi: 10.1128/iai.11.3.517-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Pearson A. D. The identification of outer membrane proteins and flagella of Campylobacter jejuni. J Gen Microbiol. 1984 May;130(5):1201–1208. doi: 10.1099/00221287-130-5-1201. [DOI] [PubMed] [Google Scholar]
- Page W. J., Taylor D. E. Comparison of methods used to separate the inner and outer membranes of cell envelopes of Campylobacter spp. J Gen Microbiol. 1988 Nov;134(11):2925–2932. doi: 10.1099/00221287-134-11-2925. [DOI] [PubMed] [Google Scholar]
- Patton C. M., Shaffer N., Edmonds P., Barrett T. J., Lambert M. A., Baker C., Perlman D. M., Brenner D. J. Human disease associated with "Campylobacter upsaliensis" (catalase-negative or weakly positive Campylobacter species) in the United States. J Clin Microbiol. 1989 Jan;27(1):66–73. doi: 10.1128/jcm.27.1.66-73.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980 Dec;12(6):732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L. The genus Campylobacter: a decade of progress. Clin Microbiol Rev. 1988 Apr;1(2):157–172. doi: 10.1128/cmr.1.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautelin H., Kosunen T. U. An acid extract as a common antigen in Campylobacter coli and Campylobacter jejuni strains. J Clin Microbiol. 1983 Apr;17(4):700–701. doi: 10.1128/jcm.17.4.700-701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E. F., Dannelly H. K., Malloy P. J., Reeves H. C. Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal Biochem. 1987 Dec;167(2):290–294. doi: 10.1016/0003-2697(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B., Benjamin J. Differentiation of enteropathogenic Campylobacter. J Clin Pathol. 1980 Nov;33(11):1122–1122. doi: 10.1136/jcp.33.11.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. I., Caldwell M. B., Lee E. C., Guerry P., Trust T. J., Ruiz-Palacios G. M. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenman W. M., Chai J., Louie T. J., Goudreau C., Lior H., Newell D. G., Pearson A. D., Taylor D. E. Antigenic analysis of Campylobacter flagellar protein and other proteins. J Clin Microbiol. 1985 Jan;21(1):108–112. doi: 10.1128/jcm.21.1.108-112.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]